Journal of Hematology & Thromboembolic Diseases
Open Access
ISSN: 2329-8790
ISSN: 2329-8790
Case Report - (2022)Volume 10, Issue 6
Allogeneic stem cell transplant is a successful treatment for severe aplastic anemia. Subsequent development of acute leukemia is a rare event. Cytogenetic/molecular analysis has shown that leukemia can originate in the host cells. In this report we describe a pediatric patient who developed donor cell acute myeloid leukemia with a del (9q) aberration and a CEBPA double mutation, 11 years after an unrelated allogeneic stem cell transplant for severe acquired aplastic anemia. Mechanisms involved in the pathogenesis are complex and several hypotheses have been proposed. Novel approaches, such as next generation sequencing to unravel the mechanisms of leukemogenesis in this category of patients, appear promising.
Donor cell leukemia; Severe aplastic anemia; Children; Del (9q) aberration; CEBPA double mutation
Donor Cell Leukemia (DCL) is a rare complication after Allogeneic Hematopoietic Stem Cell Transplant (Allo-HSCT). Rare cases have been described in patients following an allo- HSCT for Severe Aplastic Anemia (SAA) [1-9]. These events occurred at different latency periods from the allo-HSCT and with different conditioning regimens including or not Total- Body Irradiation (TBI), employment of Cyclosporine A (CsA), antimetabolite drugs and Granulocyte-Colony-Stimulating-Factor (G-CSF). Several scenarios have been proposed to explain the DCL physiopathology, but the mechanisms remain largely unknown. In patients transplanted for SAA, defects of the recipient microenvironment, and/or of the immune surveillance may induce a leukemic transformation in donor hematopoietic cells, but also pre-transplant conditioning chemotherapy, radiation damage, viral transfection telomere shortening, and occult leukemia in the donor cells have been implicated [10].
We hereby report a pediatric case with SAA who developed an Acute Myeloid Leukemia (AML) with del (9q) and double CEBPA mutation, 11 years after an unrelated allo-HSCT.
In January 2009, a 5-year-old boy received an SAA diagnosis (Hb 6.3 g/dl; WBC 0.8 × 109/L; platelets 7.0 × 109/L). The child had no history of infections. Physical examination was normal except for cutaneous hemorrhages. Viral serological tests (EBV, CMV, hepatitis, HIV) were negative. Bone marrow aspirate and biopsy documented a deep hypoplasia and absence of leukemia. Immunophenotyping ruled out the presence of a Paroxysmal Nocturnal Hemoglobinuria (PNH) clone. The Diepoxybutane Test (DEB) was negative. Cytogenetics showed a normal male karyotype and excluded the presence of monosomy 5, 7 and 8, and other chromosomal aberrations. No TERC, TERT, TINF2 gene mutations were found. No compatible sibling was identified, so the patient received Immunosuppressive Treatment (IST) with equine Anti-Thymocyte Globulin (ATG), CsA, prednisone and G-CSF, according to the AIEOP (Associazione Italiana Ematologia e Oncologia Pediatrica) guidelines, but remained transfusion-dependent. The clinical outcome was further complicated by severe gastrointestinal aspergillosis. Twelve months later, a bone marrow biopsy showed persistent hypoplasia. In September 2010, 20 months from the initial diagnosis, an unrelated compatible donor allo-HSCT was carried out. Conditioning regimen consisted of fludarabine and cyclophosphamide; Graft-Versus-Host (GVHD) prophylaxis included methotrexate, CsA and ATG. A total of 7.26 × 106/kg CD34-positive, nucleated bone marrow cells were infused. Engraftment occurred on day 20; there was no evidence of GVHD. On day 28, a bone marrow examination showed normal trilinear regenerating marrow; cytogenetics revealed a normal male karyotype (46XY). Chimerism, periodically tested using Short-Tandem-Repeat Polymerase-Chain Reaction (STRPCR), resulted 100% donor. Periodical hematologic checks resulted normal for 10 years after allo-HSCT. In May 2021, a routine peripheral blood count showed-Hb 13.1 g/dl, platelets 107.0 × 109/L and WBC 7.2 × 109/L with 30% myeloid blasts. Coagulation profile, renal and liver functions were normal. A bone marrow aspirate revealed 65% myeloid leukemia cells with characteristic phenotypic abnormalities. The most striking finding was the presence of a single long and slender Auer rod, granulocytic lineage vacuolation and erythroid dysplasia (Figure 1A).
