Journal of Hepatology and Gastrointestinal disorders
Open Access
ISSN: 2475-3181
ISSN: 2475-3181
Research Article - (2022)Volume 8, Issue 1
Objectives: Determination of the sustained virologic response in chronic HCV infected patients with HCV related nephropathy receiving direct acting antiviral drugs and assessment of the immunological, clinical and renal responses to DAAs in this group of patients.
Methods: This prospective observational study was carried out over a period of two years and included 34 patients diagnosed with HCV nephropathy for whom virological, immunological, clinical and renal responses were assessed pre and 12 weeks post treatment who were planned to be treated with Direct Acting Antivirals (DAAs) (in the form of combination of OBV-PTV/r with RBV, combination of SOF/SIM, or combination of SOF/DCV with or without RBV according to the availability).
Results: Thirty four HCV nephropathy patients were observed while receiving DAAs and for 12 weeks after. Renal biopsy was done in thirty patients while four refused. The mean age of patients was 53.1 ± 10.1 years and 55.9% of them were males. Membranoproliferative lesion was detected in 83% of biopsied cases. Sustained virological response 12 weeks after treatment detected in 97%. The median eGFR and proteinuria were improved after treatment, However, Partial and complete improvement of eGFR and proteinuria was detected in 57% and 44% of patients, respectively. Clinical response of other extra renal manifestations and immunological response either complete or partial were detected in 59% and 47% respectively.
Conclusion: The use of DAAs was highly effective and tolerable in Egyptian patients infected with HCV and has-associated Nephropathy, with high virological response rates in these patients. This was associated with amelioration of renal disease in half of the nephropathic patients. The occurrence of AKI with DAA regimens used in the study was independent of other AKI precipitating factors, transient and reversible after the end of treatment denoting renal safety of these drugs in patients with normal renal function.
HCV nephropathy; DAA; HCV; Kidney; Egyptians
Hepatitis C Virus (HCV) infection is the leading cause of chronic liver disease and its serious complications including cirrhosis, liver failure, end-stage liver disease, Hepatocellular Carcinoma (HCC) and liver related death [1]. HCV causes chronic hepatitis in 60%- 80% of the patients, from whom 20% can develop cirrhosis over 20-30 years of infection. About 5% of the cirrhotic patients may develop HCC and 6% may decompensate during the following years. The risk of mortality after decompensation is 15%-20% in the following year [2].
HCV may also induce ExtraHepatic Manifestations (EHMs); including renal, hematological, musculoskeletal and dermatological disease [3]. Sabry [4] reported that there was a strong and causal relationship between chronic HCV infection and glomerular disease. Several types of renal disease with HCV infection have been described including Mixed Cryoglobulinemia (MC), Membranoproliferative Glomerulonephritis (MPGN), Membranous Nephropathy (MN) and polyarteritis nodosa. Crescentic Glomerulonephritis (GN) may be superimposed on any of these glomerular lesions [5].
The most common renal pathological pattern in HCV patients with nephropathy is type-I MPGN, which is strongly linked to type II cryoglobulinemia. Renal manifestations range from slight proteinuria and hematuria to nephrotic and nephritic syndromes, as well as renal insufficiency in a lesser extent. Hypertension develops in most of cases [6]. Chronic HCV is independently associated with the development of CKD [7]. A meta-analysis published in 2015 demonstrated that chronic HCV infection was associated with a 51% increase in the risk of proteinuria and a 43% increase in the incidence of CKD [8]. There is also a higher risk of progression to End-Stage Renal Disease (ESRD) in persons with chronic HCV infection and CKD, and an increased risk of all- cause mortality in persons on dialysis [9].
The better understanding of the pathogenetic mechanisms of HCV associated glomerular disease, which is believed to a great extent, to be caused by the underlying infection which leads to immune complex formation and glomerular injury, has made antiviral therapy one of the important corners in the treatment of this condition. The goal of antiviral therapy in patients with HCV- associated nephropathy is to achieve viral clearance, ameliorate renal injury and minimize the need for immunosuppressive drugs [10]. The use of Direct Acting Antivirals (DAAs) has greatly improved the virological clearance in HCV patients. However, there is not much data about the virological, renal, immunological and clinical responses to these drugs in patients with HCV-associated nephropathy.
