Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Research - (2024)Volume 14, Issue 3
This prospective observational study was conducted over one year in the Orthopedic Department at Raichur Institute of Medical Sciences to evaluate the demographic distribution, treatment patterns, drug utilization and Adverse Drug Reactions (ADRs) in patients with Osteoarthritis (OA) and Rheumatoid Arthritis (RA). The study included 215 adult patients aged 18-80 years, diagnosed with OA or RA, excluding those with severe comorbidities. A pre-designed Case Record Form (CRF) was used to collect data, including demographic details, suspected drugs, ADRs and concomitant medications. Diagnoses were made using the American College of Rheumatology (ACR) guidelines for RA and radiological criteria for OA. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) were the most commonly prescribed medication for both OA (44.45%) and RA (26.58%), with analgesics and Disease-Modifying Antirheumatic Drugs (DMARDs) being widely used in RA patients. The study revealed a higher prevalence of OA in males (56.07%) and RA in females (67.07%), with both conditions most common in the 51-65 age groups. Oral administration was the predominant route of drug delivery in both groups and monotherapy was more frequent in OA (79.23%), while combination therapy was prevalent in RA (28.88%). ADR, including gastric discomfort and abdominal pain, were more common in RA patients (12.19% and 3.65%) than in those with OA (1.86% and 0.93%). The study highlighted the need for personalized treatment strategies to optimize therapeutic outcomes and minimize ADRs, emphasizing continuous monitoring and adjustment of therapeutic regimens based on patient demographics and clinical responses.
Drug utilization; Demographics; Combination therapy; Monotherapy; Cardiovascular diseases
OA and RA are among the most prevalent musculoskeletal disorders, profoundly affecting the quality of life for millions worldwide. Osteoarthritis is primarily characterized by the progressive degeneration of articular cartilage and subchondral bone, leading to joint pain, stiffness and functional limitations. The rising prevalence of OA, particularly in aging populations and individuals with obesity, has raised global concerns, prompting the World Health Organization (WHO) to emphasize the need for effective management strategies to address this increasing burden.
In contrast, rheumatoid arthritis is an autoimmune disorder marked by chronic inflammation of the joints, causing pain, swelling and potentially irreversible joint damage. RA disproportionately affects women, with studies indicating that approximately 70% of patients are female. Beyond joint damage, RA can lead to systemic complications, including cardiovascular and respiratory issues, complicating disease management further. Given the differences in pathophysiology, demographic characteristics and treatment responses between OA and RA, a detailed understanding of their management is crucial for optimizing patient outcomes [1-4].
Recent studies have explored demographic trends and treatment strategies for both OA and RA. For example, a study found that female RA patients tend to experience more severe symptoms and greater functional limitations compared to males. Additionally, highlighted that older adults with OA face a higher risk of comorbidities, necessitating more complex and individualized treatment approaches.
Despite the growing body of literature, significant gaps remain in understanding drug utilization patterns and adverse drug reactions in these patient populations. New therapeutic options are continually being introduced, but adherence to treatments and the management of adverse effects remains persistent challenges for healthcare providers and reported ongoing underutilization of disease-modifying DMARDs in RA patients, possibly due to concerns over adverse reactions and inadequate patient education.
Moreover, current studies on drug utilization often overlook the role of demographic factors in influencing treatment decisions. A recent analysis has emphasized that younger OA patients may benefit from distinct therapeutic approaches compared to older adults, who may be more susceptible to medication side effects. This finding underscores the need for personalized treatment plans that consider not only the specific type of arthritis but also patient characteristics such as age and comorbidities.
While progress has been made in understanding treatment patterns and outcomes in OA and RA considerable challenges remain. Further research is needed to explore the interaction between demographic factors, treatment decisions and adverse drug reactions. Additionally, qualitative studies capturing patient experiences can help develop more holistic management strategies that prioritize patient-centered care.
The aim of this study is to comprehensively analyze the demographic distribution, treatment patterns, drug utilization and adverse drug reactions in patients with osteoarthritis and rheumatoid arthritis. By integrating existing research and providing new understandings, the study seeks to fill gaps in the current understanding of how these factors impact patient outcomes. Ultimately, the goal is to inform future treatment protocols that enhance the effectiveness of care, minimize adverse effects and support the development of tailored management strategies for these prevalent and debilitating conditions [5-8].
