Journal of Genetic Syndromes & Gene Therapy
Open Access
ISSN: ISSN: 2157-7412
ISSN: ISSN: 2157-7412
Review Article - (2024)Volume 15, Issue 1
Duchenne Muscular Dystrophy (DMD) is a severe congenital disorder caused by DMD gene mutations, which results in muscular degeneration and movement difficulties, eventually leading to death. Whereas its less severe form, Becker Muscular Dystrophy (BMD), also caused by the DMD gene mutations, shows slower patterns of progression with much later onset. This review discusses the various mutations resulting in DMD, its genetic basis, the diagnostic tools for performing a genetic diagnosis of having DMD and also advocates the necessity of having genetic therapies and employing nutraceuticals for therapy in the context of this disease. Furthermore, we have also highlighted several treatment options, such as antibacterial drugs, AON-mediated exon skipping therapy, Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR-Cas9) gene-editing technology, nutraceuticals and other significant approaches that have resulted in more promising and durable cures. However, these methods are still under clinical trials. As a whole, our review presents a holistic view of this muscle malady.
Duchenne Muscular Dystrophy (DMD); Becker Muscular Dystrophy (BMD); Dystrophin; Exon skipping; Diagnosis; Therapy; Nutraceuticals
DMD: Duchenne Muscular Dystrophy; BMD: Becker Muscular Dystrophy; ECM: Extracellular Matrix; ORF: Open Reading Frame; CR: Cysteine-Rich; CT: C-Terminal; ABD: Actin-Binding Domain; FDA: Food and Drug Administration; AONs: Antisense Oligonucleotides; PMOs: Phosphorodiamidate Morpholino Oligomers; MLPA: Multiple Ligation Probe Assay; CGH: Comparative Genomic Hybridization; CNVs: Copy Number Variations; MRI: Magnetic Resonance Imaging; EMG: Electromyography; CMRI: Cardiac Magnetic Resonance Imaging; MLPA: Multiplex Ligation-dependent Probe Amplification; CGH: Comparative Genomic Hybridization; mRNA: messenger Ribonucleic Acid; DAPC: Dystrophin-related Protein Complex; 2`OMePS: 2`-O-Methyl-Phosphorothioate; PPMO: Peptide-Conjugated PMO; HDACs: Histone Deacetylase; DGC: Dystrophin- Glycoprotein Complex; KMTs: Histone Lysine Methyl Transferases; HAC: Human Artificial Chromosome; AAV: Adeno-Associated Virus; RCs: Regulatory Cassettes; DSBs: Double-Stranded Breaks; NHEJ: Non-Homologous End-Joining; HDR: Homology-Directed Repair; GC: Gallocatechin; EC: Epicatechin; ECG: Epigallocatechin; EGCG: Epigallocatechin Gallate; GTE: Green Tea Extract; CK: Creatine Kinase; NOS: Nitric Oxide Synthase; BBI: Bowman Birk Inhibitor; EDL: Extensor Digitorum Longus; NGS: Next-Generation Sequencing; CRISPR-Cas9: Clustered Regularly Interspaced Short Palindromic Repeats; F-actin: Filamentous actin; PCR: Polymerase Chain Reaction; Grb-2: Growth Factor Receptor-bound protein 2; DNA: Deoxyribonucleic Acid; PNA: Peptide Nucleic Acid; MAPK: Mitogen-Activated Protein Kinase
Duchenne Muscular Dystrophy (DMD) is one of the most prevalent genetic disorders in humans, impacting one out of every 3500-5000 newborn males [1].
The DMD gene is the longest in humans, with 79 exons and a transcript length of roughly 14 kb. Dystrophin protein is encoded by DMD, which manifests itself in the cardiac and skeletal muscle fibers' sarcolemma and it binds the muscle fibers’ cytoskeleton with the Extracellular Matrix (ECM). Though present at birth, symptoms of DMD usually begin to appear at 2-5 years of age and include difficulty walking, with children becoming wheelchair-bound by 12 or 13 years of age in the majority of instances. Mutations in the DMD gene cause this X-linked recessive condition. Because of the out-of-frame mutation, dystrophin cannot be produced and the Open Reading Frame (ORF) is disrupted. Dystrophin connects the Filamentous actin (F-actin) cytoskeleton to the ECM in muscle through its Amino terminus and Carboxyl terminus (N- and C-terminal) domain regions. Dystrophin has four binding domains through which it interacts with the sarcolemma. Dystrophin's Cysteine-Rich (CR) and C-Terminal (CT) domains, along with the spectrin-like repetitions R1-3 and R10-12, interact with the sarcolemma. The Actin-Binding Domain (ABD) is responsible for the binding of dystrophin and actin. In certain cases, the ORF remains intact even when a mutation (referred to as an in-frame mutation) is present, resulting in a shortened but functioning dystrophin and a milder variant of the illness. This is called Becker Muscular Dystrophy (BMD) [2]. DMD in women is extremely rare (fewer than one million times) and is only known from cases of persons suffering from turner syndrome, DMD translocations or bi-allelic DMD mutations. Female carriers (individuals carrying a single X chromosomal DMD mutation) are normally asymptomatic, but they can be similar to male carriers in rare situations. Around 2.5 to 19% of carrier’s experience symptoms associated with skeletal muscle, with 7.3 to 16.7% developing expanded pupils. Additional cardiac symptoms, such as an abnormal echocardiogram, may be present in carriers of cardiomyopathy [3]. 2016 witnessed the conditional approval of Eteplirsen, a medication developed by Sarepta Therapeutics, by the US Food and Drug Administration (FDA) for use in treating DMD. This drug targets exon 51 and is effective in about 13% of individuals [4].
For screening the exon deletions, samples of DMD/BMD patients were collected from several parts of India and clinical diagnoses were done using multiplex Polymerase Chain Reaction (PCR). Exon skipping therapy uses Antisense Oligonucleotides (AONs) to restore the ORF and knock up (rescue) the target protein, albeit this method is not suitable for mutations in the important domains of dystrophin since skipping the exons, without affecting the functionality of dystrophin is not possible. Exon skipping strategy mediated by Antisense Oligonucleotide (AONs) is a new treatment option for people with DMD. By exons skipping in DMD, AONs can create transcripts within frames and functional proteins. For 47% of individuals affected, the targeted skipping of exons 8, 44, 45, 50, 51, 52, 53 and 55 of DMD was anticipated to be beneficial [5]. It has been demonstrated that exon skipping completely rescues nonsense, duplication, splice site and deletion mutations. Exon- skipping therapy's main goal is to halt the progression of DMD by interfering with splicing events, resulting in milder symptoms similar to those seen in BMD. One of the most promising AONs is Phosphorodiamidate Morpholino Oligomers (PMOs). PMOs are made unrecognizable by nucleases by chemically substituting the phosphodiester backbone with phosphorodiamidate linkages. This significantly increases PMOs' stability [6]. Genome editing using CRISPR-Cas9 technology offers the ability to alter disease development by restoring the expression of a modified dystrophin gene [7].
