Journal of Psychology & Psychotherapy
Open Access
ISSN: 2161-0487
ISSN: 2161-0487
Research Article - (2024)Volume 14, Issue 5
Introduction: Autism Spectrum Disorder (ASD) is characterized by impairments in social communication and the presence of repetitive and stereotyped behaviors. Data indicate a prevalence of 2.7% in childhood, and early interventions have a significant effect when administered before the age of 4.
Objectives: To review the scientific literature on screening scales for ASD developed for children up to 36 months, identify the instruments, compare their accuracies, and organize them according to their screening levels 1 (universal screening) and 2 (diagnostic support).
Method: Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol, two judges independently performed article selection and data extraction. Searches were conducted in the PubMed, Virtual Health Library (VHL), Scielo, PsycINFO, and Google Scholar databases, with filter for the period between 2013 and 2022. A total of 815 articles were found, of which 22 were included in this review. The study was registered in PROSPERO: “CRD42022343562”.
Results: The Modified Checklist for Autism in Toddlers, Revised with Follow-Up (M-CHAT-R/F) emerged as the most accurate instrument at level 1, while at level 2, the Autism Diagnostic Observation Schedule-Toddler Module (ADOS-2-T) stood out. The Social Attention and Communication Surveillance-Revised (SACS-R) showed the best indicators regardless of the screening level. In the Brazilian context, the M-CHAT-R/F is the only fully adapted instrument. Scales that assess more specific age ranges and involve interactive follow-up interviews by professionals are more suitable for screening purposes. These findings can contribute to clinical practice guidelines and research on early diagnosis of ASD.
Autism spectrum disorder; Early diagnosis; Data accuracy; ROC curve
Autistic Spectrum Disorder (ASD) comprises a series of differences in the neurodevelopment of a population. Individuals on the spectrum show impaired socialization and communication, as well as repetitive and stereotyped behaviors, in a heterogeneous set of symptoms, commonly recognized in children between 12 and 24 months [1]. A systematic review of retro and prospective studies corroborated with strong evidence that ASD symptoms manifest in the first two years of life [2].
The early diagnosis of autism should be guided by a clinical evaluation that involves, among other actions, an interview with caregivers, observation of the child and the use of screening instruments [3]. Despite the evidence that autism can be safely diagnosed at around 24 months [4-6], American data indicated that, in 2018, the average age at diagnosis was 50 months, ranging from 36 to 63 months [7]. Another common aspect in the ASD evaluation process is the long wait that occurs between the search for care after the first signs and the diagnostic definition. In the United States of America, this time is 12 months on average [8]. Such barriers oppose the scientific consensus that early diagnosis and intervention, preferably initiated before four years old, are the most significant factors for a better prognosis of the disorder [9-11].
Since 2007, the American Academy of Pediatrics (AAP) has recommended that all children be screened for ASD between 18 and 24 months, a position that has recently been reaffirmed [12]. However, the AAP, as well as the Centers for Disease Control and Prevention (CDC), do not endorse any specific instrument for screening purposes. Research contributes to this position, since it has shown that there is not enough evidence to recommend a single universal instrument [13-15]. In the Brazilian context, the situation is repeated. Federal law 13.438/2017 made it mandatory to apply a protocol for risk screening to all children in their first 18 months of life, especially for ASD. However, the Brazilian Ministry of Health does not indicate a reference instrument.
Given this scenario, the support instruments, the focus of this review, when properly accurate and feasible, can provide greater speed and safety to the diagnostic process [16]. The protocols also provide early access to an intervention, combat false negatives and reduce the occurrence of false positives, avoiding unnecessary interventions. Thus, improving the early identification of ASD is a relevant goal with numerous challenges, making research on instruments that screen the disorder significant.
As a result of encouraging ASD screening, a wide variety of instruments are suggested in the literature [3,16,17]. However, with specific regard to early screening, there is a shortage of protocols developed exclusively for diagnosis before four years old [14,18]. A systematic search in four databases identified 59 tools used to detect ASD. Of these, only nine instruments were applied in non-English speaking populations, and only five could be considered specific for the early detection of autism [19]. Another review located 11 early screening instruments, but concluded that there was no gold standard among the selected tolos [14]. A large study conducted in Europe provided an overview of ASD screening studies, seeking to identify the variables that influenced the results. At the time of the research, more than 70,000 children had been screened using 18 different protocols. Compiling the factors with the greatest influence on the results, the authors concluded, at that time, that it was impossible to choose the screening method that best adapts to a specific context [13]. It is pertinent to highlight that the reviews found analyzed several instruments that are currently outdated.