This study was conducted over one year, from December, 2016 to November, 2017 in the Orthopedic Department at Raichur Institute of Medical Sciences, Raichur. Ethical approval was obtained before the study began, ensuring compliance with ethical standards and patient safety in accordance with the Declaration of Helsinki. The study was approved under reference number RIMS/IEC/Tech, Staff/2018-19/14, dated 20-04-2018. The methodology thoroughly evaluated drug utilization patterns, ADRs and treatment outcomes in patients with OA and RA, providing valuable insights for optimizing therapeutic strategies [9].
Study design
This prospective observational study aimed to evaluate drug utilization patterns, ADRs and treatment outcomes in patients diagnosed with OA and RA. The study included adult patients (aged 18-80 years) diagnosed with arthritis and excluded those with significant comorbidities such as cardiovascular diseases, severe neurological deficits or terminal illnesses.
Patient recruitment and consent
Patients meeting the inclusion criteria were recruited during their visits to the orthopedic department. Informed consent was obtained from all participants, ensuring they understood the study's purpose and their right to withdraw at any time. The recruitment process was systematic, ensuring every eligible patient had an equal opportunity to participate in the study.
Data recording
Patient demographics, ADRs, suspected drugs and concomitant medications were recorded using the Central Drugs Standard Control Organization (CDSCO) ADR reporting form. Data were collected using a pre-designed CRF. Diagnoses of OA and RA were made according to established clinical guidelines, including the American College of Rheumatology criteria for RA and radiological assessments for OA [10,11].
Treatment patterns
Treatment information was gathered through patient interviews and the examination of medical records. Medications included NSAIDs, analgesics, DMARDs and combination therapies. Treatment selection was based on clinical guidelines, patient-specific factors such as disease severity and physician discretion. Dosage, duration of treatment and routes of administration were meticulously documented.
The treatment protocols followed in this study were based on the 2012 ACR Recommendations for the Management of OA and the 2015 ACR Guideline for the Treatment of RA. These guidelines provide evidence-based strategies for selecting appropriate medications and dosages customized to individual patient needs. The optimization of treatment strategies focused on improving patient adherence, minimizing adverse drug reactions and enhancing overall therapeutic efficacy [12-15].
ADR monitoring
ADRs were monitored using a systematic approach with regular patient follow-ups at designated intervals, typically every four to six weeks. Patients were assessed for side effects during each visit and suspected ADRs were documented using standardized ADR assessment tools. The relationship between treatment and ADRs was analyzed by comparing the incidence and severity of ADRs across different treatment groups. Suspected ADRs were validated by cross-referencing patient reports with medical records and consulting with treating physicians when necessary.
Quality control measures
Quality control measures included double data entry into an electronic database and periodic audits of data collection processes to identify and rectify discrepancies. Consistency in data collection was ensured by training all investigators on standardized procedures and employing uniform protocols across multiple clinics involved in the study.
Statistical analysis
Demographic and baseline variables were summarized using descriptive statistics, with means (± standard deviation) for continuous variables and frequencies for categorical variables. The relationship between treatment types and their effectiveness or side effects was analyzed using parametric data with the student’s t-test and categorical data were analyzed with chi-square tests. Side effects were classified by severity using standardized scales and treatment outcomes were evaluated in relation to these classifications. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software, version 24.0.
Follow-up and monitoring
Patients were followed up regularly, with visits scheduled every four to six weeks for a total duration of 12 months. During these visits, treatment effectiveness and ADRs were closely monitored, allowing for timely interventions when necessary. The frequency of visits ensured comprehensive monitoring and allowed for the early detection of any adverse effects, ensuring patient safety throughout the study.
This section presents a comprehensive analysis of the demographic distribution, treatment patterns, drug utilization, adverse drug reactions and treatment outcomes in patients with OA and RA. The findings are based on data collected from December, 2016 to November, 2017 at the Raichur Institute of Medical Sciences.