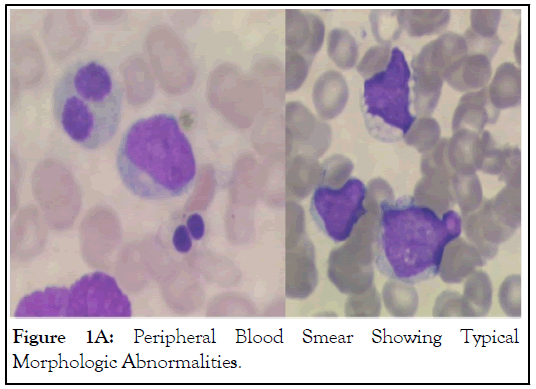
Figure 1A: Peripheral Blood Smear Showing Typical Morphologic Abnormalities.
At immunophenotype, the leukemic cells were CD45/ MPO/CD34/CD117/HLA-DR/CD13/CD15/CD38/CD33/ CD133-positive with CD7 aberrant expression. CD56/CD4/CD16/CD11b/CD14/CD19/CD2/CD3/CD79a were negative. Cytogenetics revealed a del (9) as sole karyotypic aberration, with the area of deletion in the 9q21-22 region (Figure 1B).
Figure 1B: GTG-Banding Revealing del (9q).
A molecular study on the bone marrow cells involving CBF, KMTD2A, NUP98, NPM1, CEBPA, DNMT3A, WT1 and FLT3 mutations resulted negative. Next-Generation Sequencing (NGS) analysis demonstrated a CEBPA double mutation (c. 196-197delGC (NTD) and c.949_951dupCTG (bZip)) [11]. Bone marrow cells chimerism resulted 97% donor, revealing the origin of the leukemic cells in the donor. The patient received standard-dose cytarabine, daunorubicin and Gentuzumab- Ozogamicin (GO), and achieved a complete hematological and immunophenotypic Complete Remission (CR). A second course of chemotherapy was administered. The patient is currently in CR after high-dose cytarabine consolidation. Bone marrow and peripheral blood cells chimerism is 100% donor.
DCL is extremely rare after allo-HSCT. Most of the data available is limited to case reports or small case series. Two large surveys assessed the frequency of DCL [1,12]. The European Bone Marrow Transplantation Registry identified 14 cases out of 10,489 allo-HSCTs [1]. The Minnesota University reported 8 cases out of 2,390 engrafted allo-HSCTs [12]. Recently, a Japanese survey identified 40 DCL cases out of 36,870 allo- HSCTs, with 0.16% incidence estimated at 15 years [13]. DCL is a relatively later event than a disease relapse, with a median latency of 31 months (range 2-312 months) [10]. Furthermore, most DCLs occurred in patients transplanted for leukemia; only 8 cases of DCL after an allo-HSCT for SAA have so far been described [2-9]. In these cases, DCL occurred from 149 days to 12 years after HSCT (Table 1).
| Author | Age (yrs) | IST | Conditioning regimen | Latency | DCL dx studies | Cytogenetics | Therapy |
|---|---|---|---|---|---|---|---|
| Browne PV, et al., 1991 | 19 | ATG+CsA | Cy+ALG | 319 days | Cytog+PCR | t (9;11) | DNR+CA+6TG |
| Lawler 2002 | 12 | CsA | Cy+HSCT/ALG+Cy+TA + 2nd HSCT | 149 days | STR+PCR | None | Ida+CA |
| Lang 2004 | 12 | None | TBI+Cy+CA | 3 yrs | RFLP | - 7 | Cy+Busulfan+ATG+2nd HSCT |
| Hashino 2006 | 23 | ATG+Cy+ CsA |
TBI+Cy | 23 mos | PCR | t(2;3; - 7 | Early death |
| Otero 2012 | 23 | None | Cy+ATG | 3 yrs | Cytog | -7 | 2nd HSCT |
| Haltrich 2014 | 2 | None | Cy+ATG | 12 yrs | Cytog | t(3;21), trisomy 12 | DLI/Busulfan+Cy+Melphalan |
| Ruiz-Delgado 2015 | 34 | CsA | Busulfan+Cy+Fludara | 38 mos | STR+PCR | HCL | NK |
| Ma, 2016 | 25 | CsA | Cy+ALG | 2.5 yrs | STR+PCR | t(8;21), trisomy 8 | DNR+CA+ IL2r |
Note: ATG: Anti-thymocyte globulin; ALG: Anti-lymphocyte globulin; CA: Cytarabine; Cytog: Cytogenetic; CsA: Cyclosporine; Cy: Cyclophosphamide; Dx: Diagnosis; Fludara: Fludarabine; HCL: Hairy cell leukemia; HSCT: Hematopoietic stem cell transplantation; Ida: Idarubicin; IL2r: Recombinant interleukin-2; IST: Immunosuppressive therapy; NK: Not-known; PCR: Polymerase chain reaction; RFLP: Restriction fragment length polymorphism; STR: Short tandem repeat; TAI: Thoraco-abdominal irradiation; TBI: Total body irradiation
Table 1: Reported Cases of Donor Cell Leukemia in Patients Transplanted for Severe Aplastic Anemia.