Study population
This prospective observational study was carried out over a period of two years from March 2016 to February 2018. Included 34 patients diagnosed with HCV nephropathy (Presented by abnormalities in kidney function (raised serum creatinine and decline of eGFR) and/ or proteinuria (more than 300 mg/day measured by 24-hours urine collection) and/or active urinary sediments (RBCs, RBCs casts) and diagnosis was confirmed by renal biopsy (It was done after patients consented and if and not contraindicated) in addition to abnormal serological markers (elevated RF and consumed C3 and C4). Enrolled cases were selected from clinics and wards of Nephrology and Hepatology units of Internal Medicine department, Mansoura University, Egypt.
Patients with ESRD, history of other renal disease (other glomerular, tubulo-interstitial, or reno-vascular disorders), severe co-morbidity as severe heart failure or malignancy, other liver disease (e.g. autoimmune hepatitis, HBV, Wilson,…) or decompensated liver disease (ascites, hepatic encephalopathy,…) were excluded from the study.
Clinical and biochemical evaluation
Baseline demographic, virological, laboratory, and clinical data were collected. Patients were subjected to clinical assessment which included number and type of organ involvement and symptoms at baseline and 12 weeks post treatment. Immunologic markers including rheumatoid factor (RF), C4 complement fraction (C4), C3 complement fraction (C3) were done before and 12 weeks post treatment. Urine analysis and Twenty-four-hour urine protein were assessed before treatment and 12 weeks post treatment, eGFR was estimated using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation before treatment and 12 weeks post treatment. Ultrasound-guided renal biopsy was done in 30 patients using a gauge 16 or 18 coaxial quick-core biopsy set (if accepted by the patient and not contraindicated). RT-PCR-HCV was done at baseline and 12 (SVR 12) weeks after the end of therapy using quantitative RT-PCR (using step one RT-PCR).
Study outcomes
Outcomes of the study in HCV nephropathy group included:
Primary outcome which is SVR 12: Was defined as achievement of sustained virological response by obtained negative RT-PCR HCV RNA at week 12 after end of treatment.
Secondary outcomes included: (1) Clinical response, (2) Immunologic response and (3) Renal response (including GFR, proteinuria and total renal responses). Clinical response was defined as complete when there was improvement of all affected organs, a partial clinical response was defined as improvement in at least half of the involved organs from baseline. All other patients were clinical non-responders. The immunologic response was complete when C3, C4 and RF were normalized, and partial when improvement of any two immunologic parameters was detected. Other patients were non responders.
Renal response included GFR response which was considered as complete if eGFR became ≥ 60 if the baseline value was less than 60 mL/min/1.73 m2, while it was considered as partial if eGFR was stable or improved (eGFR increased by ≥ 20% of baseline). Worsening of eGFR was recorded as non-response. Proteinuria response was defined as complete if the level of proteinuria decreased to 0.5 g/day or less. Partial response means decrease of the proteinuria >50% of the baseline, while no response was considered when proteinuria decreased <50% of baseline or worsened.
Total renal response was defined as complete in case of combination of normalization of renal function (eGFR ≥ 60) when abnormal before treatment (eGFR<60) and proteinuria of 0.5 g/day or less. A partial response was defined as a stable or improved renal function (eGFR increased by ≥ 20% of baseline) and/or a reduction of at least 50% of proteinuria. No response was defined as worsening of renal function (eGFR reduced by ≥ 20% of baseline) not attributable to other causes and/or proteinuria increase or a reduction insufficient for the definition of complete or partial response.
Statistical analysis
The statistical analysis was performed using SPSS program (Statistical Package of Social Sciences) version 22 for Windows (SPSS, Inc., Chicago, IL, USA). Data were presented using mean and standard deviation (SD) or median and interquartile (IQ) range for quantitative values, while in number (percentage) for qualitative values. The significance of statistical differences between continuous variables was assessed using either independent samples (T) test for normally distributed variables or Mann-Whitney test (U) for the data that were not normally. distributed. Chi-square test or Fisher exact.
