Orthopedic & Muscular System: Current Research
Open Access
ISSN: 2161-0533
ISSN: 2161-0533
Research Article - (2022)Volume 11, Issue 2
Objective: The aim of this study is to evaluate the analgesic effect of the new lipogel formulation based on Cannabis sativa oil enriched in CBD, escin, bromelain, Boswellia extract, glucosamine sulphate, MethylSulfonylMethane (MSM) and methylsalicylate (Cibides lipogel®), in patients with localized pain related to acute minor musculoskeletal conditions.
Methods: In this study 60 patients with pain due to one of the following conditions: tendinitis of the upper or lower limbs, low back pain, knee and ankle sprains/contusions, sport-related soft-tissue injury (sprains, strains and contusions) of upper or lower limbs, cervicalgia (neck pain), myalgias, arthrosis, carpal-tunnel syndrome, were included in the evaluation. Patients were allocated to treatment with the new lipogel formulation three times a day for 7 days and were evaluated at 5 timings: T0 (baseline), T1 (30 minutes after first application), T2 (1 day after first application), T3 (3 days after first application) and T4 (7 days after first application) by assigning a score in a 100-mm Visual Analogue Scale (VAS). The primary objective was to assess the analgesic effect by using the reduction of VAS score from T0 to T4. The secondary outcome was to obtain information on the safety of locally applied product.
Results: The results showed a statistically significant VAS score reduction versus baseline for each time point evaluated, with a mean reduction from 77.52 registered at T0 to 30.55 registered at T4 (P<0.001). The mean VAS score from T0 to T4 was reduced of about 47%. Regarding the secondary safety outcome, the gel application was well tolerated, and no adverse events were reported.
Conclusion: This study confirmed the analgesic effect of a new topical composition based on Cannabis sativa oil enriched in CBD, escin, bromelain, Boswellia extract, glucosamine sulphate, MethylSulfonylMethane (MSM) and methylsalicylate (Cibides lipogel®), in patients with localized pain related to acute minor musculoskeletal conditions.
SSRI; Physical activity; Muscle; Serotonin; Kynurenic acid; Kats; PGC1α
Fluoxetine is one of the important antidepressant of selective serotonin reuptake Inhibitors (SSRI) group, widely used to treat various mental health disorders, such as Moderate-to-severe depression and anxiety [1,2]. Symptoms contribute to insomnia, loss of Appetite, lack of motivation, and increased physical fatigue [3–5]. All these mentioned Symptoms may however, influence physical performances in athletes.
Fluoxetine elevates serotonin and tryptophan synaptic levels, an essential serotonin. Precursor [6,7]. Evidence shows that serotonin has central and peripheral effects. Kynurenine Pathway (KP) of tryptophan (TRP) metabolism is thought to be involved predominantly invariations of serotonin levels in both areas, but less is known in the periphery [8]. A Combination of endurance, physical exercise, and fluoxetine treatment has been shown to enhance muscular performance and induce the transcription factor PGC1 α [9,10]. Subsequently, PGC1 α regulates the expression of kynurenine aminotransferase (KAT) gene, an important KP enzyme in skeletal muscle [9]. Further, this combination of physical endurance exercise and fluoxetine treatment also decreases inflammation and, through this mechanism, decreases gene expression for others KP enzymes as tryptophan 2,3-dioxygenase (TDO), indoleamine 2,3-dioxygenase (IDO), and kynurenine 3-monooxygenase (KMO), increases the tryptophan consumption toward serotonin synthesis [11].
On the other hand, some recent studies confirmed that fluoxetine treatment ameliorates skeletal muscle oxidative capacity by enhancing mitochondrial enzyme. Activity [12]. A body of evidence suggests that PGC1 α was modified in both fiber-types of skeletal muscle by a long-term endurance exercise [13]. Among KP metabolites, the kynurenic acid (KYNA) has protective, while others have toxic roles [14,15]. Kynurenine and KYNA can Cross into muscle fibers, but the majority is intramuscularly produced. The amounts of KYN and KYNA locally produced after an endurance exercise are nevertheless not known.
We therefore present a study on the mutual effects of fluoxetine, exercise and combination in plasma and soleus through the involvement of skeletal muscle KYN Pathway.