To assess duplications and deletions in DMD, a quantitative method like the Multiple Ligation Probe Assay (MLPA) or microarray-based Comparative Genomic Hybridization (array- CGH) is utilised. MLPA is the most extensively utilized quantitative approach currently available. In a multiplex PCR-based approach, Copy Number Variations (CNVs) are detected by analysing all the exons of the DMD gene at the same time [8]. This article presents a thorough overview of the DMD, including everything from the genetic basis to the structure of the DMD gene. Several diagnostic tools are also discussed for DMD patients to improve their quality of life. In addition to this, approved therapeutic modalities and others are reviewed in clinical development.
Epidemiology
DMD is the most prevalent muscular dystrophy among children and is considered one of the most common severe congenital myopathies. Despite the X-linked inheritance pattern of DMD, males are more likely than females to be affected. The expected incidence of this disease is 1 out of every 3600 newborn males. Study reports have shown that the prevalence of DMD in the US is 2 per 10,000 [9]. A French study indicated that for individuals born before 1970, the average life expectancy was 25.77 years, while for those born after 1970, it was 40.95 years, indicating that patients with DMD are now more likely to survive. The clinical picture of male DMD and BMD patients includes cardiomyopathy significantly [10].
DMD gene
The DMD gene is situated in the X chromosomes p-arm (Xp21) and is usually expressed in striated and cardiac muscle. In addition to this, it is also present in the brain and retina, but its distribution is less in the brain than in that of muscle [11]. It is a recessive characteristic that is X-linked. It covers 2.2Mb of intronic sequence, with the biggest isoform's coding sequence totaling 11,058 bases across 79 exons. It is the longest gene across the human genome [12]. Dystrophinopathies that result from dystrophin gene mutations include three forms: DMD, BMD and an intermediate form. The mutation causes reduced production of dystrophin protein with the loss of muscle membrane integrity and ultimately results in necrosis.
Mutations mostly include deletions and duplications (70-80%) and point mutations are seen only in 20-30% of DMD individuals [9]. DMD is caused owing to malfunctioning or the absence of dystrophin protein, whereas BMD occurs due to mutations that only partially reduce the gene's function or quantity of product. The muscle isoform of this protein is encoded by the Dystrophin Protein isoform of 427 kDa (Dp427m) mRNA of DMD. 17 transcript variants of DMD are produced by eight unique alternative promoters, alternatively spliced exons and an alternative polyadenylation site, which are expressed and translated in muscles as well as in other types of cells throughout the body [12].
Clinical diagnosis of DMD
DMD mostly occurs in boys below the age of five years. They mostly have a previous family history of the disease, but it can also affect disregarding family history. With the three main aspects of suspicion, we can early diagnose the disease. Firstly, any abnormalities in muscle function of the lower limbs of the child. Secondly, a generalized blood test shows an abrupt increase in serum creatinine kinase level and finally, an elevation of transaminases (Figure 1). The production of transaminases is done by both muscle cells and liver cells. So, DMD diagnosis can be done prior to liver biopsy [13].
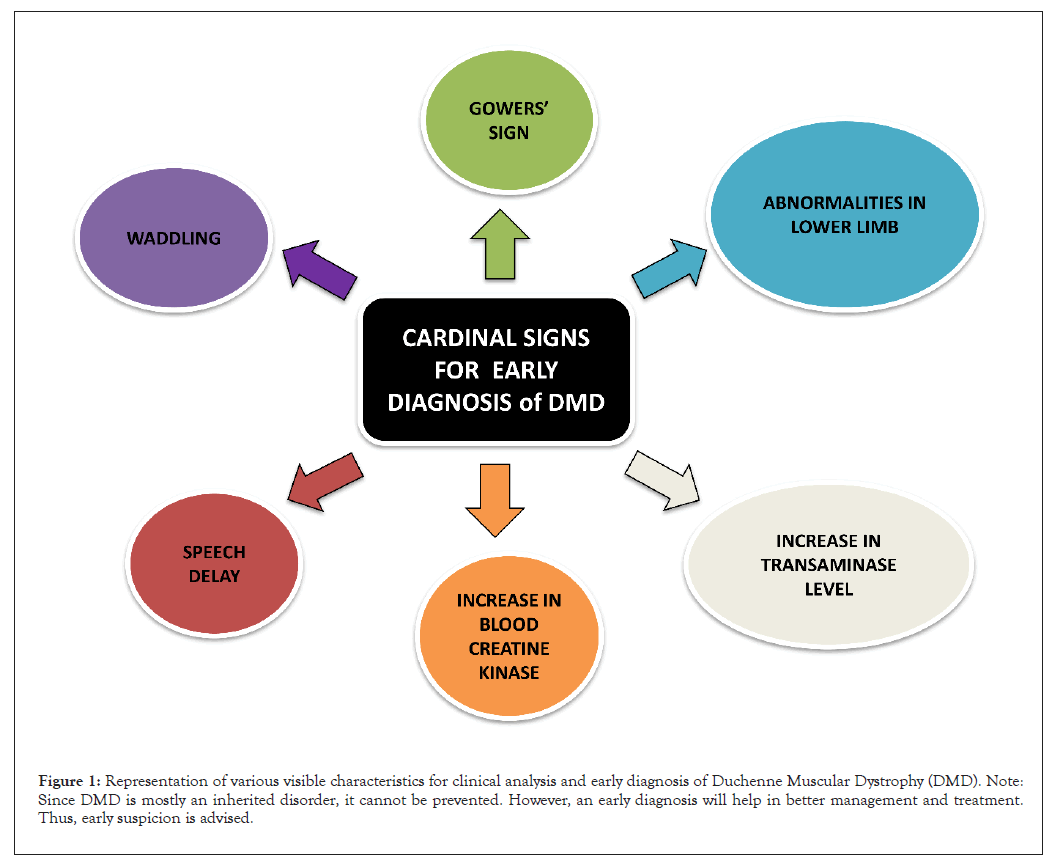
Figure 1: Representation of various visible characteristics for clinical analysis and early diagnosis of Duchenne Muscular Dystrophy (DMD). Note: Since DMD is mostly an inherited disorder, it cannot be prevented. However, an early diagnosis will help in better management and treatment. Thus, early suspicion is advised.
