Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research - (2024)Volume 14, Issue 6
The misfolding and self-assembly of α-synuclein into fibrillar aggregates are closely linked to Parkinson's Disease (PD) and related dementia. Emerging evidence indicates that intermediate states, particularly small oligomers, formed during the misfolding process are the major toxic species. Despite the importance of characterizing these small oligomers to better understand the molecular mechanisms of misfolding, their biophysical study has been hindered by the absence of dependable methods for generating diverse oligomeric forms. In this work, we describe distinct small misfolded oligomers generated from Wild-Type (WT) and pathogenic variants (A53T and G51D ) of α-synuclein, which were produced by using lipid vesicles at pH 7.4. Our findings indicate that single-point mutations affect the aggregation behavior of α-synuclein under the influence of Cardiolipin (CL)-containing vesicles, a key component of mitochondrial membranes. Structural analysis via Circular Dichroism (CD) and Nuclear Magnetic Resonance (NMR) revealed that these oligomers display distinct structural and dynamic characteristics. These structurally unique oligomers will be of great use in investigating the structure-function relationship of misfolded oligomers in neurodegenerative diseases.
Oligomer; Misfolding; α-synuclein; Mutation; Cardiolipin
DOPC: 1,2-Dioleoyl-sn-Glycero-3-Phosphocholine; DLB: Dementia with Lewy Bodies; HSQC: Heteronuclear Single-Quantum Coherence; DOPE: 1,2-Dioleoyl-sn-Glycero-3-Phosphoethanolamine; PD: Parkinson’s Disease; DLS: Dynamic Light Scattering; TEM: Transmission Electron Microscopy; CL: Cardiolipin; CD: Circular Dichroism; ThT: Thioflavin T; DMPS: 1,2-Dimyristoyl-sn-Glycero-3-Phospho-L-Serine; NMR: Nuclear Magnetic Resonance
Understanding the biophysical characteristics of misfolded small oligomers is essential to resolve the complex molecular mechanisms underlying protein misfolding and aggregation processes, which are essential for the development of therapeutic agents and biomarkers aimed at reducing the progression of neurodegenerative diseases. The preparation of well-defined oligomeric species, essential for the biophysical characterization, poses a significant challenge due to the tendency for misfolding and aggregation to occur through multiple pathways, resulting in a diverse arrangement of oligomeric conformations [1-6].
The misfolding and self-assembly of α-synuclein are influenced by numerous factors. Among these factors are negatively charged lipids, metal ions, various amyloidogenic proteins such as tubulin associated unit (tau) and β-amyloid, and even Deoxyribonucleic Acid (DNA) [7-11]. The cofactors can interact with α-synuclein and modulate its aggregation tendency [12,13]. These interactions may also promote nucleation, accelerate fibril elongation or stabilize intermediate oligomeric species, thereby facilitating the formation of diverse α-synuclein aggregates with varying sizes, structures and toxicity levels. Our recent cryo-Electron Microscopy (cryo- EM) structural studies have provided insights into the interplay between α-synuclein and cofactors, tau and phospholipids [14]. Specifically, these studies have revealed that tau can induce the formation of homogeneous α-synuclein fibrillar species. Moreover, in the presence of a model lipid vesicle composed of DMPS, untwisted α-synuclein filaments were detected, indicating that the interaction between α-synuclein and cofactors may steer the polypeptide toward a particular aggregation pathway [15].
More recently, we utilized phospholipid vesicles as a model system that can mimic mitochondria membranes [16]. α-synuclein has been found to localize to mitochondria, and there is growing evidence suggesting a link between α-synuclein and mitochondrial dysfunction [17-19]. Mitochondrial membranes are unique due to the presence of negatively charged CL [20].
The interaction of CL with α-synuclein has been identified as an essential factor contributing to α-synuclein aggregation and mitochondrial impairment [21-25]. Small non-toxic and toxic oligomeric species of WT α-synuclein could be prepared using phospholipid vesicles containing CL [16].