Tests were used for comparing qualitative variables, when appropriate. The Cochran-Armitage test of trend was used to determine whether there is a linear trend in binomial proportions across the levels of an independent ordinal variable. The Wilcoxon signed-rank test was used for the comparison between 2 paired samples. Significance was considered for P values of <0.05 for all statistical analyses in the present study.
Base line demographic data
The Patients mean age was 53.1 ± 10.1 years and 55.9% of them were males. The main clinical manifestations were hypertension (64.7%) which was the most dominant manifestation, edema (52.9%), fatigue (50%), vasculitic rash (20.6%), arthralgia (11.8%) and neuropathy (2.9%). Proteinuria was present in all cases, eGFR was <60 ml/min in 65% of cases. All cases had elevated RF, while 25 cases (73.5%) had consumed C4 and 24 cases (70.6%) had consumed C3 (Table 1).
| Parameter | Statistic (Mean ± SD or no (%) |
|---|---|
| Age | 53.1 ± 10.1 |
| Gender | |
| Male | 19 (55.9%) |
| Female | 15 (44.1%) |
| BMI | 30.7 ± 4.9 |
| CKD Stage | |
| Stage 1 | 3 (8.8%) |
| Stage 2 | 9 (26.5%) |
| Stage 3a | 4 (11.8%) |
| Stage 3b | 12 (35.3%) |
| Stage 4 | 5 (14.7%) |
| Stage 5 | 1 (2.9%) |
| Clinical findings | |
| Edema | 18 (52.9%) |
| Hypertension | 22 (64.7%) |
| Controlled on 1 drug | 13 (38.2%) |
| Controlled on 2 drugs | 8 (23.5%) |
| Controlled on 3 or more | 1 (2.9%) |
| Vasculitic rash | 7 (20.6%) |
| Weakness | 17 (50%) |
| Arthralgia | 4 (11.8%) |
| Neuropathy | 1 (2.9%) |
| Laboratory findings | Statistic (Median (IQR) or mean ± SD or no (%)) |
| S. bilirubin (mg/dL) | 0.8 (0.7-1.2) |
| INR | 1.08 (0.9-1.1) |
| PCR RNA HCV ( × 105 IU/mL) | 4.9 (0.9- 9.4) |
| AST (IU/L) | 59.2 (34.8-96.5) |
| ALT (IU/L) | 44 (30-85.3) |
| Albumin (g/dL) | 3.5 (3.3-4.1) |
| WBCs (/cmm3) | 5.5 (4.5-7.5) |
| HB (g/dL) | 12.5 ± 1.8 |
| Platelet (PLT) (/cmm3) | 150 (114.8-182.5) |
| Creatinine (mg/dL) | 1.55 (1.08-1.8) |
| Proteinuria (mg/day) | 1742.5 (1337.5-2462.5) |
| RBCs in urine (RBC/HPF) | 21.5 (10-35.8) |
| RBCs casts n (%) | 5 (14.7%) |
| eGFR (mL/min/1.73 m2) | 42.3 (31.9-72.8) |
| RF (IU/ml) | 64 (32-136) |
| C3 (mg/dL) | 80 (70-90.8) |
| C4 (mg/dL) | 8 (6.9-9.6) |
| Antiviral Treatment regimens | No (%) |
| SOF/DCV | 3 (8.8%) |
| SOF/DCV/RBV | 13 (38.2%) |
| OBV-PTV/r +RBV | 14 (41.2%) |
| SOF/SIM | 4 (11.8%) |
| Treatment duration (from total 12 weeks) | |
| 12 weeks | 31 (91.2%) |
| 10 weeks | 1 (2.9%) |
| 7 weeks | 1 (2.9%) |
| 6 weeks | 1 (2.9%) |
Table 1: Baseline characteristics of patients before treatment.