DMD diagnosis can be broadly categorized into two categories (Figure 2). One enlisting the molecular basis and the other dealing with the genetic complexities. The molecular diagnosis includes the following:
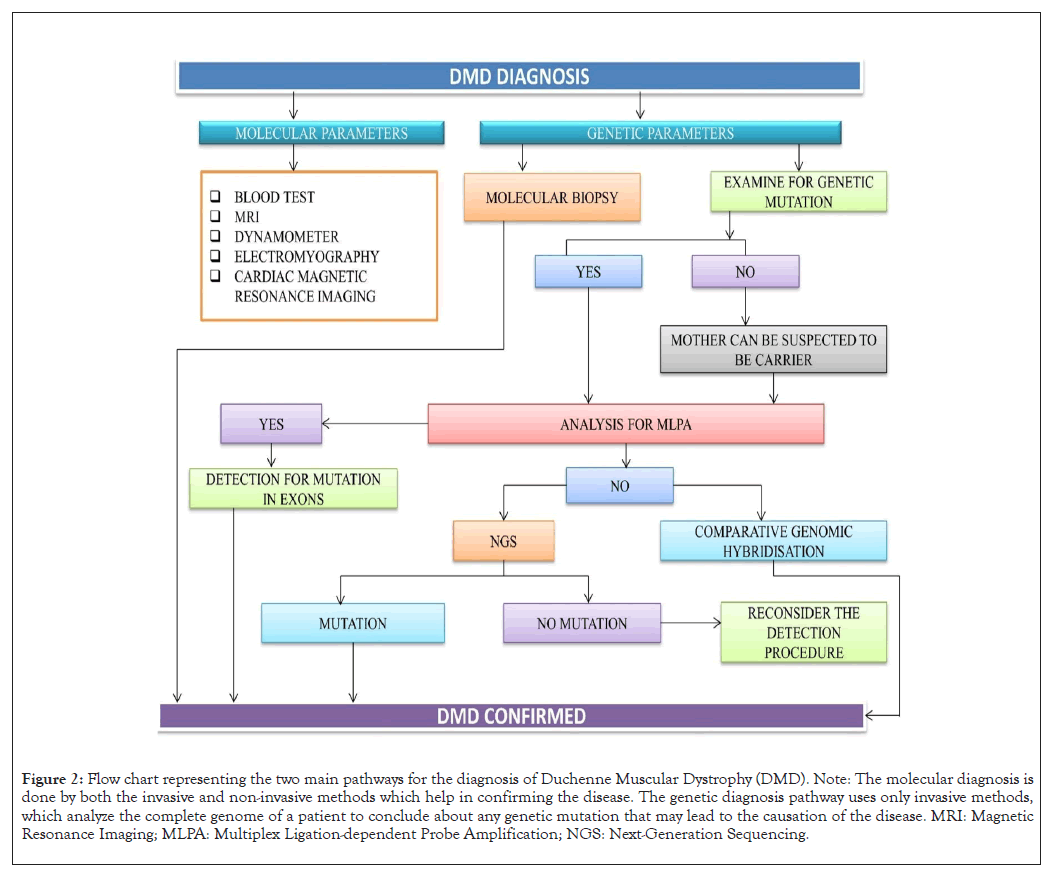
Figure 2: Flow chart representing the two main pathways for the diagnosis of Duchenne Muscular Dystrophy (DMD). Note: The molecular diagnosis is done by both the invasive and non-invasive methods which help in confirming the disease. The genetic diagnosis pathway uses only invasive methods, which analyze the complete genome of a patient to conclude about any genetic mutation that may lead to the causation of the disease. MRI: Magnetic Resonance Imaging; MLPA: Multiplex Ligation-dependent Probe Amplification; NGS: Next-Generation Sequencing.
Blood test: This proves to be the easiest mode of preliminary diagnosis of DMD since it is more accessible and cost-effective than other specialized molecular diagnostic tests that demand sophisticated instruments. The components that are diagnosed by blood tests are elevated serum creatine kinase from muscle damage and elevated serum aldolase and myoglobulin which acts as a marker for muscular dysfunction [14].
Magnetic Resonance Imaging (MRI): This is a non-ionizing, non- invasive clinical method for detecting DMD. It detects muscular abnormalities and defects in connective tissue. A quantitative analysis of lipid fractions and metabolic products within the muscle can be done by magnetic resonance spectroscopy, which is another non-invasive sampling technique. This technique collaborates with MRI for better results.
Dynamometer: This is an instrument that gives measures of isometric muscle strength. The universal level objective grip strength read by a dynamometer is about 46 kg of force for males and 23 kg for females (below 20 years). Any fluctuation from this level will indicate chances of DMD occurrence [15].
Electromyography (EMG): This tests the synergy between the working of the muscle and nerves by measuring electrical impulses along with nerve and muscle tissues. The oscilloscope measures the change in electrical impulse as a wave. The characteristics of the wave throw light on the ability of muscles to respond when stimulated by nerves. A lower amplitude waveform in the oscilloscope indicates weakness in muscle activity.
Cardiac testing: One of the main causes of mortality in DMD is cardiomyopathy. It decreases the left ventricular systolic function and hence requires immediate diagnostic intervention. Cardiac Magnetic Resonance Imaging (CMRI) is used for the diagnosis of myocardial damage and function related to DMD. This is a non-invasive non-radiating technique that will ease our diagnostic procedure.
The genetic component of diagnosis
Suspected cases with impaired muscle function, inability to climb stairs, Gower’s signs, frequent falls and delayed speech should be positively diagnosed with DMD. These are mainly caused due to the absence of dystrophin. The most important genetic cause includes the different mutation cases of which deletion accounts for 65% and remains the major cause of DMD followed by point mutation (25%), duplication (6-10%) and complex mutations (2%). The worldwide used quantitative testing method is Multiplex Ligation-dependent Probe Amplification (MLPA). It detects each of the DMD gene's 79 exons and finds out the CNVs in a multiplex PCR. The MLPA method finds out the specific exon for the deletion duplication in patients and also in carriers. However, this method has a demerit, it does not give us any knowledge about intronic mutations [8]. The second is another quantitative method which is an oligonucleotide- based array, named Comparative Genomic Hybridization (CGH). This method covers the backlogs of the MLPA method. It vividly gives us the exact analysis of copy number variation which includes the intronic region and 3’-5’ flanking region [16]. For this reason, it is one of the most important diagnosis methods which we use for all the complex rearrangements intronic mutations and point breaks. With advancements in scientific technologies, sanger sequencing has been introduced. But it is laborious and expensive at the same time. In the near future, all these diagnostic methods can be replaced by whole-genome sequencing.
Molecular aspects of DMD
DMD, a recessive genetic disorder related to the sex chromosome, is a consequence of the existence of a defective “dystrophin” protein encoding the DMD gene. Although large deletions and duplications are frequently seen, minor mutations have also been discovered. The DMD gene is the longest in humans, 2.2 Mb in length, containing 79 exons. More areas of this gene contain an intronic sequence and it has eight distinct alternative promoters, alternatively splicing exons and a site for alternative polyadenylation. Minor mutations are also seen in DMD patients, but the mutation rate is fairly high and is typically brought on by the deletion or duplication (Figure 3) of one or more exons [17]. Although deletion, duplication or pathogenic variant are seen, there have also been reported major chromosomal rearrangements between a "X chromosome and an autosome" in some cases. A few instances of translocations involving DMD have also been documented as chromosomal abnormalities [18]. Both in males and females, DMD is brought on by the X-linked and autosomal reciprocal translocations [18]. These duplications and deletions can happen randomly within the gene but are most frequently seen between exons 2-10 and exons 45-55, respectively [19]. The cryptic or pseudo-exons in this disease disrupt the reading frame through incorporation into the messenger Ribonucleic Acid (mRNA). The cryptic exons may also contain stop codons that impede the synthesis of functional dystrophin. The few documented missense mutations in DMD patients in a domain of dystrophin protein bear mainly cysteine. This mutation breaks the proteoglycan and extracellular matrix binding [19]. Dystrophin has a crucial structural role in muscle function. Dystrophin attaches itself to the Dystrophin-related Protein Complex (DAPC) at the sarcolemma. When dystrophin is genetically mutated, the DAPC is disrupted. This alternation of proteins gradually causes membrane leakage and fiber damage. Growth Factor Receptor-Bound protein 2 (Grb- 2), which interacts with syntrophins in the cytosol, is an adaptor protein that functions in the DAPC and participates in cellular signaling. Loss of this protein adds to the progression of the disease [20,21]. Mutations that result in deletion and duplication might have two different effects. The reading frame won't be disrupted if the number of nucleotides in the exons that are mutated is an integer of 3. The insertion of abnormal amino acids can change the reading frame in a condition where the number of nucleotide counts in mutated exons is non-integer of 3 and produces functionally unstable dystrophin, which induces DMD pathogenesis [17].