Building upon these findings, we extended our investigation to include pathogenic variants (G51D and A53T) linked to early- onset familial PD [26]. Our biophysical studies revealed that these pathogenic variants altered the α-synuclein aggregation properties, resulting in the formation of structurally distinct oligomers compared to WT α-synuclein. The distinct α-synuclein oligomers derived from WT and pathogenic variants of α-synuclein associated with different disease phenotypes will help us investigate structure-function relationship of misfolded protein oligomers.
Expression and purification of α-synuclein
A pET21 a plasmid (#51486) containing the full-length human α-synuclein was utilized to produce recombinant α-synuclein. Bacterial expression was performed using BL21 (DE3) competent cells, following the established protocol [27]. The Site-Directed Mutagenesis (SDM) kit (GeneArtTM, Invitrogen) was used to prepare the pathogenic variants (G51D and A53T). Both WT and mutant α-synuclein were expressed and purified in the same manner. Briefly, an isolated colony was selected and grown in a 5 ml LB medium at 37°C for 3 h to create a starter culture. The small culture was subsequently transferred into 0.5 l of LB medium containing carbenicillin at a concentration of 100 mg/l. In the case of an expression of isotopically 15N-labeled α-synuclein, the LB medium was replaced with the M9 minimal medium (M9 media) supplemented with 15NH4Cl. The cells were incubated at 37°C with shaking (250 rpm). To induce α-synuclein overexpression, Isopropyl-beta-D-Thiogalactopyranoside (IPTG) at a final concentration of 0.5 mM was added at Optical Density600 (OD600) of 0.8, and the cells were then grown overnight at 25°C. After the overnight expression, the cells were spun down at 5000 g (4°C).
The cell pellet was dissolved evenly using the 20 mM Tris buffer (pH 8) with a protease inhibitor cocktail. The cells were then sonicated for five cycles (30 s on and 30 s off). The resulting lysate was harvested via centrifugation at 10,000 g for 20 min at 4°C. ammonium sulfate at a final concentration of 30% (w/v) was added to precipitate nucleic acids, and the resulting precipitate was centrifuged at 10,000 g. The remaining supernatant was subjected to further precipitation using 50% (w/v) ammonium sulfate and the α-synuclein pellet was obtained by centrifugation at 10,000 g for 30 min.
The protein pellet was resuspended using 10 mM Tris buffer (pH 8.0) and then dialyzed overnight at 4°C to remove salts and impurities. The protein solution was purified in two stages: Initially through anion exchange chromatography with a HiTrap Q HP column, followed by Gel Filtration Chromatography (GFC) using a HiLoad 16/60 Superdex 200 pg column, both performed at 4°C.
Preparation of α-synuclein oligomers
To generate α-synuclein oligomers, monomeric α-synuclein (60 μM) was mixed with lipid vesicles (100 μM) at pH 7.4 and incubated at 37°C for three weeks. The solution containing the oligomers was then concentrated by 20 times using a 50 kDa cut off centrifugal filter. After concentration, 15% ammonium sulfate was added to precipitate the oligomers, and the resulting precipitate was centrifuged at 4°C.
Transmission Electron Microscopy (TEM)
A 3 μl aliquot of the protein samples was applied to glow- discharged carbon-coated formvar copper 300 mesh grids. After a 30 s incubation of the grid, the excess sample was gently washed away by filter paper and distilled water was applied to wash the grid. The samples were then subjected to negative staining with 1% uranyl acetate for 30 s. Following the staining process, the excess staining solution was gently blotted off, and the grids were allowed to air-dry. The grids were subsequently examined using a Philips CM12 TEM at 80 kV.
Preparation of lipid vesicles
The three lipids like CL, DOPE and DOPC, solubilized in chloroform were combined in a molar ratio of 1.0:1.3:2.0. The chloroform was then removed under vacuum, and the lipid mixture was air dried under vacuum overnight. The dried lipid mixture was rehydrated in 10 mM phosphate buffer (pH 6.5) to a total lipid concentration of 3 mM. The lipid suspension was kept at 45°C for 2 h with vertexing every 10 min.
The lipid solution was subjected to five freeze-thaw cycles using dry ice and a water bath set at 45°C. Large Unilamellar Vesicles (LUVs) with a diameter of 100 nm were prepared by extruding the lipid solution 10 times through a 100 nm membrane filter at 45°C. A Dynamic Light Scattering (DLS) equipment (DynaPro NanoStar) was utilized to examine the distribution of vesicle sizes, ensuring that a consistent final size range of 90 nm to 110 nm was attained.