On the other hand, the literature points to two scales considered the gold standard in ASD screening: The Autism Diagnostic Interview-Revised (ADI-R), based on a parental report; and the Autism Diagnostic Observation Schedule-Second Edition (ADOS-2), based on the observation of a person with suspected autism [3,20-25]. However, none of these instruments was developed exclusively for early screening. In addition, they are not clinical unanimity, considering the continuity of production of new scales [24,25]. As a barrier, the need for training and clinical experience of the applicator was pointed out, in addition to the high acquisition cost, which can make its use unfeasible, especially in low-income countries [21].
In the Brazilian scenario, few instruments are available [26,27], especially when considering only instruments based on Diagnostic and Statistical Manual of Mental Disorders (DSM-5), as well as those purely developed for children up to 36 months. This worrying Brazilian context is confirmed observing the absence of protocols in the database of the Psychological Tests Assessment System (SATEPSI), a body of the Federal Council of Psychology that evaluates the evaluation instruments, according to their psychometric properties.
Faced with this variability of tools, it should be noted that the diagnostic support instruments are presented in the literature divided into two large groups: Levels 1 and 2. In level 1 screening, the objective is to point out risk for ASD and other impairments in the development of the population in general (universal screening). In level 2 screening, the previously screened diagnostic hypothesis of autism is considered, where the instrument aims to support diagnostic confirmation or exclusion [14,28].
Thus, considering the DSM-5 diagnostic criteria, the operational logistics of the diagnostic instruments, their objectives and the need for early identification of ASD, the general objective of the present study was to verify the accuracy of the diagnostic support instruments developed specifically for ASD screening. ASD in children up to 36 months. In addition, to improve clinical practice, this work also aimed to organize the tools according to their screening levels, formats, application times, age scopes, need for training and use of original materials, as well as the verification of adaptations to Brazil.
Registration design and protocol
This is a systematic literature review, structured according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA), registered on the PROSPERO systematic reviews platform, under the identification “CRD42022343562” on July 11, 2022.
Eligibility criteria
The research was filtered by productions from 2013, in which the instruments and the outcome (screening or diagnostic assessment of ASD) should be based on the DSM-5. As for the language, no restrictions were applied. Furthermore, the following inclusion criteria were observed: Original scientific articles published in peer-reviewed journals; longitudinal, cross-sectional, case-control studies and clinical trials; and instruments developed exclusively for ASD screening up to 36 months. Among these, the following were excluded: Instruments that are not in their latest version (outdated); tools based on high technology, biomarkers or imaging tests; and studies that did not present instrument accuracy data.
Systematic review search strategy
According to PRISMA recommendations, the research was divided into four phases: Identification, screening, eligibility and inclusion. The data sources included in the study were: PubMed, Virtual Health Library (VHL), Scielo, PsycINFO and Google Scholar, the latter being the first 200 occurrences. In search of effectiveness and for relevant evolutionary reasons, Boolean operations were performed with the following combinations: Autism or autistic and early diagnosis and accuracy or validation or roc curve. In addition, potentially relevant references to experts on the subject were requested by electronic correspondence. The research was carried out between July and August 2022. The search resulted in 815 productions, distributed as follows: Pubmed=360, VHL=117, Scielo=1, PsycINFO=137, Google Scholar=200.
Selection of studies
The studies were blindly considered by two reviewers, who determined whether the studies met the inclusion criteria. Publications were weighted independently using the platform Rayyan and disagreements were resolved by consensus among the authors of this review. First, the evaluators selected the articles by title and abstract and, later, by full text. All articles that did not meet the search criteria were excluded, as shown in Figure 1.