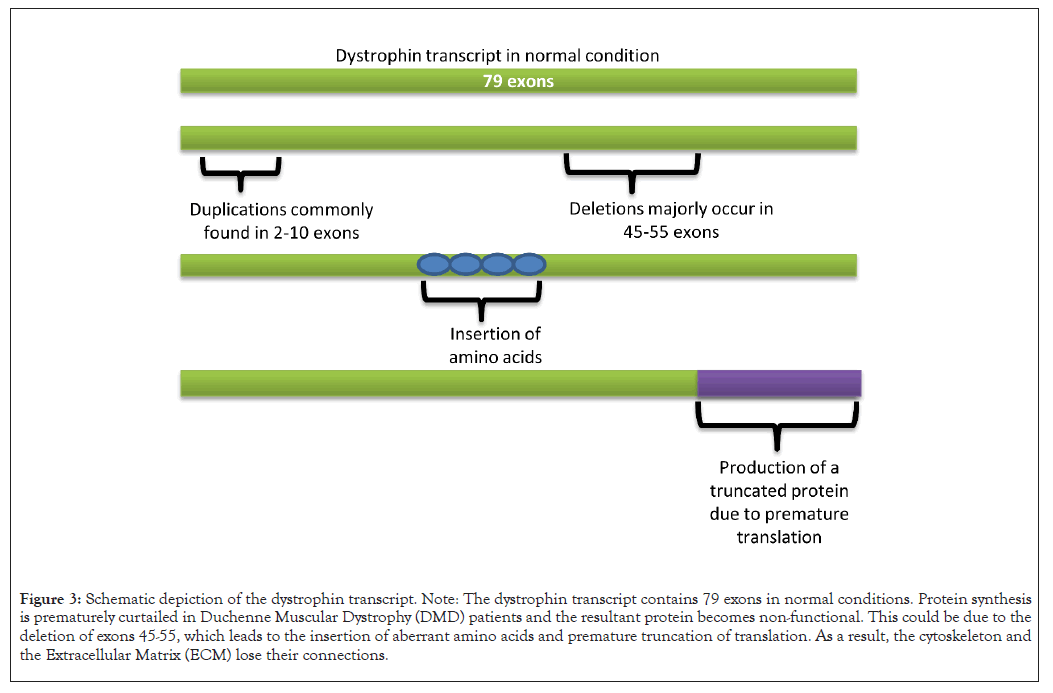
Figure 3: Schematic depiction of the dystrophin transcript. Note: The dystrophin transcript contains 79 exons in normal conditions. Protein synthesis is prematurely curtailed in Duchenne Muscular Dystrophy (DMD) patients and the resultant protein becomes non-functional. This could be due to the deletion of exons 45-55, which leads to the insertion of aberrant amino acids and premature truncation of translation. As a result, the cytoskeleton and the Extracellular Matrix (ECM) lose their connections.
Signs and symptoms of DMD
DDMD typically first appears in early childhood. In children affected, the muscles closest to the trunk or proximal muscles, such as those in the shoulder area and upper arms, as well as the upper legs and pelvic area, weaken and atrophy. Certain other muscles, on the other hand, appear to be disproportionately large, possibly due to fat deposition [22].
Early signs of DMD in children include difficulties meeting developmental milestones involving sitting or standing without aid, toe walking, a peculiar waddling gait, trouble climbing stairs or getting out of a seated posture (Gower's sign) and frequent falls. Due to muscular scarring, young toddlers and kids might appear weird and clumsy with unnatural calves’ expansion (pseudo hypertrophy). A noticeable improvement between the ages of three and five could mistakenly encourage parents, even though this can be the result of natural development and growth. Additional abnormalities such as increased spine curvature (scoliosis or lordosis, osteoporosis), atrophy of pectoral and thigh muscles and certain joints may not be properly fixed as the condition advances (contractures).
DMD may coexist with additional potentially fatal conditions in the late teens, such as cardiac muscle deterioration and weakening (cardiomyopathy). Cardiomyopathy causes irregular heartbeats (arrhythmias) and heart failure. Dystrophin mutations have been linked to a higher prevalence of cardiomyopathy and potential responsiveness to therapy. Patients with DMD frequently exhibit sinus tachycardia and they have higher heart rates as compared to other muscular dystrophies [23]. Muscle weakness and degeneration in the rib cage are other significant consequences associated with DMD [24]. This may result in a higher chance of lung infections (like pneumonia), trouble in coughing and eventually failure of respiration, as shown in Figure 4.
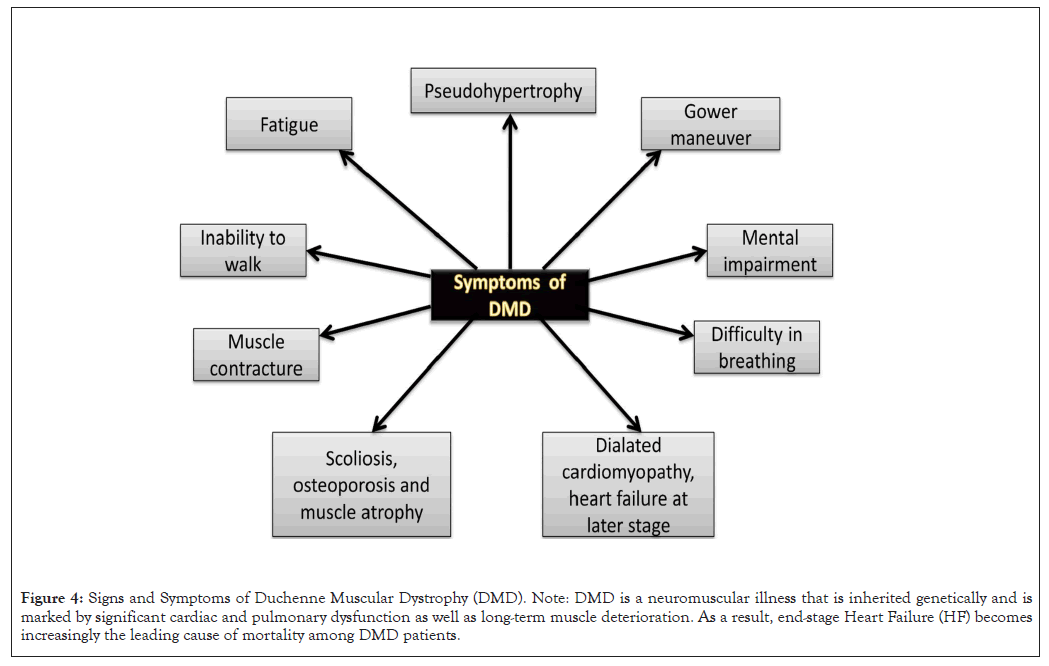
Figure 4: Signs and Symptoms of Duchenne Muscular Dystrophy (DMD). Note: DMD is a neuromuscular illness that is inherited genetically and is marked by significant cardiac and pulmonary dysfunction as well as long-term muscle deterioration. As a result, end-stage Heart Failure (HF) becomes increasingly the leading cause of mortality among DMD patients.