Aggregation assay
The aggregation kinetics of α-synuclein were analyzed using a Thioflavin T (ThT) fluorescence assay on a SpectraMax® microplate reader. Monomeric α-synuclein (60 μM) was incubated with ThT (50 μM) in the presence and absence of lipid vesicles (100 μM). Two hundred microliters of each sample, in duplicate, were transferred to a 96-well black clear-bottom microplate, which was then tightly sealed. The microplates were incubated in a stationary state at 37°C, and ThT fluorescence emission at 482 nm was measured periodically, with excitation set at 440 nm.
CD spectroscopy
A Jasco 815 spectrometer equipped with a 0.1 cm path length quartz cuvette was used to acquire the CD spectra for α-synuclein oligomers. A 0.2 mg/ml α-synuclein oligomeric sample in 10 mM phosphate buffer (pH 7.4) was used for the CD experiment and 30 scans were recorded and averaged for each sample.
Solution-state NMR
15N-labeled α-synuclein monomers (60 μM) in the presence of CL-containing lipid vesicles (100 μM) was incubated for 3 weeks at 37°C. Following the incubation period, TEM was used to check for the presence of α-synuclein oligomers. The NMR samples were prepared by concentrating the oligomers 10-20 fold using a 50 kDa membrane filter. Two-dimensional 1H/15N HSQC NMR spectra of the isotopically 15N-labeled α-synuclein oligomers were recorded using a 5 mm cryoprobe on an 800 MHz NMR spectrometer (Bruker).
The aggregation of both WT α-synuclein and its pathogenic variants (G51D and A53T) was investigated with lipid vesicles designed to mimic the composition of mitochondria inner membranes (Figure 1). These lipid vesicles consisted of a mixture of CL, DOPE and DOPC, with a molar ratio of 1.0:1.3:2.0 [20]. The experiments were conducted under physiological conditions, with a pH of 7.4, to closely resemble the cellular environment.
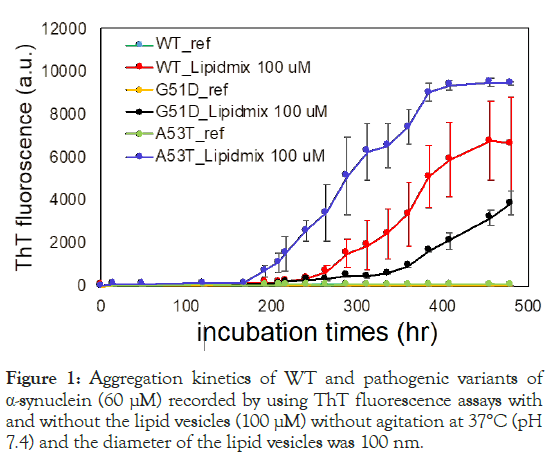
Figure 1: Aggregation kinetics of WT and pathogenic variants of α-synuclein (60 μM) recorded by using ThT fluorescence assays with and without the lipid vesicles (100 μM) without agitation at 37°C (pH 7.4) and the diameter of the lipid vesicles was 100 nm.
The aggregation kinetics of α-synuclein, both with and without lipid vesicles, were examined using the ThT fluorescence assay. After prolonged incubation, the ThT fluorescence increased gradually (blue, red and black), while no significant increase was detected even after extended incubation without lipid vesicles. Notably, the presence of negatively charged CL in lipid vesicles accelerated α-synuclein aggregation at the neutral pH, indicating that lipid-protein interactions play an important role in modulating aggregation kinetics. Furthermore, the assay revealed distinct effects of specific amino acid substitutions in the N-terminal region of α-synuclein on its aggregation kinetics. The A53T mutation was found to accelerate aggregation, leading to a faster increase in ThT fluorescence compared to WT α-synuclein. Conversely, the G51D mutation slowed down aggregation kinetics relative to WT α-synuclein.