Regarding renal histopathology, MPGN was the most common pathological pattern (25 cases, 83.3%) Other pathology included mesangio-proliferative GN (6.7%), Tubulointerstitial Nephritis (TIN) (6.7%) and FSGS (3.3%) (Table 2) (Figure 1).
| Pathological pattern | Number (%) |
|---|---|
| MPGN | 25 (83.3%) |
| MGN | 2 (6.7%) |
| TIN | 2 (6.7%) |
| FSGS | 1 (3.3%) |
| Intra-capillary thrombi | 17 (56.7%) |
| Inflammatory leukocytes | 18 (60%) |
| Fibrinoid necrosis | 4 (13.3%) |
| Crescents | 12 (40%) |
| Acute tubular necrosis (ATN) | 11 (36.7%) |
| Interstitial inflammation | |
| No | 9 (30%) |
| Mild | 10 (33.3%) |
| Moderate | 7 (23.3%) |
| Severe | 4 (13.3%) |
| Blood vessel intimal thickening | |
| No thickening | 16 (53.3%) |
| Mild | 13 (43.3%) |
| Moderate | 1 (3.3%) |
| Tubular atrophy percent | 30 (20-40) |
| Interstitial fibrosis percent | 30 (20-40) |
| Immunofluorescence | |
| IgA | 1 (3.3%) |
| IgM | 21 (70%) |
| IgG | 13 (43.3%) |
| C3 | 26 (86.7%) |
Table 2: Renal biopsy characteristics of the HCV nephropathy group.
Figure 1: Survival curve (in months) depending on whether or not complete recanalization of portal thrombosis is obtained.
Patients received DAAs in the form of SOF/DCV (8.8%), SOF/ DCV/RBV (38.2%), OBV-PTV/r+RBV (41.2%), SOF/SIM (11.8%). A total 31 (91.2%) patients completed the treatment duration, while 3(8.8%) patients discontinued their treatment (one of them received SOF/DCV/RBV and stopped after 10 weeks due to rising serum creatinine, two received OBV-PTV/r+RBV, one of them stopped after 6 weeks due to severe anemia, while the other stopped after 7 weeks due to severe persistent vomiting). The baseline characteristics of this group of patients are shown in Table 1.
Clinical and laboratory date after therapy
On comparing clinical data, and laboratory findings before and after treatment (Table 3), the RF, C3, C4 and ALT improved significantly (p=0.001, 0.002, <0.0005 and <0.0005, respectively). Variables of renal involvement; namely proteinuria (from 1742.5 (1337.5-2462.5) to 1100(187.5-2182)), RBCs in urine (From 21.5 (10-35.8) to 5.5(3.5-20)) and serum creatinine (from 1.55(1.1-1.8) to 1.2(1.1-2.2)) were decreased after treatment, while eGFR was increased (From 42.3(31.9-72.8) to 52(29.7-68.7)).
| Parameter | Pre-treatment | Post-treatment | p value |
|---|---|---|---|
| Number (%) or median (IQR) | |||
| Edema | 18 (52.9%) | 13 (38.2%) | *0.125 |
| Vasculitic rash | 7 (20.6%) | 2 (5.9%) | *0.063 |
| Arthralgia | 4 (11.8%) | 0 (0%) | *0.125 |
| Neuropathy | 1 (2.9%) | 1 (2.9%) | *1.000 |
| Weakness | 17 (50%) | 9 (26.5%) | *0.008 |
| Renal involvement | |||
| Proteinuria (mg/day) | 1742.5 (1337.5-2462.5) | 1100 (187.5-2182) | **0.075 |
| RBCs in urine | 21.5 (10-35.8) | 5.5 (3.5-20) | **0.001 |
| eGFR (ml/min/1.73m2) | 42.3 (31.9-72.8) | 52 (29.7-68.7) | **0.973 |
| S. creatinine (mg/dl) | 1.55 (1.1-1.8) | 1.2 (1.1-2.2) | **0.402 |
| RF (IU/ml) | 64 (32-136) | 16 (11-89.1) | **0.001 |
| C3 (mg/dL) | 80 (70-90.8) | 110 (78.8-125) | **0.002 |
| C4 (mg/dL) | 8 (6.9-9.6) | 15 (8.9-20.3) | **<0.0005 |
| ALT (IU/L) | 40 (30-85) | 21 (19.8-29.3) | **<0.0005 |
Note: P value by (*) McNemar test, (**) Wilcoxon Signed Ranks Test
Table 3: Clinical and laboratory finding before and after DAAs.