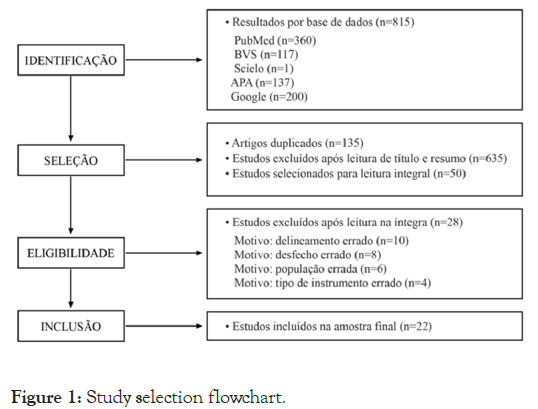
Figure 1: Study selection flowchart.
Data extraction
Both researchers were involved in the data extraction process. The following information was collected: Instrument name, application format, screening level, type of respondent, age scope, need for training and original materials and the existence of adaptation to Brazil. The following data were examined: Sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), accuracy and Area Under the Curve (AUC), always considering the sample size for discussion purposes. The outcome was the presence of a suspected diagnosis of autism confirmed through clinical evaluation, following the DSM-5 criteria.
22 articles were found that analyzed 13 different instruments, all developed exclusively for the population aged 0 to 36 months: Autism Detection in Early Childhood (ADEC), Autism Diagnostic Observation Schedule Second Edition- Toddler Module (ADOS-2-T), Brief Autism Detection in Early Childhood (BADEC), Checklist for Autism in Toddlers-23-item (CHAT-23), Early Screening for Autism and Communication Disorders (ESAC), Early Social Responsiveness (ESR), Modified Checklist for Autism in Toddlers Revised with Follow-up (M-CHAT-R/F), Quantitative Checklist for Autism in Toddlers (Q-CHAT), Quantitative Checklist for Autism in Toddlers-10- item (Q-CHAT-10), Rapid Interactive Screening Test for Autism in Toddlers (RITA-T), Social Attention and Communication Surveillance-Revised (SACS-R), Systematic Observation of Red Flags (SORF) and Toddler Autism Symptom Inventory (TASI).
As for the forms of application, six instruments are based on interaction and direct observation of the child, three are self- administered questionnaires, three are mixed and one is of the interview type. Regarding screening levels, six tools are dedicated to level 2, four to level 1 and three to both levels, that is, when the instrument proposes to screen both in general populations and in those with previously identified risk.
The age scope ranged from 11 to 36 months. Regarding the need to acquire original materials for application, nine do not need it, while four do. In terms of training, this data is the opposite, as nine instruments require prior training and only four do not.
In five instruments, the main respondents are health and education professionals, while four are dedicated to caregivers. Another four protocols combine parental responses with professional completion. Finally, of the 13 instruments, only two have studies of adaptation to Brazil, the ADOS-2, M-CHAT-R/F [29,30]. It is noteworthy that this review analyzed the ADOS-2-T, a specific ADOS-2 module for children aged up to 36 months. The compilation of these data can be found in the Table 1.
| Instrument | Application format | Level | Respondents | Age in months | Materials/ training | Brazil adaptation | Articles (reference) |
|---|---|---|---|---|---|---|---|
| ADEC | Interactive assessment of 16 items (scale likert of 3 points) | 2 | Professionals | 12 to 36 | Yes/Yes | N/F | [30] |
| ADOS-2-T | Interactive and observational evaluation of 9 semi-structured activities | 2 | Professionals | 12 to 30 | Yes/Yes | Partially | [31] |
| BADEC | Interactive assessment of 5 items (scale likert of 3 points) | 2 | Professionals | 12 to 36 | Yes/Yes | N/F | [32] |
| CHAT-23 | Self-administered questionnaire with 23 yes/no questions, and observation of 5 items by professionals | 1, 2 | Parents and professionals | 18 to 24 | No/Yes | N/F | [33] |
| ESAC | 46-item self-administered questionnaire (scale likert of 3 points) | 1 | Parents | 12 to 36 | No/No | N/F | [6] |
| ESR | Observational evaluation of the child's interaction with parents in 25 semi-structured activities | 2 | Professionals | 13 to 24 | Yes/Yes | N/F | [34] |
| M-CHAT-R/F | Self-administered 20-item yes/no scale, with follow-up interview by a professional | 1 | Parents and professionals | 16 to 30 | No/No | Yes | [29,35-42] |
| Q-CHAT | Self-administered 25-item scale (scale likert of 5 points) | 1 | Parents | 18 to 24 | No/No | N/F | [43] |
| Q-CHAT-10 | Self-administered 10-item scale (scale likert of 5 points) | 1 | Parents | 18 to 24 | No/No | N/F | [39] |
| RITA-T | 9-item interactive assessment (Yes/No or likert of 5 points) | 2 | Professionals | 18 to 36 | No/Yes | N/F | [44,45] |
| SACS-R | Observational assessment with interview and self-administered module of 40 items (rarely/frequently) | 1, 2 | Parents and professionals | 11 to 30 | No/Yes | N/F | [46] |
| SORF | 22-item home and clinical observational assessment (scale likert of 4 points) | 1, 2 | Parents and professionals | 16 to 24 | No/Yes | N/F | [47,48] |
| TASI | Semi-structured 37-item parental interview conducted by a professional | 2 | Parents | 12 to 36 | No/Yes | N/F | [17] |
Note: Parents=Primary caregivers; Professionals=Health or education workers and N/F=Information not found.