Therapeutic aspects of DMD
DMD counts among the severe dystrophinopathies with no proper cure available to date. However, the journey of finding a cure has led researchers to investigate the prevention of the disease by restoring functional dystrophin protein. Several strategies have been employed through years of research and are still under constant trials to come up with better treatment options. Some of these strategies utilize certain antibacterial medications and chemical analogs that produce functional protein by stop codon read-through procedures. Another approach is AON-mediated exon skipping therapy in which pre-designed oligonucleotide helps in generating a working version of the dystrophin gene. Other major approaches that resulted in more potential and permanent therapies include vector-mediated functional DMD transfer, utilization of CRISPR-Cas9 gene-editing tool and employment of several histone deacetylase inhibitors.
Stop codon read-through therapy
One of the major obstacles to normal muscle development and lack of functional dystrophin genes in DMD patients is a nonsense mutation. This is primarily due to an early termination codon being present, resulting in a non-functional protein in approximately 10% of DMD cases [25]. Moreover, the mRNA generated encounters degradation through nonsense-mediated mRNA decay [26].
Read-through therapy employs small ribosome interacting molecules that bring about conformational changes by inserting an alternative amino acid in place of the premature stop codon. This missense mutation allows the segment of RNA to be re-coded and read through the previous termination codon. This generated comparatively functional dystrophin protein and based on this principle several medications have evolved.
Antibacterial agent-mediated stop codon read-through: Gentamicin, the potent aminoglycoside antibiotic, contains major and minor aminoglycoside components with variable read-through capability. Different components of gentamicin show different pharmacokinetic properties which explain the basis of its variable curing effects. However, the side effects may put patients in danger of nephrotoxicity, cytotoxicity, neurotoxicity and bacterial resistance [27].
Ataluren mediated stop codon read-through: Ataluren, known as oxadiazole, is a novel orphan drug that suppresses nonsense mutations in DMD patients. It is suggested that ataluren has an increased potency with lesser toxicity and other adverse effects. Furthermore, it shows a better tolerance. However, it has reported inconclusive results in clinical trials. Consecutively, the drug was rejected twice by the FDA and is currently under further research to determine the safety and pharmacokinetics of the drug [28].
Exon-skipping mediated treatment
Patients with DMD show variation in terms of specific reading frame mutation, involving specific exons, then patients who have BMD and show somewhat reduced symptoms. This is primarily due to the partially functioning internally truncated protein that is synthesized in BMD patients. Exon 45 deletion and reading frame disruption have been frequently associated with DMD whereas deletion of exons 44 and 45 in BMD patients produces functional truncated protein. This finding has provided the foundation of exon skipping mechanisms to decrease the intensity of complications in DMD patients by targeting specific exons using a pre-designed Antisense Oligonucleotide (AON) which manipulates the splicing of pre-mRNA and recuperates the affected reading frame as is found in BMD patients. For instance, AON designed to target exon 44 in DMD would cause exons 43 and 46 to join together and generate a BMD-like reading frame.
Similarly, skipping of exon 51 through AON targeted splicing modulation restores the reading frame disrupted by exon 48- 50 deletion [29]. Therefore, in terms of DMD research, AON- mediated therapy has been leading the way, with the potential to target approximately 80% of DMD cases [30]. However, this is highly mutation-specific and several medications are required to treat a wider range of patients [31]. Some of these widely used and FDA-approved drugs are discussed in further sections.
Phosphorodiamidate Morpholino Oligomer (PMO) modification: Synthetic Deoxyribonucleic Acid (DNA) analogues known as PMO antisense oligonucleotides are based on six- sided morpholine ring structures, joined by phosphorodiamidate linkages. PMOs confer better tolerability because of the uncharged backbone and are available in greater quantity in the serum for cells to uptake and direct to target mRNA. Along with serum stability, PMOs also have lesser off-target effects and immune responses [32]. These advantages are employed in the consecutive development of FDA-approved drugs such as Eteplirsen and Golodirsen targeted for specific exons. However, Pharmacokinetic evidence regarding PMOs has demonstrated a brief exon-skipping impact, quick elimination from circulation and restricted cellular absorption [32]. This is primarily because PMOs do not bind plasma proteins and thus, are rapidly excreted through urine. Consequently, it offers low toxicity. However, it is unable to activate the complement system and therefore requires a repeated dose of administration to acquire the desired effect [33].
Eteplirsen: Eteplirsen is a drug that received conditional approval from the FDA in April 2016 and targets exon 51 in nearly 14% of DMD cases through the exon-skipping mechanism [29]. An enhanced quantity and improved function of the faulty DMD gene are achieved as the neutrally charged backbone of the drug reduces erroneous targeting and immune response, hence providing a favorable tolerability profile and restoring muscular strength and mobility.
However, in due course of several clinical trials and tests, the FDA has highlighted no correlation between the increase in dystrophin with its clinical benefit. Moreover, an error has been found in the outcomes of western blot and immuno-fluorescence used to confirm the level of dystrophin in muscle biopsies. Nevertheless, further research will provide more valuable insights into understanding the structural significance of morpholino AONs that would lead to improved potency and outcome.
Golodirsen: Golodirsen is a 25-mer AON that benefits about 8% of DMD patients by targeting the skipping of exon 53. This drug is administered intravenously, which results in an overexpression of the dystrophin gene and was approved in the US in 2019 [27].
Like the above-mentioned AONs, viltolarsen also causes skipping of exon 53 and was thus, approved in Japan. This 21-mer oligonucleotide is currently under clinical trial in the US and Canada [34]. Another oligonucleotide, casimersen targets exon 45 is also under clinical development [35].
2`-O-Methyl-Phosphorothioate (2`OMePS) modification: 2`OMePS is a first-generation antisense oligonucleotide that has a modified phosphorothioate backbone where the ribose ring's 2 position is methylated. The oligonucleotide is designed to replace its phosphate group's non-bridging oxygen with a Sulphur atom to prevent it from the nuclease attack [32]. However, the potential side effects and toxicity associated with off-target exposure of 2`OMePS are mainly due to the polyanionic nature and high water solubility. Furthermore, they bind to plasma proteins with low affinity resulting in their sequestration and inactivation. Thus, very little AON is excreted through urine and feces, allowing the major bulk to be distributed to tissues. It has been suggested that in the case of humans, the major portion of the antisense oligonucleotide gets accumulated in proximal tubular cells in the kidney [32]. Consequently, careful monitoring of renal functions is necessary for patients receiving 2`OMePS treatment.