In our child with SAA, DCL occurred 11 years after the transplant. DCL etiology remains largely unknown; pathogenetic hypotheses include transfer of oncogenic material from the host to donor cells, inadequate patient immune surveillance, and leukemic transformation of engrafted cells and, finally, occult leukemia in the donor cells [10]. The DCL management poses ethical dilemmas regarding donor notification and opens potential concerns over the future health of the donor. We communicated our patient’s event to the Italian Bone Marrow Donor Registry (IBMDR), and we discovered that his donor is currently in good health. The type of leukemia occurring in our patient is worthy of attention. He developed an AML with a del (9q) combined with CEBPA double mutation. Del (9q) aberration in AML is a rare abnormality reported in approximately 2% of adult de novo AMLs. The concomitant CEBPA double mutation in del (9q) AML with noncomplex aberrant karyotype occurs in approximately 13% of cases [14,15]. In 2008, the World Health Organization (WHO) myeloid malignancies classification considered del (9q) as an aberration presumptive of a Myelodysplastic Syndrome (MDS) [16]. In AML, with the sole del (9q), some mutations that could influence the patients’ prognosis, such as NPM1, DNMT3A and CEBPA, have been found more frequently than in other AMLs, classified as intermediate-II risk, according to the European Leukemia Net (ELN) AML classification. On the contrary, FLT3-ITD seems a rare event in these patients. Therefore, the mutational pattern of AML with sole del (9q) is very peculiar. In 2016, given this evidence, in the revised WHO AML classification, AML with sole del (9q) was no longer considered as a myelodysplasticrelated cytogenetical abnormality [16,17]. In our patient, a double CEBPA mutation without a concomitant WT1 mutation was detected, as frequently occurs. Additional molecular studies have highlighted a Minimally Deleted Region (MDR) composed of 7 genes (GKAP1, KIF27, C9ORF64, HNRNPK, RMI1, SLC28A3, NTRK2) common to del (9q) AML [18], as found in our patient. The HNRNPK is the most frequently studied gene. The gene product HNRNP-K is a multifunctional protein which binds DNA and RNA, targets CEBPA, and plays a major role in the erythroid maturation and inflammation, as an mRNA regulator. A recent study has hypothesized its key-role in leukemogenesis. In humans, the HNRNP-K can influence the expression of C/EBPα with reduced levels of its mRNA, altering transcriptional and post-transcriptional regulation of this pathway. This study also confirmed the elevated expression of MDR genes in del (9q) CEBPA mutated samples by mutant C/ EBP α , suggesting that this might contribute to an improved prognosis [15,18]. CEBPA-double-mutated cases usually bear Nand C-terminal mutations, typically on different alleles, and are associated with a favorable clinical outcome. Identification of CEBPA alterations can be very challenging, due to technical issues concerning the high Guanine Cytosine (GC) content of the gene, which frequently correlates with failure of the PCR, artifacts, and the cost effectiveness of sequencing procedures. Moreover, CEBPA mutations are involved in a specific AML setting called CEBPA-associated familial AML, defined as the presence of a heterozygous germline CEBPA pathogenic variant in an individual with AML, and/or family in which more than one individual has AML. However, our patient carried biallelic CEBPA mutations not listed in the notable familial acute myeloid leukemia-associated CEBPA germline pathogenic variants [19,20].
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
Citation: Testi AM, Kaiser F, Iori AP, Barberi W, Rocca UL, Moleti ML, et al. (2022) Donor Cell Acute Myeloid Leukemia with Del (9q) after a Transplant for Severe Aplastic Anemia: A Case of a Pediatric Patient. J Hematol Thrombo Dis. 10.503.
Received: 01-Sep-2022, Manuscript No. JHTD-22-19471 ; Editor assigned: 05-Sep-2022, Pre QC No. JHTD-22-19471 (PQ); Reviewed: 19-Sep-2022, QC No. JHTD-22-19471 ; Revised: 26-Sep-2022, Manuscript No. JHTD-22-19471 (R); Published: 03-Oct-2022 , DOI: 10.35248/2329-8790.22.10.503
Copyright: © 2022 Testi AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.