The morphological features of the α-synuclein aggregates prepared using the lipid vesicles was investigated by TEM. Figure 2, displays the TEM images capturing small oligomeric species with a diameter of ~10 nm. It is notable that both WT and pathogenic variants of α-synuclein form the small oligomers despite their different aggregation kinetics under the influence of CL-containing lipid vesicles.
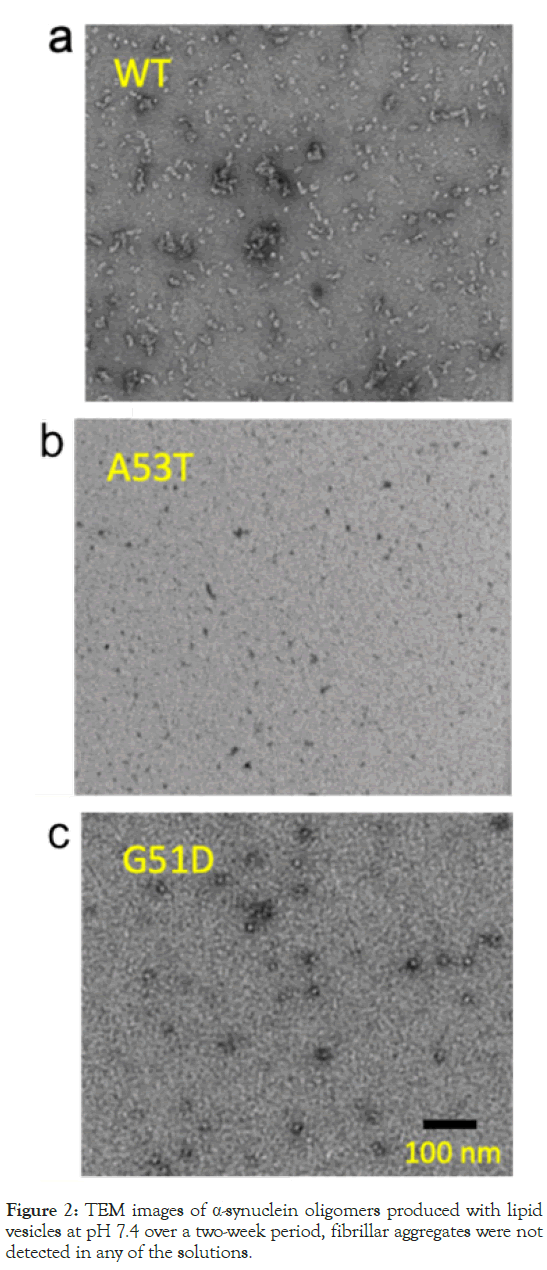
Figure 2: TEM images of α-synuclein oligomers produced with lipid vesicles at pH 7.4 over a two-week period, fibrillar aggregates were not detected in any of the solutions.
Given that both monomeric and oligomeric species may coexist in equilibrium, it's necessary to separate misfolded oligomers from native monomers for biophysical characterization. Our previous studies revealed that oligomeric species derived from WT α-synuclein and Transthyretin (TTR) can be isolated from the native proteins using a reduced amount of ammonium sulfate [16,28]. This method exploited the fact that misfolded oligomers possess a stronger aggregation property compared to native monomers. This difference in aggregation tendency allows for the selective precipitation of misfolded oligomers while leaving native monomers in solution when using a reduced amount of ammonium sulfate [29].
In order to prepare α-synuclein oligomers with ammonium sulfate, two steps were undertaken. Initially, the protein solution, with a concentration of 60 μM, was concentrated by about 20 times using membrane filters (50 kDa cut off). Then, ammonium sulfate at a concentration of 15% was utilized to isolate the oligomeric species, as the monomeric protein starts to precipitate at concentrations of 35%–40%. The stability of the oligomer samples was assessed after being precipitated, confirming that the precipitated samples comprised the oligomeric species, as depicted in Figure 3.