Table 1: Categorization of instruments.
Segmented by levels, (Tables 2-4) compile instrument accuracy data. This separation became relevant in view of the relevance that each dimension analyzed has for the purpose of the tool. Level 1 tracking, for example, benefits from increased sensitivity.
| Instrument | Author (year) | n | Se. | Sp. | PPV | NPV | Accuracy | AUC | Observation |
|---|---|---|---|---|---|---|---|---|---|
| ESAC | [6] | 159 | 86,0 | 82,0 | 64,0 | 94,0 | N/F | 91,0 | 12 to 17 months |
| 187 | 87,0 | 85,0 | 76,0 | 92,0 | N/F | 93,0 | 18 to 23 months | ||
| 249 | 88,0 | 84,0 | 81,0 | 90,0 | N/F | 93,0 | 24 to 36 months | ||
| M-CHAT-R/F | [36] | 100 | 100,0 | 83,3 | N/F | N/F | N/F | N/F | - |
| [37] | 7928 | 96,3 | 86,5 | 91,0 | N/F | N/F | 96,7 | - | |
| [38] | 1549 | 66,0 | 97,0 | 31,0 | 99,0 | N/F | 86,5 | M-CHAT-R | |
| 95,0 | 84,0 | 72,0 | 97,0 | N/F | 92,7 | M-CHAT-R/F | |||
| [30] | 75 | 88,2 | 53,6 | N/F | N/F | 63,0 | 63,0 | - | |
| [39] | 3529 | 82,0 | 99,0 | 47,0 | 99,0 | N/F | N/F | 14 to 22 months | |
| [40] | 3096 | 75,0 | 99,0 | 30,0 | 99,0 | N/F | N/F | 23 to 36 months | |
| 408 | 73,0 | 66,0 | 28,0 | 93,0 | N/F | N/F | M-CHAT-R | ||
| 368 | 36,0 | 89,0 | 36,0 | 89,0 | N/F | N/F | M-CHAT-R/F | ||
| [41] | 312 | 86,0 | 94,0 | 53,0 | 99,0 | N/F | 94,0 | - | |
| [42] | 3052 | 74,3 | 97,4 | 42,3 | 99,3 | N/F | N/F | - | |
| [43] | 110 | 88,9 | 94,6 | 76,2 | 97,8 | N/F | 99,0 | ||
| Q-CHAT | [44] | 315 | 72,7 | 92,1 | N/F | N/F | N/F | 89,5 | ASD vs. TD |
| 72,7 | 76,0 | N/F | N/F | N/F | 79,3 | ASD vs. NonASD | |||
| Q-CHAT- 10 | [40] | 406 | 34,0 | 95,0 | 54,0 | 89,0 | N/F | N/F | Standard version |
| 63,0 | 79,0 | 35,0 | 92,0 | N/F | 75,0 | Ordinal version |
Note: Se=Sensitivity; Sp=Specificity; N/F=Information not found; TD=Typical development and NonASD=Other developmental conditions or disorders. The values of the accuracy criteria are presented in percentage.