Vasculitic rash resolved in the majority of the patients and arthralgia resolved in all patients. Edema improved in 6 out of 18 patients. On the other hand, one patient was noticed to develop edema with progressive proteinuria and decline of eGFR after treatment. The patient with symptoms of peripheral neuropathy did not show clinical improvement of these symptoms.
Assessment of virological response
At the end of the study, thirty-three patients (97.1%) achieved sustained virological response. The only case that did not achieve SVR-12 (2.9%), did not show neither clinical nor renal response. Hypertension worsened in 6 patients (27.3%). About 52.9% of the patients did not achieve immunological response. GFR was normalized (eGFR improved to >60) in 29.4% of cases and improved but did not reach >60 in 17.6% of patients, on the other hand the eGFR worsened in 35.4%. The proteinuria improved in 44% of patients; however, it was worsened in 56%. As regards total renal response, 53% achieved both complete and partial response, while 47% were non-responders. On assessment of clinical response, there was no response in 41% (Table 4) (Figure 2).
| Parameter | Number (%) |
|---|---|
| SVR 12 | 33 (97.1%) |
| Hypertension | |
| Improved | 1 (4.5%) |
| Stable | 15 (68.2%) |
| Worsened | 6 (27.3%) |
| Immunologic response: | |
| Complete | 9 (26.5%) |
| Partial | 7 (20.6%) |
| No response | 18 (52.9%) |
| GFR response | |
| Stable Normal eGFR>60 | 6 (17.6%) |
| Complete (normalization of abnormal eGFR) | 10 (29.4%) |
| Partial (stable or improved eGFR but not normal) | 6 (17.6%) |
| No response (worsening of eGFR) | 12 (35.4%) |
| Proteinuria response | |
| Complete (normalization) | 14 (41.1%) |
| Partial (>50% decrease but not normal) | 1 (2.9%) |
| No response (<50% decrease or worsening) | 19 (56%) |
| Total renal response | |
| Complete | 9 (26.5%) |
| Partial | 9 (26.5%) |
| No response | 16 (47%) |
| Clinical response | |
| Complete | 9 (26.5%) |
| Partial | 11 (32.4%) |
| No response | 14 (41.1%) |
Table 4: Different responses after therapy with DAAs.
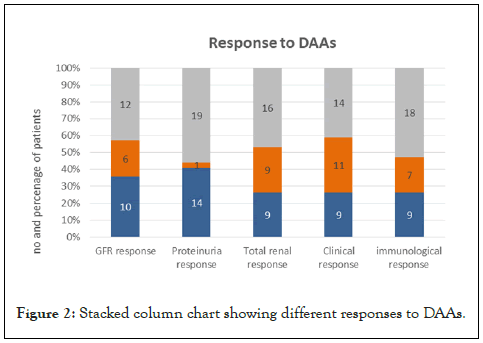
Figure 2: Stacked column chart showing different responses to DAAs.
There was a significant relation between the immunological response and the total renal response. About 68.8% of the patients who achieved immunological response achieved either complete (50%) or partial (18.8%) total renal response. On the other hand, the immunological non-response was associated with renal non- response in 61.1% of patients (Figure 3).
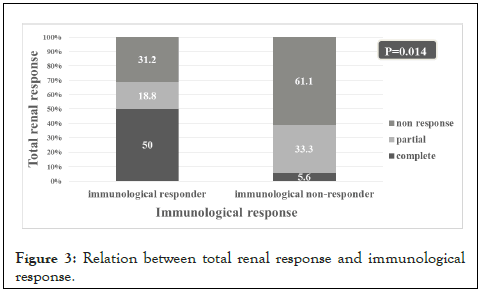
Figure 3: Relation between total renal response and immunological response.