Animals
Six-week old adult Balbc/j male mice were purchased from Janvier Labs (Le Genest Surl’Isle, France) weighing 21 g-25 g at the beginning of the study. They were randomly assigned as five mice per cage in a temperature (21 ±1°C) controlled room with a 12 h light: 12 h dark cycle (lights on at 06:00 h). Food and water were provided ad libitum. The protocols involving animals and their care were conducted in conformity with the institutional guidelines that comply with national and international laws and policies (Council directive #87-848, October 19, 1987, Ministère de l’Agriculture et de la Forêt, Service Vétérinaire dela Santé et de la Protection Animale, permissions # 92-373 to FC) and in compliance with protocols approved by the Institutional Animal Care and Use Committee (CEE26 authorization #6195).
Drugs
Fluoxetine hydrochloride (18 mg/kg per day in the drinking water for six weeks) was purchased from Anawa Trading (Zurich, Switzerland).
Treadmill-running
Exercise training consisted of running on a 6-lane rodent motor- driven treadmill (Ugo Basile®, Gemonio, Italy) equipped with UB X-Pad software version 1.0.01 that automatically recorded the distance, velocity, and time of animal running.
To reduce their stress, mice were faced with a treadmill for one-week adaptation (20 min/day, 15 m/min). To determinate individual maximal aerobic running speed, treadmill band speed was increased from 6 m/min by 0.03 m/s every 2 min until their exhaustion. Subsequently, mice underwent incremental exercise training for six weeks. Mice were divided into four groups, no exercise-saline, no exercise-fluoxetine, exercise-saline, and exercise-fluoxetine (n=11-12 animals per group). Bodyweight was assessed at the beginning of each week during the six weeks. After the last physical performance determination, animals were sacrificed by cervical dislocation before tissue harvest. Soleus muscle from both legs was dissected and mass was measured just after sampling. Tissues were frozen in liquid nitrogen and stored with plasma at -800°C until further analysis.
Kynurenine pathway evaluation:
KP-related metabolites evaluation by LC-MS/MS: To 190 μL of plasma or calibrator or controls, 10 μL of each internal standard (IS) was added. Samples were mixed for about 10 s, and 700 μL of methanol, 79 g/L zinc sulphate in water (80/20, v/v) containing 0.05% trifluoroacetic acid were added. After mixing, the samples were placed at 4°C for 15 min and centrifuged at 10,000 g for 15 min at 4°C. Seven hundred microliters of clear supernatant were transferred into a new tube and dried under a nitrogen stream. Residues were reconstituted with 30 μL of water containing 0.08% ASC. All pipetting was manual. LC–MS/MS was performed using a dionex ultimate 3000 liquid chromatography instrument (ThermoFisher, Waltham, USA) connected to a Sciex API 4000 tandem mass spectrometer (SCIEX, Ontario, Canada). Data acquisitions were made using SCIEX Analyst software (v.1.5.2). All compounds were separated over a Kinetex C18 column (100 mm × 2.1 mm i.d. column; 5 μM particle size) with a 2.1 mm C18 guard column, both obtained from Phenomenex (Torrance, USA). The mobile phases consisted of water containing 0.1% formic acid (FA) (mobile phase A) and acetonitrile (ACN) containing 0.1% FA (mobile phase B) (Figures 1A and 1B).
Figure 1: Kynurenine (KYN) to tryptophan (TRP) ratios of concentrations determined by LCMS/MS in plasma (A) or homogenates of soleus muscle (B). Male Balbc/j mice were treated by fluoxetine (18 mg/kg/day, p.o., 6 weeks) or saline and either submitted to six weeks treadmill training exercise (exercise group) or untrained (no exercise group)(n=11–12 mice per group). Data were presented as mean ± SEM and the statistical significance level was set at p<0.05. Note: ( ) Saline, (
) Saline, ( ) Fluoxetine.
) Fluoxetine.
The compounds were separated using a linear gradient that started at A/B=100/0 and ended at 7 min with A/ B=72/28. The mobile phase was then held at A/B =0/100 for 3 min and the LC column re equilibrated with A/B=100/0 for 5 min. To avoid detector saturation with tryptophan, we used a split between LC and MS/MS that began at 4.15 min and ended at 6.3 min. As only 4% of the flow arrived at MS/MS during the split, we also used a column shunt starting at 4.75 min for 1.6 min at a flow rate of 2.5 mL/min (schematic representation in Figure 2A).