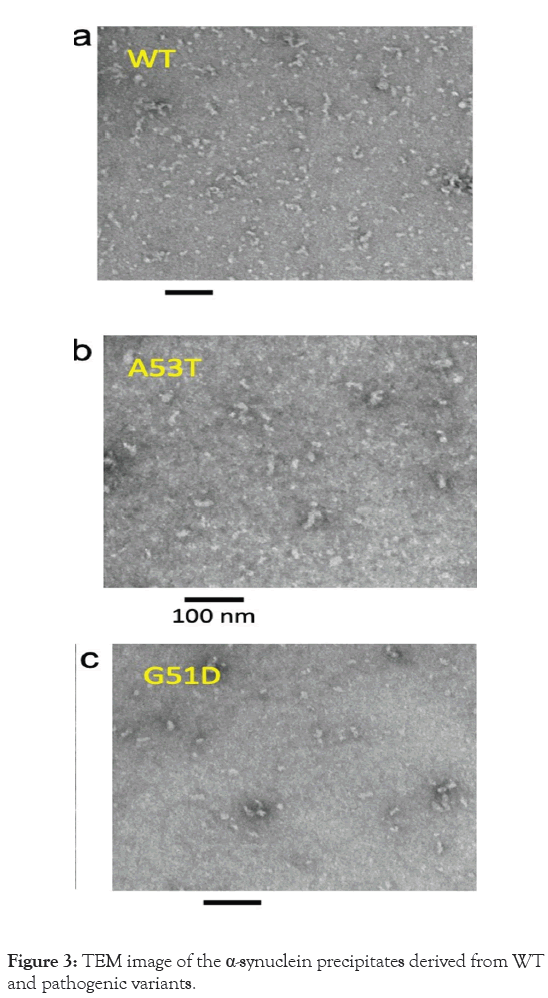
Figure 3: TEM image of the α-synuclein precipitates derived from WT and pathogenic variants.
The structural characteristics of the oligomers were examined using CD spectroscopy, as illustrated in Figure 4. Analysis of the CD spectra revealed that the WT oligomers have helical characteristics, as evidenced by the significant intensity observed at around 210 nm in Table 1. The CD spectra obtained from the variant oligomers display different characteristics, indicating that the mutant oligomers may adopt distinct molecular conformations compared to the WT oligomers (Figures 4a-4c). These differences in CD spectra suggest variations in the secondary structure elements (Table 1) and overall conformational properties of the oligomers induced by specific amino acid substitutions associated with the variants.
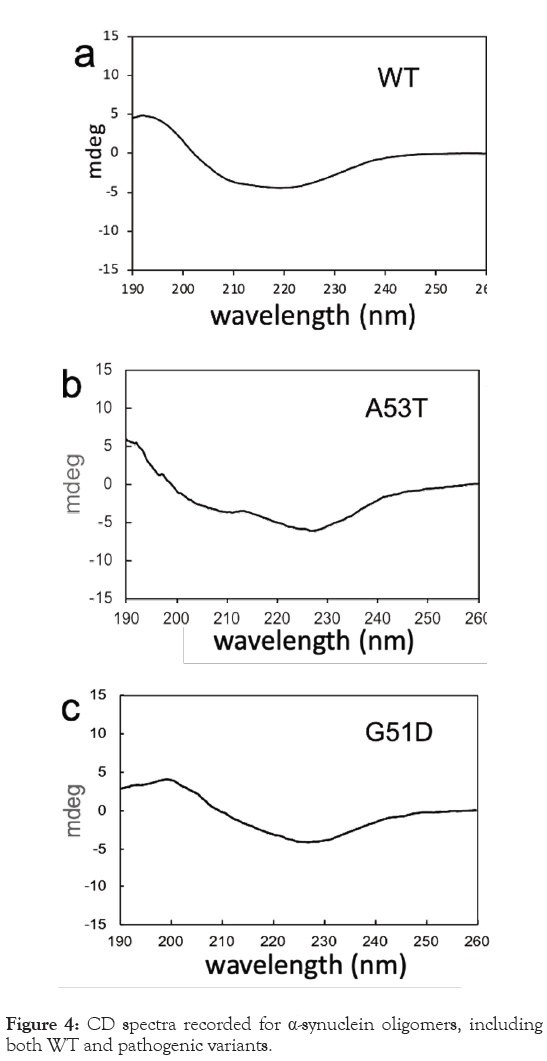
Figure 4: CD spectra recorded for α-synuclein oligomers, including both WT and pathogenic variants.
| α-synuclein oligomers | α-helix (%) | β-sheet (%) | Disordered (%) |
|---|---|---|---|
| WT | 9 | 38 | 53 |
| A53T | 8 | 45 | 47 |
| G51D | 0 | 53 | 47 |
Table 1: Secondary structural analyses of α-synuclein oligomers formed in the presence of CL-Small Unilamellar Vesicles (CL-SUVs) under different pH conditions using DichroWeb.