Adverse events
The adverse events during treatment with DAAs were recorded (Table 5). The frequency of occurrence of anemia was recorded 3 times during the administration of DAAs. The degree of drop of hemoglobin was recorded as a comparison between the baseline hemoglobin and both the lowest level during the follow up as well as at the end of the study. Anemia was the most obvious adverse event as it occurred in 32 cases (94.1%) at any time during the study. The hemoglobin level decreased to <8.5 g/dl in 5 cases (14.7%). Erythropoietin therapy was needed in 8 cases (23.5%), while 4 cases (11.8%) required blood transfusion. Surprisingly, anemia occurred with all DAAs regimens used in the study. However, it was more evident in the patients who received RBV containing regimens. RBV was stopped in 6 out of 27 patients (22.2%) due to persistent anemia (2 cases were CKD stage 2, 2 cases CKD stage 3b and 2 cases CKD stage 4 (data are not shown)). RBV was stopped in first month in 4 cases and in second month in 2 cases. Fatigue developed in 4 cases during treatment (11.8%). Four cases (11.8%) had vomiting, one of them was persistent and consequently, she had to stop treatment after only 7 weeks. Flu like symptoms was noticed in 3 patients (8.8%).
| Adverse event | Number (%) |
|---|---|
| Deterioration of HB anytime during study | 32 (94.1%) |
| Lowest HB level anytime during study | |
| HB>10 g/dL | 19 (55.9%) |
| 8.5-10 g/dL | 8 (23.5%) |
| <8.5 g/dL | 5 (14.7%) |
| Change of HB at end of study from baseline: | |
| HB not changed or increased | 3 (8.8%) |
| HB decreased<10% | 9 (26.5%) |
| HB decreased ≥ 10% | 22 (64.7%) |
| Anemia within each treatment regimen | |
| SOF/DCV (HB>10 g/dl) | *3 (100%) |
| SOF/DCV/RBV | *12 (92.3%) |
| HB>10 g/dl | 7 (53.8%) |
| 8.5-10 g/dl | 5 (38.5%) |
| OBV-PTV/r+RBV | *14 (92.9%) |
| HB>10 g/dl | 6 (42.9%) |
| 8.5-10 g/dl | 3 (21.4%) |
| <8.5 g/dl | 5 (35.7%) |
| SOF/SIM (HB>10 g/dl) | *3 (75%) |
| Anemia requiring erythropoietin therapy | 8 (23.5%) |
| Anemia requiring blood transfusion | 4 (11.8%) |
| RBV stoppage | 6 (22.2%) |
| Flu like symptoms | 3 (8.8%) |
| Fatigue | 4 (11.8%) |
| Vomiting | 4 (11.8%) |
| Occasional | 3 (8.8%) |
| Persistent | 1 (2.9%) |
Note: (*) % in each treatment regimen group.
Table 5: Adverse events with DAAs during treatment.
The introduction of DAAs in treatment of HCV-infected patients has dramatically improved the virological response in general population. The goals of DAAs for treatment of HCV nephropathy are achieving SVR and remission of symptoms and minimizing the need for immunosuppression [11]. However, few data are available regarding the efficacy of DAAs in achieving and maintaining virological, immunological and renal response in chronic HCV patients with HCV related nephropathy. Although, some studies have evaluated the efficacy of DAAs in HCV patients, they included few numbers of patients with renal involvement and renal biopsy was done in a fewer number of patients in these studies. So, the renal response was not studied in relation to biopsy finding.
In the present study, the aim was to determine the SVR in chronic HCV infected patients with HCV related nephropathy receiving DAAs and to assess the immunological, clinical and renal responses to DAAs in this group of patients.
The current study results showed that the majority (97.1%) of the 34 nephropathic patients achieved SVR 12 weeks after the end of treatment. Similar results were reported by Fabrizi [10], who administered similar DAA regimens to chronic HCV patients with glomerular diseases in their study. On the other hand, A lower SVR 12 response rate (83%) was reported by Sise [12] in patients with renal involvement which may be due to very small number of patients (12 patients) and earlier protocols (SOF-RBV) used by Sise which were much weaker than those used in our study.
Other studies showed SVR 12 comparable to that of the present study; although these studies included few patients with renal involvement [13-15] where SVR results reported in these studies were 100%, 89% and 100% respectively. Interestingly, in the current study SVR 12 was achieved in three patients who did not continue the whole duration of treatment (12 weeks).