Figure 2: Kynurenic acid (KYNA) to kynurenine (KYN) ratios of concentrations determined by LCMS/MS in plasma (A) or homogenates of soleus muscle (B). Male Balbc/j mice were treated by fluoxetine (18 mg/kg/day, p.o., 6 weeks) or saline and either submitted to six weeks treadmill training exercise (exercise group) or untrained (no exercise group)(n=11–12 mice per group). Data were presented as mean ± SEM and the statistical significance level was set at p < 0.05.Note: ( ) Saline, (
) Saline, ( ) Fluoxetine.
) Fluoxetine.
Mass Spectrometry detection was carried out in positive ion mode using electrospray ionization (ESI) source. The MS/MS parameters were optimized for each single standard metabolite by infusing each single solution (2 μg/mL) into mobile phase flow with a syringe pump (Hamilton Company, Reno, USA). The main metabolites (Trp, KYN and KYNA) were analyzed in positive ESI mode with several multiple reaction monitoring (MRM) scans used as quantifier or qualifier.
Gene-related gene evaluation by Real-time qPCR assay: Total RNA was isolated from cells or tissues (gastrocnemius, soleus, hippocampus and cortex) were extracted using Qiagen RNAeasy Mini Kit (Qiagen, USA) and following all steps recommended by the manufacturer. At the last step, RNA was re-suspended in 15 μl of RNAse-free water to allow for a high final concentration of RNA. RNA concentrations and quality were determined using a NanoDrop spectrophotometer (NanoDrop technologies, Wilmington, USA) and a RNA LabChip® 6000 Nano kit (Agilent Technologies, Santa Clara, USA). RNA quality cut off value was OD ratio 260/230 > 1.6 nm and OD ratio 260/280 ratio>1.7 nm, and RIN (RNA Integrity Number)>7.0. 0.5 μg of total RNA was then reverse transcribed and converted into double-stranded cDNA using the qScript cDNA Synthesis Kit (Quanta Biosciences, Gaithersburg, Maryland).
In brief, small PCR products (70–160 base-pairs) were amplified in quadruplets on a BioRad real-time PCR machine (CFX-96), using universal PCR conditions (65°C–59°C touch down, followed by 35 cycles (15 s at 95°C, 10 s at 59°C and 10 s at 72°C)). The cDNA was amplified in 20 μl as involved by the reactions (3 mm MgCl2, 200 nM dNTPs, 200 nM primers, 0.5 unit Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA). Primers were designed using Primer 3 Plus.
Results were calculated as the geometric mean of relative intensities compared to three internal controls (ACTIN, GAPDH and PPIG). Protocols were in accordance with the MIQE guidelines. KAT1, KAT2, PGC1α1 and NRF1 were studied in all tissues.
Statistical analyses
Prism computer software (GraphPad, San Diego, USA) was used to conduct statistical analysis. Two-way analysis of variance (ANOVA) followed by post-hoc Tukey’s multiple comparison tests were used to compare test groups to control groups: no exercise-saline, no exercise-fluoxetine, exercise-saline and exercise-fluoxetine. Data were presented as mean ± S.E.M. and the statistical significance level was set at p<0.05.
Metabolites evaluation
Tryptophan (TRP) and two KP metabolites, kynurenine (KYN) and kynurenic acid (KYNA), were assayed by LC-MS/MS in plasma and homogenates of soleus muscle. To simplify the results and prevent from further figures, data were presented as ratio to their respective precursor.
The plasma and soleus KYN/TRP ratios (Figures 1A and 1B) were significantly decreased after a physical exercise in the fluoxetine- treated group, (-45.7%, p<0.01, -55.2%, p<0.05, respectively). However, ratios were insignificantly decreased by physical exercise in saline-injected mice. The fluoxetine treatment vs saline did not significantly modify the KYN/TRP ratios in trained or untrained groups.