Drisapersen is a 2`OMePS compound that skips exon 51 and has been proven to enhance muscle activity in several tests. However, no evidence of a treatment-related overexpression of the dystrophin gene was indicated by western blotting or immunofluorescence, leading to an inconclusive outcome [36].
Peptide PMO (PPMO): PPMOs are modified PMO that has short peptide fragment rich in arginine (Peptide Nucleic Acid Internalization Peptides (Pip)) attached to either the 5` or 3` position of PMO through chemical conjugation directly and helps in easy penetration through the cell membrane [32]. This addresses the low PMO delivery efficiency in the heart muscles. Cardiac muscles are the primarily affected tissue by faulty dystrophin function and are a primary cause of death for people with DMD. Pip5 and Pip6 are therefore specifically designed to target the activity of the cardiac muscles. It is suggested that PPMO has more serum stability and efficient uptake by the cells than PMO [26]. However, higher toxicity is associated with the arginine residues of PPMO because of its positive charge and results in lethargy, weight loss and renal toxicity [37]. This has been countered with modified Pips that have reduced the number of arginine residues. The possibilities of PPMO as a potent drug for DMD treatment have considerably drawn the attention of researchers and further investigation may help in overcoming the limitations.
AON-mediated exon skipping efficacy and safety: A considerable effort has been made to date in drug discovery to optimize drug affinity and selectivity and at the same time identification of drug toxicity in preclinical or clinical developments [38]. Therefore, maintaining a balanced profile between efficacy and safety is essential for AON-mediated exon skipping. Numerous investigations have been carried out to create more effective AON modifications, including arginine-rich peptide PMO, 2`OMePS and PPMO and the use of adjunctive components like glycine that facilitate more target-tissue uptake [39].
However, when considering DMD treatment targeting the cardiac muscles for improvement in dystrophin production by exon skipping method is essential. Cardiomyopathy has been DMD sufferers' primary cause of mortality and it is suggested that the efficacy of AON-mediated exon skipping is negligible [40]. Hence, extensive effort has been devoted to increasing the efficacy of antisense drugs including tricycle-DNA, Peptide Nucleic Acid (PNA)/ Phosphorodiamidate Morpholino Oligonucleotide (PMO) internalization peptides, octa guanidine morpholino and nanoparticles.
The considerably increased expression of dystrophin in cardio myocytes has resulted only from the employment of PPMO treatment. However, the main complication associated with PPMO is toxicity due to arginine residues. Therefore, reducing AON drug toxicity yet preserving activity with proper dose administration is a matter of further research.
Histone deacetylase inhibitors
Severe muscular atrophy characterizes advanced DMD patients. Following dystrophic muscle degeneration, a number of harmful and compensatory processes occur [41]. Therefore, using pharmaceuticals to stop DMD from getting worse would be the best way to encourage a strong and effective regeneration response in damaged muscles [42]. Targeting epigenetic modifiers could be beneficial for pharmaceutical methods aimed at enhancing the capacity for regeneration in dystrophic muscles since epigenetic pathways affect the muscle stem cells’ regenerative potential.
Histone Deacetylase (HDACs) act as epigenetic silencers in undifferentiated cells, preventing muscle-specific genes from being inappropriately activated transcriptionally. Both HDAC1 as well as 2 (class I HDACs) are associated with Myogenic Differentiation 1 (MyoD) and are localized in the nucleus and HDAC4 and 5 (class II HDACs) move amid the cytoplasm and the nucleus, acting as repressors of mRNA that is dependent on Myocyte Enhancer Factor 2 (MEF2) [43]. It was found that pharmacological inhibition of HDAC can induce muscle gene expression. Studies inspired by this idea done on mice models of DMD have shown the positive impact of HDAC inhibition in muscle development. The justification for using epigenetic medicines stems from experimental findings revealing that HDACs pharmacological inhibition enhances both in vivo and in vitro muscle growth [44]. HDAC inhibitors have been shown to have positive effects on the physiological and morphological restoration of dystrophic musculature in mouse models of DMD, according to preclinical and clinical research. Several investigations have revealed a molecular link between chromatin and the Dystrophin-Glycoprotein Complex (DGC). The beneficial effects of both Nitric Oxide (NO) and HDACI donors require the development of myofibers, which are greater than typical myofiber size and the follistatin, a myostatin antagonist, activating transcription [45,46]. Histone Lysine Methyl Transferases (KMTs) perform significant functions in the control of transcription throughout the course of development and are becoming increasingly crucial in controlling cellular differentiation, including myogenesis.
Satellite cell stemness is correlated with low levels of H3K27me3, but old quiescent satellite cells appear to have an increase in H3K27me3, suggesting that as they age, their capacity for renewal improves [47]. Thus, research suggests that epigenetics controls the stemness of muscle stem cells, under the influence of environmental cues. ITF2357 (Givinostat) and Trichostatin A are the most potent HDACIs. Givinostat's application in clinical studies for various disorders convinced the researchers at the start. Several experiments to examine Givinostat's preclinical efficacy in mouse models of DMD. One anticipated impact of Givinostat in DMD muscles is to induce regeneration, given that HDACI can drive regeneration at the cost of fibro-adipogenic deterioration [48]. Bettica, et al., have evaluated Givinostat's favorable histological effects on twenty DMD youngsters. Givinostat treatment significantly enhanced the percentage of muscle tissue found in biopsies while decreasing the fraction of fibrotic tissue. Additionally, it significantly decreased fatty replacement and tissue necrosis. Overall, the medication was well-tolerated and risk-free. Furthermore, no improvement in functional testing was seen, although the study's sample size was insufficient to draw clear conclusions.
Vector-mediated gene therapy
This method employs the replacement of faulty genes with artificial genes or alteration of their sequence or way of expression, possibly curing a variety of genetic illnesses associated with loss of function.
Artificial chromosome-mediated dystrophin transfer: Human Artificial Chromosome (HAC) created by engineering or de novo synthesis from native chromosomes, has the ability to deliver patients the entire DMD gene. As an additional genomic copy, HAC can replicate during mitosis and dystrophin-HAC from DMD patients' induced Pluripotent Stem Cells (iPSCs) can be created. Dystrophin is expressed when these iPSCs differentiate into myogenic progenitors which are mesoangioblast-like. Nevertheless, it is unknown if the entire dystrophin gene can be transferred into dystrophic muscles by this iPSC-HAC. On the contrary, any immunological rejections caused due to foreign genomes must also be considered [49].