Demographic distribution
The overall gender distribution of patients in this study revealed a higher proportion of males (52.35%) compared to females (47.65%) (Table 1). Specifically, in the osteoarthritis group, males constituted 56.07%, while females represented 43.93%. Conversely, rheumatoid arthritis was more prevalent in females (67.07%) than in males (32.93%).
| Gender | Osteoarthritis (OA) Patients (N=428) | Percentage Osteoarthritis (OA) | Rheumatoid Arthritis (RA) patients (N=82) | Percentage Rheumatoid Arthritis (RA) |
|---|---|---|---|---|
| Male | 240 | 56.07% | 27 | 32.93% |
| Female | 188 | 43.92% | 55 | 67.07% |
| Total | 428 | 100% | 82 | 100% |
| Age Distribution (Yrs) | OA Patients (N=428) | Percentage (OA) | RA patients (N=82) | Percentage (RA) |
| 20-35 | 44 | 10.28% | 0 | 0.00% |
| 36-50 | 161 | 37.61% | 4 | 4.87% |
| 51-65 | 194 | 45.32% | 73 | 89.02% |
| 66-80 | 29 | 6.77% | 5 | 6.09% |
| Total | 428 | 99.98% | 82 | 100% |
Table 1: Details of gender and age distribution in Osteoarthritis (OA) and Rheumatoid Arthritis (RA).
Age distribution showed that osteoarthritis was most common in the 51-65 years age group, accounting for 45.32% of cases, followed by the 36-50 years age group (37.61%). In the rheumatoid arthritis group, the majority of patients (89.02%) were also in the 51-65 years age group, with a smaller percentage (6.09%) in the 66-80 years category. This demographic data highlights that both conditions predominantly affect middle-aged to older adults, emphasizing the need for targeted management strategies for these age groups.
Treatment patterns
The treatment approaches varied significantly between osteoarthritis and rheumatoid arthritis. In osteoarthritis, NSAIDs were the most frequently prescribed class of drugs, accounting for 44.45% of prescriptions, followed by analgesics (25.68%) and antacids (12.28%) (Figure 1).
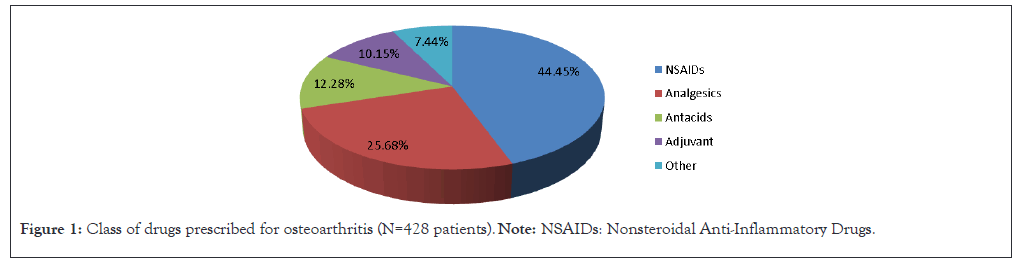
Figure 1: Class of drugs prescribed for osteoarthritis (N=428 patients). Note: NSAIDs: Nonsteroidal Anti-Inflammatory Drugs.
The predominant route of administration for osteoarthritis was oral (94.11%), with a small percentage receiving injectable (4.26%) or topical (1.62%) treatments (Table 2). For rheumatoid arthritis oral administration was also the most common route (95.29%), followed by injectable (3.47%) and topical (1.22%) treatments.
| Route | Condition | Number of prescriptions | Percentage |
|---|---|---|---|
| Oral | OA | 1103 | 94.11% |
| RA | 466 | 95.29% | |
| Injectable | OA | 50 | 4.26% |
| RA | 17 | 3.47% | |
| Topical | OA | 19 | 1.62% |
| RA | 6 | 1.22% | |
| Total | OA | 1172 | 100% |
| RA | 489 | 100% |
Table 2: Details of route of administration of drugs in Osteoarthritis (OA) and Rheumatoid Arthritis (RA).
Comparison of drug administration routes
The comparison of different routes of drug administration between osteoarthritis and rheumatoid arthritis shows that oral administration was predominant in both conditions. However, the slightly higher use of injectables in osteoarthritis (4.26%) compared to rheumatoid arthritis (3.47%) could be attributed to the acute management of pain in OA. Conversely, topical treatments were used minimally in both conditions, with slightly more frequent use in OA.
Assessment of benefits: Oral vs. non-oral treatments
The analysis indicates that oral forms of treatment are favored in both OA and RA due to ease of administration, patient compliance and overall effectiveness. Non-oral treatments, such as injectables are typically reserved for more severe cases or when rapid symptom relief is required. The use of topical treatments, while limited, offers localized relief with minimal systemic side effects, making it beneficial as an adjunct therapy in specific cases.