The KYNA/KYN ratios (Figures 2A and 2B) were significantly increased after a physical exercise in the fluoxetine-treated group, when compared to the sedentary treated or untreated groups (+37.0%, p<0.01, +132.0%, p<0.05, respectively). Ratios were also significantly increased after an exercise in saline-treated group (+29.7%, p<0.05, + 136.2%, p<0.05, respectively).
Gene expression evaluation
The KAT1, KAT2 (Figures 3A and 3B) and PGC1 α , NRF1 (Figures 4A and 4B) gene expressions were measured in soleus muscle. Exercise in saline-treated animals significantly induced significant changes in both gene expressions (p<0.001). We observed significantly increased KAT2 gene expression levels after fluoxetine treatment in untrained or trained animals compared to saline-treated group (+581%, p<0.001, +57.7%, p<0.01, respectively). Combination had synergistic effect (+1240%, p<0.001).
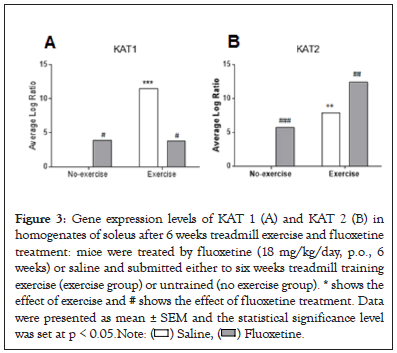
Figure 3: Gene expression levels of KAT 1 (A) and KAT 2 (B) in
homogenates of soleus after 6 weeks treadmill exercise and fluoxetine
treatment: mice were treated by fluoxetine (18 mg/kg/day, p.o., 6
weeks) or saline and submitted either to six weeks treadmill training
exercise (exercise group) or untrained (no exercise group). * shows the
effect of exercise and # shows the effect of fluoxetine treatment. Data
were presented as mean ± SEM and the statistical significance level
was set at p < 0.05. Note: ( ) Saline, (
) Saline, ( ) Fluoxetine.
) Fluoxetine.
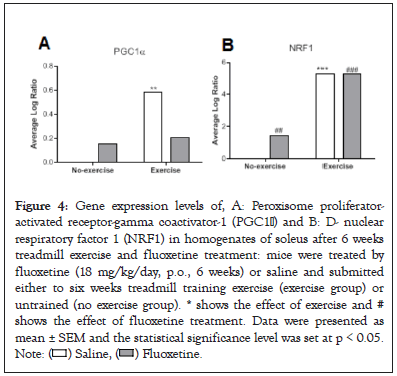
Figure 4: Gene expression levels of, A: Peroxisome proliferator-
activated receptor-gamma coactivator-1 (PGC1α) and B: D- nuclear
respiratory factor 1 (NRF1) in homogenates of soleus after 6 weeks
treadmill exercise and fluoxetine treatment: mice were treated by
fluoxetine (18 mg/kg/day, p.o., 6 weeks) or saline and submitted
either to six weeks treadmill training exercise (exercise group) or
untrained (no exercise group). * shows the effect of exercise and #
shows the effect of fluoxetine treatment. Data were presented as
mean ± SEM and the statistical significance level was set at p < 0.05.
Note: ( ) Saline, (
) Saline, ( ) Fluoxetine.
) Fluoxetine.
Concerning PGC1 α gene expression, fluoxetine consumption increased its expression in untrained animals (+ 16.0%, p<0.01) but was decreased in trained animals (- 64.4%, p<0.01). Combination of exercise and fluoxetine consumption insignificantly increased expression.
Concerning NRF1 gene expression, fluoxetine consumption significantly increased its expression only in untrained animals (+147%, p<0.001). Exercise with a saline or a fluoxetine treatment very significantly increased NRF1 gene expression (+528% and +259%, p<0.001) [16-23].
All these results confirmed the role of fluoxetine in skeletal muscle remodeling after physical exercise. To validate, testing the effects of other SSRI as escitalopram in skeletal muscles, with other methods using cell specific deletion of 5-HT and TRP hydroxylase1 receptors should be used. Whereas, this work is preliminarily enough, and many challenges were not predicted, a new project is strongly suggested to highlight the related factors and the real mechanism involved behind these observed consequences.