AAV-mediated mini-/micro-dystrophin transfer: Numerous vehicles have been investigated for potential applications in gene therapy; however, recently, the focus has been on using Adeno- Associated Virus (AAV)-generated shuttle vectors to transfer artificial gene constructs. Because of its robust neuromuscular tropism and reduced occurrence of elevated levels of patients' pre-existing, neutralizing AAV9 antibodies, AAV9 is majorly the most commonly used vector in neuromuscular gene therapy [50]. The biggest obstacle to gene therapy mediated by AAV is the enormous size of the DMD transcript (14 Kb). AAV vectors have a much smaller carrying capacity (5 Kb) for genes and Regulatory Cassettes (RCs) than DMD full-length mRNA, which is a drawback of employing them. To overcome this barrier, shorter transgenes that encode mini- and micro-dystrophin, which are shorter proteins and can be included in AAV, were developed [51].
After intravascular administration of AAV micro-dystrophin, several investigations confirmed pan-body expression and this technique's therapeutic effects. Three clinical trials in DMD boys are presently underway; they use micro-dystrophins and the US launched it in December 2017 and are currently being carried out in Europe. In both ambulatory as well as non-ambulatory patients with DMD, a single intravenous infusion of PF-06939926, a transfer of micro- dystrophin mediated by an AAV9, was tested for dosage, safety and tolerability (NCT03362502 and NCT04281485). Solid biosciences studied an AAV9-mediated transfer of micro-dystrophin via SGT- 001 (NCT03368742).
Recently, the trial was reactivated with a modified clinical protocol and second-generation technology was used to create SGT-001, after being suspended due to safety concerns [52]. Sarepta Therapeutics, Inc. proposed the first open-label phase trial I/II (NCT03375164) of the AAV transfer's third form by examining the efficacy and safety of IV infusion of rAAVrh74.MHCK7 micro-dystrophin via SRP-9001. The first 11 people enrolled in research SRP-9001–103 (NCT04626674), another open-label Phase I study being undertaken in collaboration with Roche, reported encouraging results with 12- week dystrophin expression and an excellent safety profile [53]. The risk of adverse immune responses to the viral vehicle is a major worry with AAV-mediated gene substitution. Indeed, at large doses, AAV vectors may induce an immunological response against the encoded transgene or the AAV capsid. Therefore, it is necessary to design efficient delivery methods to lessen this immune reaction as well as to closely monitor immune system responses to the vector or transgene [51].
CRISPR/CAS9-mediated gene editing
The most effective gene therapy strategy, genome editing, aims to resolve a genetic issue permanently on a genomic level. Numerous approaches for DMD gene editing were investigated, all of which worked similarly. Particularly in the field of fundamental scientific research, CRISPR/Cas9 systems, a type of programmable nucleases is precise, effective and adaptable genome editing technologies. Specific modifications result by employing these nucleases in regions of interest in the genome by causing targeted Double-Stranded Breaks (DSBs) on certain sections of DNA, thereby activating DNA repair systems. Non-Homologous End-Joining (NHEJ), which is error-prone and Homology-Directed Repair (HDR), which is error- free, can be used to repair nuclease-induced DSBs. They enable the production of genomic variants (e.g., insertions, deletions or substitutions in the targeted location) that can be used to interrupt, remove or rectify genes [7].
It can be both ex vivo and in vivo. Due to X-linked inheritance and the ability of internally shortened "BMD" proteins to function normally, DMD mutations appear to be an attractive target for genome editing. In most cases, CRISPR/Cas9 editing was intended for the ORF of DMD to get restored, by replicating an AON- skipping effect but operating at the genetic level permanently. Recent research has shown that the defective DMD gene can be reframed using AAV-delivered CRISPR genome-editing tools, which may enable dystrophin restoration in vitro and in short-term mouse trials. However, clinical usage of CRISPR/Cas9 is hindered by issues with off-target mutagenesis, geno toxicity, delivery and immune reactions to AAV vectors and gene-editing technologies and these common concerns are yet to be addressed [54,55].
Nutraceuticals in treating DMD
Nutraceuticals are food items or components there of that provide health or pharmaceutical benefits, including the ability to prevent and cure illness. It is said to be a more natural method to get therapeutic benefits with few negative effects [56]. These also include vitamins and naturally occurring substances like ginseng, garlic and other herbal goods like phytochemicals that are derived from plants, which contain both soluble as well as insoluble fibres. It is believed that several nutraceuticals contain anti-inflammatory or antioxidant properties. These nutraceuticals might offer some therapeutic benefit for DMD, considering that oxidative stress and inflammation exacerbate dystrophic development.
Green tea extract
Gallocatechin (GC), Epicatechin (EC), Epigallocatechin (ECG) and Epigallocatechin Gallate (EGCG) are the catechins that make up the majority of the polyphenols found in Green Tea Extract (GTE) and are in part responsible for its medicinal effects. EGCG is the most prevalent catechin and is also responsible for the majority of its therapeutic effects [57].
The supposed medical benefits of green tea, notably its antioxidant and anti-inflammatory qualities, have led to substantial research over the past few decades. Reduced Nuclear Factor kappa B (NF- kB) pathway signaling is one of the mechanisms that mediate these advantages against inflammation and antioxidants. The NF-kB pathway is also crucial for cell proliferation and differentiation, as well as for inflammation. It has been reported that NF-kB and its downstream pro-inflammatory cytokine targets are up-regulated in the muscles of DMD patients as well as in mdx mice [58].
In one of the trials, mdx mice were given daily subcutaneous injections of 5 or 10 mg/kg GTE starting at three years of age for 5 weeks. Mice given the larger dose (25 mg/kg) did not improve significantly, whereas mice given the lower dose (5 mg/kg) exhibited a 50% drop in serum Creatine Kinase (CK). This decrease in CK levels translated into a 30% increase in locomotor activity in the group that was treated with the lower dosage. Surprisingly, the high- dosage group showed the best functional improvement, although the pathology remained unchanged [59].
GTE possesses cardio protective qualities that may be helpful for DMD patients because the primary cause of death in DMD is cardiomyopathy [60]. About GTE and its potential advantages in DMD, there is still much to discover. Preclinical research suggests that preventing necrosis in its early phases may be beneficial for DMD patients. To ascertain whether there are long-term advantages, extended treatment regimens should be assessed. These studies show a lot of variation in terms of the GTE types employed, how it was purified, the doses used and the muscles examined.
Melatonin
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone that is produced by both plants' and animals’ pineal glands. It is essential for several homeostatic activities, including blood pressure control, immune system activation, circadian rhythm modulation and seasonal regulation of reproductive activity [61]. Melatonin has been shown to inhibit enzymes like Nitric Oxide Synthase (NOS) that produce endogenous free radicals, leading to oxidative injury. This, in turn, stimulates the synthesis of antioxidant enzymes and reduces the production of free radicals in the mitochondria. In a pre-clinical study, Hibaoui, et al., [62] showed a reduction in oxidative stress in the muscles of treated mdx5Cv mice. As reported by Chahbouni, et al., [63] a clinical trial done on DMD patients showed a reduced Superoxide Dismutases (SOD) level and a serum Creatine Kinase (CK) level indicative of less muscle inflammation. Melatonin therapy decreased pro-inflammatory cytokines like Tumor Necrosis Factor Alpha (TNF-α) and Interferon‐Gamma (IFN-γ) and indicators of oxidative stress like Interleukin-1 (IL- 1), Interleukin-2 (IL-2) and Interferon‐Gamma (IFN-γ). However, more long-term studies need to be performed to determine whether melatonin can be used as a supplement.