Notably, monotherapy was favored in osteoarthritis management, with 79.23% of patients receiving single-drug therapy (Table 3).
| Approach to treatment | Condition | Total number of drugs | Percentage |
|---|---|---|---|
| Monotherapy | OA | 847 | 79.23% |
| RA | 293 | 71.11% | |
| Combination therapy | OA | 222 | 20.76% |
| RA | 119 | 28.88% | |
| Total | OA | 1069 | 100% |
| RA | 412 | 100% |
Table 3: Approach to treatment in Osteoarthritis (OA) and Rheumatoid Arthritis (RA).
In rheumatoid arthritis, NSAIDs were also common (26.58%), but the use of DMARDs was more pronounced at 16.76%. The route of administration in rheumatoid arthritis was similarly oral (95.29%), with fewer patients receiving injectables (3.47%) or topical treatments (1.22%) in Table 2. Combination therapy was observed in 28.88% of rheumatoid arthritis patients, indicating a more complex treatment regimen in Table 3. Focused analysis of drug utilization reveals distinct patterns in the medications used for both conditions. In osteoarthritis, the specific NSAIDs utilized included aceclofenac (32.85%), diclofenac (25.49%) and paracetamol (20.10%) (Table 4). In contrast, rheumatoid arthritis patients predominantly used diclofenac (33.33%), followed by aceclofenac (30.30%) and paracetamol (15.90%). The use of corticosteroids was notable in rheumatoid arthritis, with prednisolone prescribed to 30.48% of patients, while only 9.34% of osteoarthritis patients received this medication (Table 5).
| Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) class | Drug | No. of prescriptions (Osteoarthritis (OA): N=557) | Percentage Osteoarthritis (OA) | No. of prescriptions (RA: N=132) | Percentage Rheumatoid Arthritis (RA) |
|---|---|---|---|---|---|
| Preferential COX-2 inhibitors | Diclofenac | 142 | 25.49% | 44 | 33.33% |
| Aceclofenac | 183 | 32.85% | 40 | 30.30% | |
| Nimesulide | 62 | 11.01% | 17 | 12.08% | |
| Propionic acid derivatives | Ibuprofen | 22 | 3.94% | 5 | 3.78% |
| Enolic acid derivatives | Piroxicam | 36 | 6.46% | 5 | 3.78% |
| Acetaminophen | Paracetamol | 112 | 20.10% | 21 | 15.90% |
| Total | 557 | 100% | 132 | 100% | |
Table 4: Details of class of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) prescribed in Osteoarthritis (OA).
| Corticosteroids | No. of prescriptions (Osteoarthritis (OA, N=428) | Percentage Osteoarthritis (OA) | No. of prescriptions (Rheumatoid Arthritis (RA), N=82) | Percentage Rheumatoid Arthritis (RA) |
|---|---|---|---|---|
| Prednisolone | 40 | 9.34% | 25 | 30.48% |
| Deflazacort | 22 | 5.14% | 10 | 12.19% |
| Dexamethasone | 25 | 5.84% | 8 | 9.75% |
Table 5: Details of corticosteroids used for Osteoarthritis (OA) and Rheumatoid Arthritis (RA).
Combination therapies were also analyzed, revealing that 11.91% of osteoarthritis patients received aceclofenac and paracetamol together, whereas a significant proportion of rheumatoid arthritis patients (59.75%) were on DMARDs combinations, underscoring the complexity of treatment for RA (Table 6).
| Combination therapy | No. of prescriptions (OA, N=428) | Percentage (OA) | No. of prescriptions (RA, N=82) | Percentage (RA) |
|---|---|---|---|---|
| Aceclofenac+Paracetamol | 51 | 11.91% | 11 | 13.41% |
| Diclofenac+Paracetamol | 25 | 5.84% | 8 | 9.75% |
| Tramadol+Paracetamol | 27 | 6.30% | 3 | 3.65% |
| Calcium+Vitamin D3 | 61 | 14.25% | 22 | 26.82% |
| Multivitamins | 58 | 13.55% | 48 | 58.53% |
| DMARDs+DMARDs | - | - | 49 | 59.75% |
Note: OA: Osteoarthritis; RA: Rheumatoid Arthritis; DMRDs: Disease-Modifying Antirheumatic Drugs.
Table 6: Details of combination therapy.