The present work revealed that the chronic administration of SSRI type antidepressant, fluoxetine, associated with exercise was able to very significantly convert the TRP metabolism toward 5-HT biosynthesis and decrease the pathway toward KYN production in plasma and soleus. The exercise alone had similar effects on TRP metabolismbut it was not significant. This suggested that the fluoxetine-induced effects on TRP metabolism were not as predominant as physical exercise. These exercise effects were also previously observed by Metcalfe et al. (2018) in human studies after a 12 weeks’ exercise. Falabregues et al. didn’t observe 5-HT modification in peripheral 5-HT–deficient (Tph1KO) mice versus wild-type mice after 10 weeks of endurance training but data before exercise were not shown. We can conclude that association of exercise with fluoxetine consumption had a synergistic effect to increase 5-HT peripheral generation.
The second observation was a significant increase of the protector KYNA production in muscle and plasma after the association of fluoxetine and physical exercise but not by fluoxetine alone. It is well concluded that these peripheral effects were significantly induced by physical exercise, which confirm previous research works in mice and in human . KYNA is antagonist of NMDA receptor and cannot pass through blood brain barrier. Thus it is well demonstrated that association of training and fluoxetine had significant synergistic effects in order to induce peripheral variation of TRP metabolism.
Association of fluoxetine and exercise was able to increase the gene expression of only KAT2 in soleus muscle. Exercise alone and fluoxetine alone increased the expression of KAT 1 and 2, PGC1a and NRF1 genes in muscle. Muscle adaptation to exercise and the impact of external context is multifactorial, and the molecular basis of regulation is not completely explained. As previously cited researchers, the effects of endurance training and fluoxetine chronic consumption is linked to an increase of PGC-1 α expression in the soleus muscle. PGC-1 α is a significant regulator that induces mitochondrial biogenesis and a fiber switch to modify muscle fiber types in the skeletal muscle. PGC-1 α controls many aspects of angiogenic improvement and oxidative metabolism, including respiration and mitochondrial biogenesis through co-activation and enhancing the expression and activity of several transcription factors, including NRF1. They are potent stimulators of the expression of nuclear genes required for mitochondrial respiratory function. An increased expression of total PGC-1 α was described15 in soleus muscle of mice on a high-fat diet, although, following a significant increase of PGC-1 α -b and PGC-1 α -c expressions through the 5-HT2a and 5-HT7 signaling pathways. It was also suggested that a 5-HT2 agonist led to an increase of PGC-1 α promoter activity, and it supports PGC-1 α expression promoter activity by serotonin in skeletal muscles. Moreover, evidence suggested that the skeletal muscle functional serotonin 5-HT2a receptor was expressed in rat myoblasts, activating intracellular phosphorylation on the plasma membrane and at the level of T-tubules in contracting myotubes. The binding of serotonin to its receptor increases the expression of genes involved in myogenic differentiation. Unexpectedly, the 5-HT2A receptor can activate another signaling pathway; it triggers rapid and transient phosphorylation of Jak2 kinase tyrosine in response to serotonin. The self-phosphorylation of Jak2 is followed by the phosphorylation of the tyrosine of STAT3 (signal transducers and transcription activators) and its translocation into the nucleus. No difference in the abundance of 5-HT2a was observed between red muscle and white muscle, suggesting that receptor expression does not correlate with the metabolic or contractile properties of the muscle fiber. Moreover, aerobic training can also modulate sensitivity of 5-HT receptors.
This project was supported by funding from the France anti- doping agency. The authors would like to thank Mrs. Indira David for her aid in western blotting, Mr Jean-Philippe Guilloux for measuring genetic activity and Valerie Domergue for her assistance in management of animal care.
Citation: Tutakhail A, Boulet L, David D, Hamid H, Gardier A, Coudore F (2022) Does Fluoxetine Induce Changes in Kynurenine Pathway of Tryptophan Metabolism after Endurance Exercise? Orthop Muscular Syst. 11:350.
Received: 17-May-2022, Manuscript No. OMCR-22-15722; Editor assigned: 20-May-2022, Pre QC No. OMCR-22-15722 (PQ); Reviewed: 03-Jun-2022, QC No. OMCR-22-15722; Revised: 17-Jun-2022, Manuscript No. OMCR-22-15722 (R); Published: 24-Jun-2022 , DOI: 10.35248/2161-0533.22.11.350
Copyright: © 2022 Tutakhail A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.