Misfolded oligomers are characterized by the presence of both dynamic and structured core regions. Solution NMR spectroscopy is particularly effective for investigating the flexible regions of these oligomers. Figure 5, displays the 1H/15N HSQC NMR spectra of the α-synuclein oligomers prepared using the lipid vesicles. The overlaid 2D NMR spectra of the α-synuclein monomers (black) and oligomers (green and red) show different NMR resonances for the oligomeric species, differentiating them from the native monomers. The less dispersed NMR resonances in the 1H dimension of the monomers (black) suggest disordered states of the protein. The different cross-peaks observed in the oligomeric species (green and red) suggest that the oligomers possess distinct structural characteristics compared to the monomers. Additionally, the surface HSQC spectra for WT and A53T oligomers exhibit different cross-peaks for the two oligomeric species (green and red), indicating that they have distinct flexible regions (Figure 5a).
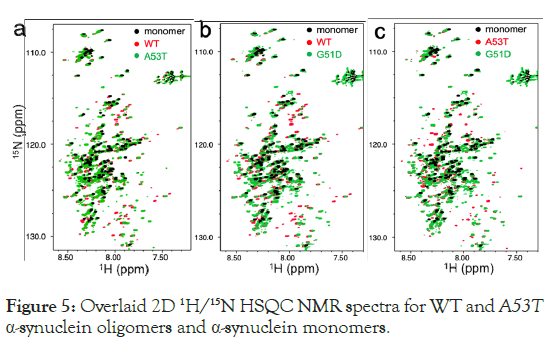
Figure 5: Overlaid 2D 1H/15N HSQC NMR spectra for WT and A53T α-synuclein oligomers and α-synuclein monomers.
The overlaid spectra for the other oligomers (red and green in Figures 5b and 5c) also show distinct cross-peaks. These NMR results suggest that the three oligomeric forms of α-synuclein (WT, A53T and G51D ) possess distinct structural properties. The NMR spectra were recorded using an 800 MHz NMR at 15°C, and sixty-four Free Induction Decays (FIDs) were recorded and averaged for the oligomer samples. The NMR samples (60 μM) were prepared by concentrating the protein solution, which had been incubated for two weeks, about 20 times using a membrane filter (50 kDa cutoff) for better signal to noise ratio. Primarily, the cross-peaks for the oligomers, which have different chemical shifts compared to the monomers, also exhibit narrow linewidths like those observed in the monomer NMR resonances. This narrow linewidth indicates that the α-synuclein oligomers formed through lipid interactions have regions of high flexibility. However, the distinct chemical shifts indicate that the flexible regions of the three α-synuclein oligomers may have different residual structures.
The preparation and biophysical characterization of diverse misfolded oligomers are important for understanding their cytotoxic nature and developing intervention strategies to block their cytotoxic effects. Although the exact molecular mechanisms lead to misfolding and aggregation within cells remain unclear, extensive studies have demonstrated that misfolding and aggregation can be induced by various cofactors in vitro. These studies have successfully prepared diverse α-synuclein oligomers of different sizes using such cofactors, indicating that they may significantly contribute to α-synuclein misfolding and aggregation in cellular environment [30]. Additionally, our previous studies demonstrated that lipid vesicles mimicking the mitochondrial membrane composition promote the formation of both toxic and non-toxic α-synuclein WT oligomers depending on pH [16]. In this study, we extended this investigation to the pathogenic variants G51D and A53T, which are associated with an earlier onset of PD, to examine the effect of these pathogenic mutations on oligomer formation under the influence of CL-containing lipid vesicles.