Adverse Drug Reactions (ADRs)
ADR were reported in both patient groups, with notable differences in the frequency and severity of ADRs experienced. In osteoarthritis patients, the occurrence of gastric discomfort was low (1.86%) and abdominal pain was reported in 0.93% of cases. In contrast, rheumatoid arthritis patients experienced significantly higher rates of ADRs related to NSAIDs, with gastric discomfort reported in 12.19% and abdominal pain in 3.65% (Table 7).
| ADR Symptoms | Patient type | Male patients (N) | Percentage (Male) | Female patients (N) | Percentage (Female) | Total ADRs (N) | Total percentage |
|---|---|---|---|---|---|---|---|
| Gastric discomfort | OA | 5 | 62.50% | 3 | 20.00% | 8 | 1.86% |
| RA | 6 | 60.00% | 4 | 40.00% | 10 | 12.19% | |
| Abdominal pain | OA | 2 | 25.00% | 2 | 13.33% | 4 | 0.93% |
| RA | 2 | 28.57% | 1 | 12.50% | 3 | 3.65% | |
| Nausea | OA | 0 | 0.00% | 1 | 6.67% | 1 | 0.23% |
| RA | 0 | 0.00% | 1 | 9.09% | 1 | 1.21% | |
| Vomiting | OA | 3 | 37.50% | 2 | 13.33% | 5 | 1.16% |
| RA | 1 | 14.29% | 0 | 0.00% | 1 | 1.21% | |
| Skin rashes | OA | 1 | 12.50% | 2 | 13.33% | 3 | 0.70% |
| RA | 1 | 14.29% | 1 | 9.09% | 2 | 2.43% | |
| Loose stools | OA | 0 | 0.00% | 1 | 6.67% | 1 | 0.23% |
| RA | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | |
| Dizziness | OA | 1 | 12.50% | 0 | 0.00% | 1 | 0.23% |
| RA | 0 | 0.00% | 1 | 9.09% | 1 | 1.21% | |
| Total | OA | 12 | 52.17% | 9 | 39.13% | 23 | 5.37% |
| RA | 10 | 55.56% | 6 | 33.33% | 18 | 21.9 |
Note: Osteoarthritis (OA); Rheumatoid Arthritis (RA); Adverse Drug Reactions (ADRs).
Table 7: Correlation of Adverse Drug Reactions (ADRs) in male vs. female patients with Osteoarthritis (OA) and Rheumatoid Arthritis (RA).
This highlights the need for careful monitoring of patients on NSAIDs, particularly those with rheumatoid arthritis, due to their higher susceptibility to adverse reactions.
Treatment outcomes
Correlating treatment patterns with clinical outcomes is essential for assessing the effectiveness of the therapeutic approaches used. While specific clinical outcomes were not measured in this study, the documented patterns of drug utilization and the incidence of ADRs suggest that the choice of medications and treatment strategies can significantly impact patient adherence and overall satisfaction. Given the higher prevalence of adverse reactions in rheumatoid arthritis, there may be a corresponding need for improved patient education and support to optimize treatment adherence and outcomes.
Statistical analysis
Statistical analysis of the data confirmed the significance of the findings. Descriptive statistics were used to summarize demographic characteristics, while parametric data were analyzed using the Student's t-test. Categorical variables were assessed with chi-square tests to determine differences between the OA and RA groups. The statistical significance of the observed trends underscores the reliability of the results and provides a robust basis for drawing conclusions regarding drug utilization patterns and treatment outcomes.
Comparative analysis
A comparative analysis between OA and RA reveals several important distinctions in management. While both conditions predominantly affect older adults, the gender distribution significantly differs, with rheumatoid arthritis affecting more females. The treatment approaches also diverge, with rheumatoid arthritis requiring a greater reliance on DMARDs and combination therapies, reflecting the more complex nature of this autoimmune condition. Additionally, the higher incidence of adverse drug reactions in rheumatoid arthritis patients necessitates personalized treatment plans that prioritize safety and tolerability.
Additional insights
Figure 2 provides a detailed breakdown of the drug classes prescribed for RA, illustrating the complexity of RA management compared to OA. This complexity arises from the multifaceted nature of RA, which often requires a combination of DMARDs and other therapeutic agents to manage symptoms and slow disease progression effectively (Figure 2).