Despite the high virological response at 12 weeks post treatment, not all the patients with virological response achieved clinical, renal or immunological response. Only half of the nephropathic patients achieved total renal response which was “complete” in only 26.5% of the whole group. This limited renal response accords with the results of the studies conducted by Emery [14] and Sollima [16] where the study by Emery et al. included Eighteen symptomatic and 65 asymptomatic patients. Sixty-six (79.5%) patients received pegIFN-free therapy (SOF/SIM, SOF/LDV, SOF/RBV and OBV- PTV/r+RBV). SVR was attained in 16(88.9%) symptomatic and 59(90.8%) asymptomatic patients. Cryoglobulins disappeared in 5(29.4%) symptomatic and 27(52.9%) asymptomatic patients. Of symptomatic patients with SVR, clinical response was complete in 7(38.8%) and partial response in 4(22.2%). Also, similar results were reported by the study by Sollima et al. which included 17 patients with HCV related MCV who received a variety of IFN- free DAA regimens (including SOF/RBV, SOF/LDV, SOF/DCV with or without RBV, OBV-PTV/r plus dasabuvir, and SOF/SIM) for 12 or 24 weeks, at post-treatment week 12, 100% of patients achieved an SVR, but only 11(64.7%) obtained a clinical response, that was complete in five (29.4%) and partial in six (35.3%) only of included patients.
However, this finding of limited renal response disagrees with the results of other studies carried out by Bonacci [11] and Gragnani [13] who showed that the majority of their patients achieved complete response. One limitation of the latter studies is the low number of their patients with renal involvement.
Regarding the GFR and proteinuria responses to DAAs, in a study by Sise [12], there was a detailed description of the changes of the renal manifestation in patients with active glomerular disease who achieved SVR where there was improvement in median eGFR and decline in median proteinuria. These findings are in agreement with our results, which revealed that there was improvement in the median eGFR and hematuria with decline in the median proteinuria. When the results were analyzed on case by case basis, the GFR was found to be improved in 40% of patients and proteinuria was noticed to be improved in 45% of patients. A study by Fabrizi [10] showed that six out of 13 patients suffered persistence of renal manifestations after SVR with DAAs; a finding that is in harmony with the present work. The latter authors discussed that their results were much pessimistic than many preceding publications.
The absence of renal response to DAAs in some patients was suggested to be possibly attributed to persistence of cryoglobulinproducing B-cell clones despite HCV clearance, which might have needed further immunosuppressive therapy to be eradicated [10]. Another explanation could be an advanced extent of organ damage which might have become established, prior to the introduction of the DAAs, so that reversibility would become unexpected [17]. Having said that, in the present study, absence of total renal response to DAAs was not different between patients with active or chronic renal biopsy lesions.
Achievement of the clinical response followed the virological response in 60% of cases. There was a high response as regard improvement of vasculitic rash, arthralgia and weakness, but it was not that optimistic as regard improvement of renal parameters. These findings suggest that the high virological improvement was not translated to improved clinical outcome in all patients. These results cope with the results of Emery [14] and Sollima [16] who reported a modest clinical response compared to their rapid and high rate of virological response to DAAs. On the contrary, a recent Egyptian study conducted by Hassan [15] reported that the majority of their patients achieved complete clinical response which exceeds the results of the present study, this difference may be due to the small number of patients with glomerular disease (7 patients only of whom 5 showed clinical improvement) included in the study by Hassan.
Immunological response was achieved in nearly half of the patients in the current study on follow up 12 weeks post treatment. Similar results have been reported by Bonacci [11] and Gragnani [13]. On the contrary, Sollima [16] reported immunological response more frequently in their studied cases (81.8% which increased to 100% during the follow-up); most of whom achieved clinical response. The persistence of the immunological activation in nearly half of the patients in the current study may be due to the continuation of the immune system in processing pathogenic immune complexes which are virus-independent leading to the progression of the process causing MC despite the viral clearance.
In patients with cryoglobulinemia, RF levels are elevated and correlate with the levels of cryoglobulins. Serum RF was routinely analyzed in nephropathic patients in this study in place of the cryoglobulins as the latter is difficult to be analyzed and needs special precautions. Elevated RF with consumed C3 and C4 were observed in the majority of patients in the current work, and they showed significant improvement after treatment with DAAs. These results agree with the results of other studies carried out recently [11,13,15,18].