Our aggregation kinetic studies revealed that CL-containing lipid vesicles accelerate the aggregation of both WT and pathogenic variants (G51D and A53T) of α-synuclein (Figure 1). These findings align with previous studies showing that the N-terminal region (residues 1–60) of α-synuclein is essential for its interactions with lipid membranes [10,31]. The single-point mutations, A53T and G51D , located in this N-terminal region, significantly alter the aggregation behavior of α-synuclein under the influence of CL-containing lipid vesicles, as was observed in the negatively charged DMPS lipid vesicles [32]. The pathogenic mutations A53T and G51D affect the amyloidogenic property of α-synuclein in distinct ways. Our results show that the A53T mutation accelerates the aggregation process. This mutation likely enhances the interactions between the lipid membranes and α-synuclein, promoting aggregation. In contrast, the G51D mutation slows down the aggregation process, suggesting that this mutation may interfere with these interactions.
The N-terminal region of α-synuclein, which is positively charged at neutral pH, may interact with the negatively charged lipid CL in the lipid vesicles. The A53T mutation appears to make these interactions more favourable, possibly by altering the local charge distribution or the conformational dynamics of the protein, thereby enhancing the aggregation process. On the other hand, the G51D mutation introduces an additional negative charge in the N-terminal region, which may hinder the interaction between α-synuclein and CL-containing lipid vesicles, thus slowing down the aggregation process.
It is notable that both WT and pathogenic variants of α-synuclein form misfolded oligomers under the influence of CL-containing lipid vesicles. Our biophysical analyses using CD and NMR spectroscopy revealed that the pathogenic mutations induce the formation of misfolded α-synuclein oligomers with distinct structural, dynamic properties compared to WT oligomers (Figures 4 and 5). These results suggest that the specific interactions with CL-containing lipid vesicles lead to the formation of structurally different types of oligomers. Interestingly, other pathogenic mutations predominantly occur in the N-terminal region of α-synuclein such as A30P, E46K, H50Q [26]. This pattern supports the main role of the N-terminal region, presumably due to its interactions with lipid membranes. The differences in structural, dynamic properties of oligomers formed by WT and pathogenic α-synuclein variants highlight the importance of the N-terminal region in mediating interactions with lipid vesicles, influencing the misfolding pathway and the resultant oligomer structures. Understanding these variations would be essential for elucidating the mechanisms of the formation of toxic oligomers and their contributions to neurodegenerative diseases.
We demonstrated that CL-containing lipid vesicles, which mimic the inner mitochondrial membrane, promote the misfolding and oligomer formation of both WT and pathogenic variants of α-synuclein. The aggregation kinetics and the structural, dynamical properties of misfolded α-synuclein oligomers were dependent on amino acid substitutions in the N-terminal region, presumably due to the differential effects of these mutations on the interactions between the lipids and N-terminal region. Pathogenic mutations in this region appear to modulate protein- lipid interactions, thereby affecting the aggregation kinetics and the structural, dynamical properties of the misfolded oligomers. Further investigations with additional mutations such as A30P, E46K and H50Q in the N-terminal region will provide more detailed understanding into the molecular basis of these interactions. Finally, misfolded oligomers with different structural, dynamical properties prepared in this study can be utilized to investigate the structure-function relationship of these misfolded species.
This work was supported in part by National Institutes of Health (NIH) R01 AG054025 (Rakez Kayed), NS097490 (Kwang Hun Lim) and NS094557 (Rakez Kayed). The NHMFL is supported by the National Science Foundation (NSF) through NSF/DMR- 2128556 and the State of Florida.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Dasari AKR, Wi S, Kayed R, Lim KH (2024). Distinct Effect of the Pathogenic Mutations on ?-Synuclein Aggregation in the Presence of Lipid Vesicles. J Phys Chem Biophys.14:410.
Received: 20-Sep-2024, Manuscript No. JPCB-24-34156; Editor assigned: 23-Sep-2024, Pre QC No. JPCB-24-34156 (PQ); Reviewed: 14-Oct-2024, QC No. JPCB-24-34156; Revised: 21-Oct-2024, Manuscript No. JPCB-24-34156 (R); Published: 28-Oct-2024 , DOI: 10.35841/2161-0398.24.14.410
Copyright: © 2024 Dasari AKR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.