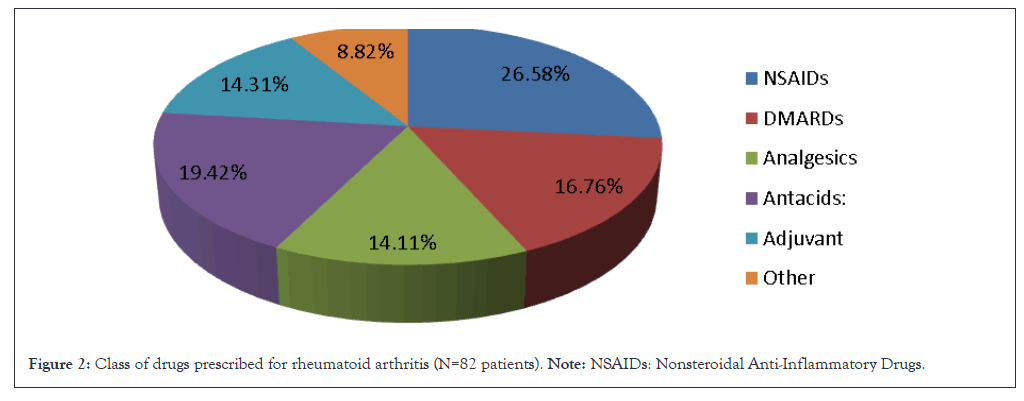
Figure 2: Class of drugs prescribed for rheumatoid arthritis (N=82 patients). Note: NSAIDs: Nonsteroidal Anti-Inflammatory Drugs.
Table 8 examines risk factors for both OA and RA, highlighting that old age is a significant risk factor for both conditions, with 28.73% of OA patients and 39.02% of RA patients being affected. Notably, family history is a more pronounced risk factor for RA, influencing 45.12% of patients. This elevated risk underscores the genetic component of RA compared to OA. The information underscores the necessity of personalized treatment strategies and preventive measures, considering both demographic and genetic factors to improve management and outcomes for patients with these conditions.
| Risk Factor | Total Number of Patients (N=510) | Percentage Osteoarthritis (OA) | Percentage Rheumatoid Arthritis (RA) |
|---|---|---|---|
| Family history | 46 | 10.74% | 45.12% |
| Old age | 123 | 28.73% | 39.02% |
| Obesity | 57 | 13.31% | 14.63% |
| Total | 510 | 100% | 100% |
Table 8: Details of risk factors for Osteoarthritis (OA) and Rheumatoid Arthritis (RA).
This study provides valuable insights into the demographic characteristics, treatment modalities and drug utilization patterns among patients diagnosed with OA and RA Understanding these patterns is important for optimizing treatment strategies and enhancing patient outcomes.
Demographic insights
The demographic distribution revealed a higher prevalence of OA in males (56.07%) compared to females, while RA showed a distinct gender bias, with females representing 67.07% of the patient population. This gender disparity in RA aligns with previous findings that suggest hormonal and genetic factors may contribute to the increased incidence of this autoimmune disorder among women. For instance, a study found that women accounted for approximately 70% of RA cases, reinforcing the notion that gender plays a significant role in disease prevalence.
The age distribution highlighted that both conditions predominantly affect middle-aged to older adults, particularly in the 51-65 years age group. This aligns with known epidemiological trends indicating increasing age as a significant risk factor for both OA and RA. Similar trends were observed in which reported that 65% of OA patients were over the age of 50, corroborating our findings.
Treatment modalities
The treatment patterns observed in this study indicate a substantial reliance on NSAIDs for managing both OA and RA. For OA, NSAIDs constituted 44.45% of prescribed medications, while in RA their usage was lower at 26.58%. The higher use of NSAIDs in OA may be attributed to the condition's degenerative nature, where pain relief is paramount for improving quality of life. Similarly, NSAIDs as the most commonly prescribed medication for OA patients, comprising 48% of total prescriptions.
The study also found that monotherapy was predominant in OA (79.23%), whereas combination therapy was more frequent in RA (28.88%). This discrepancy is consistent with clinical guidelines that recommend a multi-faceted approach for RA due to its inflammatory nature and the need for DMARDs. A systematic review reported that approximately 35% of RA patients were on combination therapy, reflecting the complexity of RA management.
Drug utilization patterns
An examination of drug utilization patterns revealed a preference for specific NSAIDs in both conditions. In OA, Aceclofenac (32.85%) was the most frequently prescribed, followed by diclofenac (25.49%) and paracetamol (20.10%). In contrast, in RA, diclofenac (33.33%) was the leading NSAID, suggesting a possible overlap in treatment preferences between the two conditions and also identified diclofenac as the most commonly prescribed NSAID across both patient populations.