The correlation between the immunological, GFR and proteinuria responses were not thoroughly studied to the best of our knowledge. The results of our study showed that the immunological response significantly correlated with the total renal response, proteinuria and clinical response. This correlation between immunological and clinical response was also shown to be significant by Bonacci [11] and Emery [14], while Gragnani [13] failed to confirm such a relation. Of note, the case with virological non-response in this study, did not achieve clinical or renal response. However, Bonacci [11] reported that two patients in their study did not clear the virus, one of them had improvement of symptoms and the other had complete clinical response.
Hypertension was the most dominant clinical manifestation in the patients in this study followed by weakness. These observations are similar to what was observed by Fabrizi [10] in their study. Blood pressure control was worsened in 27% of hypertensive patients, all of whom showed worsening of eGFR and total renal non-response. In previous literature there has been no clear discussion about the occurrence or fate of hypertension following DAAs.
The most common pathological patterns in this study were MPGN followed to a lesser extent by MGN, TIN and FSGS; findings that are similar to other studies [10,19-21]. Immunofluorescence showed dominance of C3, IgM and IgG, these findings cope with findings of Rossi [22] and Mesquita [21].
The relation between improvement in renal response-proteinuria and GFR and the findings of the renal pathology was studied in our work; The GFR response to DAAs was worsened in nearly one third of patients with active biopsy lesions and half of patients with mixed or chronic biopsy lesions. Proteinuria level improved in more than one third of patients with active biopsy lesions while improved in half of patients with mixed or chronic biopsy lesions. A literature search did not reveal similar studies that looked into the relation between the renal biopsy lesions and response to DAAs.
The most frequent adverse event in our cohort was anemia which occurred with all DAA regimens, but it was more severe with RBV containing regimens. Erythropoietin therapy was needed in eight patients while four needed blood transfusion. Gragnani [13], Bonacci [11] and Fabrizi [10] reported similarly that anemia was the most encountered adverse event in their studies. Bonacci [11] reported that four cases needed erythropoietin and no cases needed neither blood transfusion nor stoppage of treatment. Fabrizi [10] reported that anemia occurred only with RBV and that three patients needed erythropoietin therapy while four patients needed blood transfusion; however, Boglione [18] stated that anemia also occurred in patients not taking RBV containing regimens.
In this study three patients did not continue treatment duration, although they achieved viral clearance. Sise [12] reported that one patient out of twelve stopped treatment. On the contrary, other studies reported that none of their patients stopped treatment [10,11,13].
The current study had some limitations which include not performing renal biopsy post treatment in nephropathic patients to assess the treatment response; however, this was difficult to be done due to patient refusal in almost all cases. Also, the patients were followed only for 12 weeks post treatment, longer follow up may show delayed response to therapy in nephropathic patients.
Overall, the current study showed that the DAAs were effective in patients with HCV associated nephropathy and achieved excellent SVR rates. DAAs were also safe where the adverse events recorded were mostly tolerable. The renal response to DAAs was reasonable as nearly half of the patients achieved renal response which could be conceived to improve on addition of immunosuppressive drugs for complete eradication of cryoglobulin producing B cells; a postulation that needs further study.
The use of DAAs was highly effective and tolerable, with high virological response rates in patients with HCV nephropathy, which was associated with amelioration of renal disease in half of the nephropathic patients.
Citation: Sabry A, Elsaeed A, Eletreby S, El-Husseini F, Ahmed NS (2022) Effect of Direct Acting Antiviral Drugs in Hepatitis C Virus infected Egyptian P atients with Nephropathy: A single Center Study. J Hepatol Gastroint Dis. 8: 197.
Received: 03-Jan-2022, Manuscript No. JHGD-22-15518; Editor assigned: 06-Jan-2022, Pre QC No. JHGD-22-15518 (PQ); Reviewed: 20-Jan-2022, QC No. JHGD-22-15518; Revised: 24-Jan-2022, Manuscript No. JHGD-22-15518 (R);; Published: 31-Jan-2022 , DOI: 10.35248/2475-3181.1000197
Copyright: © 2022 Sabry A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.