Table 2:Accuracy data of level 1 instruments.
| Instrument | Author (year) | n | Se. | Es. | VPP | VPN | Accuracy | AUC | Observation |
|---|---|---|---|---|---|---|---|---|---|
| ADEC | [31] | 79 | 93,0 | 64,0 | 78,0 | 88,0 | N/E | 90,0 | - |
| ADOS-2-T | [32] | 412 | 98,3 | 75,4 | 93,9 | 92,0 | 93,6 | 92,0 | - |
| BADEC | [33] | 107 | 77,0 | 86,0 | 82,0 | 82,0 | N/E | 82,0 | - |
| ESR | [35] | 120 | 76,9 | 83,2 | 35,7 | 96,7 | 82,5 | 78,1 | Total sample |
| 37 | 90,9 | 92,3 | 83,3 | 96,0 | 91,9 | N/E | Validation sample | ||
| RITA-T | [45] | 61 | 100,0 | 84,0 | 88,0 | 100,0 | NE | N/E | - |
| RITA-T | [46] | 81 | 82,0 | 100,0 | 100,0 | 71,0 | N/E | N/E | - |
| TASI | [18] | 204 | 88,7 | 81,5 | 62,7 | 95,4 | N/E | 92,0 | Total sample |
| 90 | 89,5 | 67,6 | 42,5 | 96,0 | N/E | 89,0 | Validation sample |
Note: Se=Sensitivity; Es=Specificity and N/E=Information not found. The accuracy criteria values are presented as a percentage.
Table 3: Accuracy data for level 2 instruments.
| Instrument | Author (year) | n | Se. | Es. | VPP | VPN | Accuracy | AUC | Observation |
|---|---|---|---|---|---|---|---|---|---|
| CHAT-23 | [34] | 4954 | N/E | N/E | 41,70 | N/E | N/E | N/E | Stage I |
| 17293 | N/E | N/E | 48,5 | N/E | N/E | N/E | Stage II | ||
| SACS-R | [47] | 13511 | 61,5 | 99,6 | 82,6 | 98,7 | N/E | N/E | - |
| SORF | [48] | 228 | 77,0 | 72,0 | 62,0 | 84,0 | N/E | 81,0 | Composite score data |
| 73,0 | 63,0 | 54,0 | 80,0 | N/E | 75,0 | Score data | |||
| “red flags” |
Note: Se=Sensitivity; Es=Specificity; N/E=Information not found. The accuracy criteria values are presented as a percentage.
Table 4: Accuracy data for instruments that track both levels.
In Table 2, the following level 1 instruments were unified: ESAC, M-CHAT-R/F, Q-CHAT and Q-CHAT-10. The M-CHAT-R/F was the instrument that reached the highest values in sensitivity, specificity, PPV, NPV, accuracy and AUC, in addition to the largest samples. This scale also had the second-highest sensitivity and AUC indicators [31].
In the age criterion, the M-CHAT-R/F showed worse accuracy in children older than 23 months, while the ESAC showed progressive improvement in the indicators as the sample approached 36 months. The most accurate data were present with follow-up interviews (M-CHAT-R/F single two-step instrument at level 1). On the other hand, two M-CHAT-R/F studies showed worsening of some criteria after the follow-up interview. The authors pointed out possible influences of age variation between the two moments. At this level, the format, training and cost of original materials were not significant variables, considering that all instruments are self-administered, free of charge and do not require prior preparation. Of this group, only the adaptation of the M-CHAT-R/F to Brazil was located [32].
Table 3, brings the accuracy data of level 2 instruments: ADEC, ADOS-2-T, BADEC, ESR, RITA-T and TASI. Because they are more specific instruments, the sample sizes are considerably smaller. The RITA-T scale showed the highest sensitivity, specificity, PPV and NPV. It is pertinent to highlight that the samples of the RITA-T validation studies are relatively small. The study that brings the accuracy of the ADOS-2-T, has an expressive sample, with 412 individuals, and significant stability of the accuracy indicators (98.3% sensitivity, 75.4% specificity, 93.9% PPV, 92.0% VPN, 93.6% accuracy and 92.0% AUC, the latter being the highest in the level). Furthermore, it is imperative to note that ADOS-2-T is the module toddler assessment, considered the gold standard for other age groups. In this group of instruments, there was a greater distribution of high accuracy rates. Except for Brief Autism Detection in Early Childhood (BADEC), all instruments obtained at least two strong quality indicators (>90.0%) [33].