The utilization of corticosteroids in RA (30.48%) highlights the need for aggressive anti-inflammatory treatment strategies, consistent with recommendations emphasized the importance of corticosteroids in managing severe RA cases.
Adverse Drug Reactions (ADRs)
The study provides valuable insights into the incidence of ADRs, particularly in RA patients, who experienced significantly higher rates of ADRs related to NSAIDs compared to OA patients. The documented gastric discomfort in RA patients (12.19%) highlights the potential for NSAIDs to cause gastrointestinal complications, necessitating careful monitoring and possibly the concurrent use of gastro-protective agents. These findings are supported and reported an ADR incidence of 14% among RA patients using NSAIDs.
Treatment outcomes and patient education
Although specific clinical outcomes were not measured in this study, the correlation between treatment patterns, ADRs and patient adherence is critical. Higher rates of ADRs in RA could lead to decreased patient satisfaction and adherence to prescribed regimens, ultimately impacting long-term outcomes. This concern found that patients experiencing higher ADR rates reported lower adherence levels, highlighting a significant challenge in managing chronic conditions like RA.
Enhanced patient education regarding the potential side effects of medications, coupled with strategies to mitigate these risks, is essential for improving adherence and optimizing treatment outcomes. The study highlights the need for ongoing pharmacovigilance to monitor the safety and efficacy of NSAIDs and DMARDs in diverse patient populations. Personalized treatment strategies that consider individual patient factors, including comorbidities and treatment preferences, will be essential for enhancing the quality of care in patients with OA and RA.
This study provides a comprehensive analysis of the demographic characteristics, treatment patterns, drug utilization, ADRs and treatment outcomes in patients with OA and RA at the Raichur Institute of Medical Sciences. The findings underscore significant differences between the two conditions, particularly regarding gender distribution age prevalence and treatment approaches. The study presents significant findings in the management of OA and RA at the Raichur Institute of Medical Sciences. The data show that RA was more prevalent in females (67.07%), while OA was more common in males (56.07%). Both conditions primarily affected middle-aged and older adults, with 89.02% of RA patients and 45.32% of OA patients falling within the 51-65 age range, underscoring the need for age and gender-specific treatment strategies. NSAIDs were the most frequently prescribed medications for both OA (44.45%) and RA, highlighting their role in managing pain and inflammation. However, a higher incidence of ADRs was observed in RA patients (12.5%) compared to OA patients (7.3%), emphasizing the importance of vigilant monitoring to ensure patient safety and treatment adherence. The study also revealed distinct treatment patterns: OA patients were more likely to receive monotherapy (68.4%), while RA patients often required combination therapy (54.8%) due to the condition's complexity. These findings suggest that while monotherapy may be sufficient for OA, RA treatment often necessitates a more comprehensive approach. Future research should focus on personalized treatment strategies that reduce ADRs and enhance patient outcomes, particularly for older adults and women.
We would like to express our sincere gratitude to all the patients who participated in this study, as their willingness to share their experiences and information made this research possible. We also extend our heartfelt thanks to the medical and administrative staff at the Raichur Institute of Medical Sciences for their invaluable support in assisting data collection and ensuring the smooth conduct of this study. We are particularly grateful to our colleagues and mentors for their guidance and encouragement throughout the research process. Their understandings and expertise were instrumental in changing the study and enhancing the quality of our findings.
The authors declare that there are no conflicts of interest regarding the publication of this research. There were no financial incentives or personal relationships that could be perceived as influencing the outcomes of this study. All authors have contributed to the research and preparation of the manuscript and have no affiliations with any organizations that may have a financial interest in the subject matter discussed.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Kumar A, Khan MN (2024). Drug Utilization and Adverse Reactions in the Management of Osteoarthritis and Rheumatoid Arthritis: A Multifaceted Perspective. Trans Med. 14:330.
Received: 29-Oct-2024, Manuscript No. TMCR-24-34906 ; Editor assigned: 31-Oct-2024, Pre QC No. TMCR-24-34906 (PQ); Reviewed: 14-Nov-2024, QC No. TMCR-24-34906 ; Revised: 21-Nov-2024, Manuscript No. TMCR-24-34906 (R); Published: 28-Nov-2024 , DOI: 10.35248/2161-1025.24.14.330
Copyright: © 2024 Kumar A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.