Vitamin D
Vitamin D is essential for bone health and it is predominantly found in fatty fish or foods such as soy milk, dairy and orange juice or is produced in the body by converting 7-dehydrocholesterol in the skin after exposure to Ultraviolet (UV) light. In terms of the role of vitamin D, calcium absorption from the small intestine is encouraged by it. In intestinal cells, the active vitamin D metabolite 1,25-dihydroxy vitamin D (1,25-D) binds to its receptor and promotes the production of calbindin, which further binds to calcium and affects the calcium ion channels [64]. DMD patients are recommended to take calcium and vitamin D supplements to preserve bone health and avoid fractures because 78% of DMD patients are vitamin D-deficient [16].
The effectiveness of vitamin D supplementation in DMD was evaluated over a 16-year period, retroactively from 1998 to 2014. They discovered that, despite being advised to take vitamin D supplements, a substantial rate of vitamin D deficiency still exists in DMD patients. The maintenance of vitamin D dosages daily (200, 400, 800, 1000 or 1500 IU) or weekly (3000 or 6000 IU) were also examined. The major results showed that optimal blood 25-(OH) D levels could only be attained with a 1500 IU dosage of vitamin D. In addition, 84% of patients on a replenishment program of 6000 IU daily for 3 months, as opposed to just 52% on a regimen of 3000 IU daily, had adequate vitamin D levels.
The key finding of this study was that patients need to be monitored every six months to keep their blood vitamin D levels in their ideal range. As a whole, these findings are extremely uplifting for patients to use vitamin D supplements to preserve bone health and guard against mobility loss brought on by fractures [65].
Soybean
Soy is said to provide a variety of nutritional benefits, although the specific chemistry behind many of these effects is not well understood. Isoflavones like genistein and the Bowman Birk Inhibitor (BBI) have the most well-known effects. The three main isoflavones in soy, i.e., genistein, daidzein and glycitein, have been shown to have antioxidant, phytoestrogen and kinase inhibitory effects. BBI is a peptide of 8 kDa that inhibits both trypsin and chymotrypsin non-competitively. It may pass through the stomach and gut wall unharmed [66,67].
A daily intraperitoneal dose of 2 mg/kg of genistein resulted in a 25% improvement in forelimb strength in mdx mice. With this gain in strength, the biceps muscle's necrosis decreased by around 40% and the area of regenerating fibres increased by about 50%. Myosin heavy chain staining intensity during development did not vary significantly; nonetheless, there was a connection between this and an increase in the number of nuclei that were positive for myogenin. Again, demonstrating reduced muscle damage, CK levels in serum were decreased by 20% in the genistein-treated animals. The NF-kB DNA binding activity in mdx mice treated with genistein was dramatically decreased, pointing to a potential inhibitory impact.
TNF-α and phospho-Jun N-terminal Kinase (JNK) expression, which is connected to the Mitogen-Activated Protein Kinase (MAPK) signaling pathway, were both considerably downregulated. In comparison to methylprednisolone administered intraperitoneally at a dose of 0.75 mg/kg/day to mdx mice, one of the corticosteroids used to treat DMD decreased necrosis and CK levels in serum in the genistein-treated group. Positive results indicate that genistein may lessen deterioration and enhance muscular function in DMD. At present, there are no clinical trials of genistein that have been filed; however, studies for DMD patients are necessary to ascertain whether it may have the similar therapeutic effect as prednisolone without unfavorable adverse effects [68].
One more study examined the administration of BBI, a soy- derived serine protease inhibitor, to mdx mice [69]. A number of pathogenic parameters were decreased by BBI. In the BBI-treated group, serum CK was lowered by around three-fold, indicating less pathology. Muscle function also improved, along with the improvement in pathology. The absolute tetanic force of Extensor Digitorum Longus (EDL) rose, while the specific force remained the same when normalized for EDL size. Treatment with BBI reduced the recovery time from an injury brought on by eccentric contraction by 25.7%.
These studies demonstrate the potential of substances derived from soybeans to help manage DMD's persistent inflammation and immunological response. Even though the outcomes in the mdx mice are encouraging, additional research is required to establish the ideal dosage as both trials only examined a single dosage and analyzed responses at a single time-point.
Despite the difficulty in DMD’s molecular diagnosis, notable advancements have been achieved in recent years, leading to the development of extensive molecular testing techniques, that might detect different mutations, such as deep intronic events, point mutations and deletion/duplication. An accurate diagnosis using modern high-throughput technologies like CGH and Next- Generation Sequencing (NGS) will allow patients prompt diagnosis and efficient treatment opportunities. Genetic counseling can be done after analyzing the clinical, biochemical and cytogenetic data and multiplex PCR has been used to check for the dystrophin gene mutation and to screen the deletions of exons for the diagnosis of DMD. For muscle biopsy, dystrophin immunocytochemistry can be carried out. Clinical trials for treatments like gene therapy, based on the systemic distribution of AAV/micro-dystrophin vectors, have extensively been conducted and appear to be becoming more viable. Ongoing research suggests that canine models for DMD exhibit similar outcomes and different types of AAV vectors are safe in non-human primate investigations and clinical trials for other genetic diseases. If the first human trials do not yield satisfactory findings, the general approach is subject to major modification. Although DMD was long thought to be an incurable condition, recent research suggests that viable gene treatments may be available soon.
Srabaita Roy, Ranjit Shaw and Gyaneshwer Chaubey conceived and designed the study. Srabaita Roy, Ranjit Shaw and Sukanya Samaddar have prepared and formatted all the figures. Srabaita Roy and Ranjit Shaw contributed equally. Srabaita Roy, Ranjit Shaw, Ankita Das, Sukanya Samaddar, Sukanya Samanta, Ritwija Maity, Puja Chatterjee, Ankita Das, Suchismita Bhaumik and Gyaneshwer Chaubey constituted the manuscript.
No funders had a role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Gyaneshwer Chaubey is supported by Faculty IOE grant BHU (6031) and ICMR;
The authors declare no competing interests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Roy S, Shaw R, Samaddar S, Samanta S, Maity R, Chatterjee P, et al. (2024) Duchenne Muscular Dystrophy: A Review on Systemic Paradigm Approaching Diagnosis to Therapy. J Genet Syndr Gene Ther. 15:413.
Received: 30-Jan-2024, Manuscript No. JGSGT-23-28172; Editor assigned: 02-Feb-2024, Pre QC No. JGSGT-23-28172 (PQ); Reviewed: 16-Feb-2024, QC No. JGSGT-23-28172; Revised: 23-Feb-2024, Manuscript No. JGSGT-23-28172 (R); Published: 01-Mar-2024 , DOI: 10.35248/2157-7412.24.15.413
Copyright: © 2024 Roy S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.