The construction study of the Toddler Autism Symptom Inventory (TASI) showed better psychometric properties in children between 24 and 36 months, when compared to a sample younger than 24 months. The ADOS-2-T study corroborates this finding, as it also points to better accuracy in older children (between 21 and 30 months) than among younger ones (between 12 and 20 months). All instruments grouped here must be applied by a professional. Furthermore, except for TASI, all are interactive with the subject. The ADOS-2-T and ESR protocols also observe the caregivers' interaction with the child. Prior training was necessary for all tools. Only RITA-T and TASI dispense original materials. No adaptation was found for Brazil. However, ADOS- 2-T has preliminary study evidence of its core module [34].
Finally Table 4, brings data from the following instruments: CHAT-23, SACS-R and SORF. These tools are intended to be screened in the general population, to the same extent that they already support the diagnosis of ASD. In the CHAT-23 study, the sample is very expressive (n=17293). However, the article presented only the PPV (48.5%) as accuracy data, insufficient for a better analysis. Also with a significant sample, the SACS-R study (n=13511) had the highest specificity values (98.6%), PPV (82.6%) and NPV (98.7%). SORF was superior in sensitivity (77.0%) and AUC (81.0%), the latter demonstrated only by this scale. None of the studies presented accuracy values [35].
In the articles of these instruments, age subgroups were not analyzed. CHAT-23 and SORF have parental response steps followed by professional follow-up. The SACS-R, on the other hand, advises that its completion be accompanied by a health or education professional at specific and sequential moments (12, 18 and 24 months). This format is unique considering all the tools analyzed so far. The SACS-R data come from a screening in three stages with a significant interval between them (6 months). All tools require training. On the other hand, no instruments require original materials. As for the application format, SORF is purely observational, while SACS-R combines observation and interview. The CHAT-23, on the other hand, has a self- administered parental questionnaire at first, prior to professional observation. No adaptations of these instruments to Brazil were located [36].
This work, in addition to updating, sought to innovate by compiling the accuracy of instruments dedicated exclusively to the diagnostic support of ASD in children up to 36 months. This fact becomes relevant, observing the historically ratified importance of early screening, in relation to the persevering panorama of late diagnoses [37].
The findings point to some convergences. First, when considering universal screening, the M-CHAT-R/F is the instrument with the largest number of studies. Its accuracy indicators were the highest. On the other hand, the authors themselves point out that the high sensitivity of the instrument, if not combined with the follow-up interview, leads to high rates of false positives which, in turn, can lead to an overload of health services, as well as unnecessary interventions. A parenthesis is in order here. Even with the adaptation of this instrument to Brazil, the recent inclusion of the M-CHAT-R in the Brazilian child health handbook without the follow-up interview constitutes a worrying scenario [38].
The correlation between age and accuracy is another relevant finding. As previously seen, in TASI, ESAC and ADOS-2-T, age closer to 36 months was more accurate. However, this data was the opposite in the M-CHAT-R/F, translating that the specificity of the age scope is an important factor to be considered for the elaboration of the instruments, indicating the need to create age- related cutoff points. Here, it is possible to infer that a broader age range impairs the result of the tool.
Another important conclusion refers to the type of instrument respondent and application format. This review found better screening results when supported by professionals, preferably in an observational and interactive way. Collected information alone, even in the face of a professional interview, does not correspond to the most effective screening. These findings, in part, may be reflections of the context that permeates autism, because, even in the face of growing awareness, ASD is still surrounded by misinformation. It is natural that parents, except those from risk groups, have little knowledge about the disorder. Thus, from level 1, the importance of the participation of a professional in the screening process becomes imperative, always observing and interacting with the assessed child [39].
At level 2, the instruments had more homogeneous performances, with high accuracy values, demonstrating that professionals have good options to support or discard the diagnostic suspicion. Among the instruments, the ADOS-2-T showed the highest accuracy (93.6%). On the other hand, it is the tool with the highest acquisition and training cost. Thus, the decision to choose the instrument involves other factors in addition to accuracy, such as, for example, the format of the service, time and available resources. Looking from this point of view, the RITA-T instrument demonstrates advantage in application in low-income countries, as it does not require the acquisition of material. For Brazil, it is important to emphasize that full adaptations of level 2 early screening tools were not found [40].
Among the instruments that propose to track both levels (CHAT- 23, SACS-R and SORF), the domain of SACS-R is wide. The study of the tool, in addition to presenting high levels of accuracy, was carried out in a very significant sample (n=13,511). Furthermore, its application does not depend on the acquisition of original materials, involving only training costs [41].
In addition to demonstrating reliability and validity for a given population, a screening instrument to become feasible in the context of public health must be inexpensive, easy to learn and apply. From this perspective, M-CHAT-R/F, RITA-T and SACS-R stand out, as they are instruments with no acquisition cost. The RITA-T and SACS-R training, according to the authors, are fast and accesible [42].
The central concept of the SACS-R (screening at 12, 18 and 24 months) is substantiated when differences in accuracy are observed according to the age group of the subject being evaluated. In addition, most children already receive regular care in the first two years of life. Taking advantage of these moments to screen for autism, in addition to being more economical, may reflect greater effectiveness when compared to a one-off assessment [43]. ASD surveillance, through observations and interactions with health and education professionals, tends to provide more accurate diagnoses. Accurate ASD tracking is not presented as a photograph, but rather a path of monitoring specific points over a more critical window (12 to 36 months) [44].
This article brought data which indicate that diagnosing autism only by parental report or interview, and based only on a moment, can result in a mistake. It should be noted that the medical and scientific community strongly rejects the idea of waiting for the diagnosis [45]. Quite the opposite. In no way does the discussion presented here have this bias, as early diagnosis is essential. With regular screenings in health services, it is expected that children at risk for ASD will obtain, when appropriate, rapid and reliable diagnoses, to the same extent that false positives and negatives are combated [46]. What is discussed are the organization and safety of this flow, which must be supported by quality instruments, as well as by professionals prepared to work in the process [47].
It is pertinent to highlight that this research was carried out in full context of changes in the ASD diagnostic criteria of the recent revision of the DSM-5. This study can contribute to the filtering of the most recent instruments that, even so, will need to be updated. It is suggested that revisions continue, as well as instrument validation studies in Brazil, since the early diagnosis process of ASD is relatively new and dynamic [48,49].
Based on this systematic review, which had the general objective of verifying the accuracy of the instruments for screening ASD in children up to 36 months of age and, taking into account all the variables discussed in the discussion and limitations of the research, it is understood that the instrument most suitable level 1 is the M-CHAT-R/F. This scale, in addition to being widely analyzed in studies with large samples, has high levels of accuracy. Regarding level 2, the instruments were balanced, highlighting the high accuracy of ADOS-2-T. Regarding the bivalent instruments (screening levels 1 and 2), the SACS-R is the one that showed the best indicators. In view of the tools located in this review, some instruments are suggested for the Brazilian context. The M-CHAT-R/F already has an adaptation study, while the toddler module of ADOS-2 still needs adaptation to the local context, as well as SACS-R. Together with the latter instrument, RITA-T emerges as an alternative to the high costs of ADOS-2 for level 2 screening.
This article had some limitations. Different methodologies may result in the wrong comparison of indicators. Some studies brought small samples, as well as absolute values, which does not correspond to the practical context. Discrepancies between indices of some instruments point to the possibility of methodological error in some studies, especially those without cultural adaptation. The incompleteness of the accuracy data was another limitation. Furthermore, the absence of important databases such as EMBASE, Institute for Scientific Information (ISI) and web of science constitutes a significant limitation. Finally, another relevant dimension not evaluated concerns the format of interaction proposed by the instrument. When it comes to early ASD screening, it is essential that the tools do not rely exclusively on verbal language, an aspect that was not considered in this article.
Author declars no conflicto of intrest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Silveira A, de Mattos Souza LD (2024). Early Screening for Autistic Spectrum Disorder: A Systematic Review. J Psychol Psychother. 14:490
Received: 29-Aug-2024, Manuscript No. JPPT-24-33735; Editor assigned: 02-Sep-2024, Pre QC No. JPPT-24-33735 (PQ); Reviewed: 16-Sep-2024, QC No. JPPT-24-33735; Revised: 23-Sep-2024, Manuscript No. JPPT-24-33735 (R); Published: 30-Sep-2024 , DOI: 10.35841/2161-0487.24.14.490
Copyright: © 2024 Silveira A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.