Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2016) Volume 4, Issue 3
The objective of this work is to demonstrate the feasibility of the application of an edible coating based on inulin (IN), gelatine (GE), whey (WH) and glycerol (GLY) with added with Lactobacillus casei Shirota (LBC) and its effect on the characteristics of texture, moisture and colour of cracker cookies. A randomised design with two factors (applied volume and period of storage) with seven different formulations and a control derived from a previous experimental study were applied. The components of the formulations were IN, GE, WH, GLY and LBC. The blend of these ingredients was applied by spraying in 1 and 2 mL proportions on cracker cookies stored at 25°C for 20 days. The three-point break method was used. The percentage of moisture and colour change was measured. To verify the survival of LBC, the standard plate count method and scanning microscopy (SEM) were used. In addition, affective sensory tests were performed. Determinations were performed in triplicate at 3, 10, 15 and 20 days of storage. The application of an edible coating with LBC to cracker cookies significantly increased (p<0.05) moisture 1.5% compared with the control, whereas the maximum strength to breaking decreased significantly (p<0.05) by 1-2 N on average. There was no significant colour change, and the observed survival of LBC by SEM and growth in CFU/g in t15, OFZ and OMY formulations during 20 days of storage. In acceptance testing, the cookie was moderately liked by 49% of tasters, whereas only 4% of tasters referred to the cookie as disgusting him. These findings suggest that the use of IN 4%, GE 3.5% and LBC 2% coatings will maintain the cracker cookie’s quality and lead to better acceptance by consumers, providing an alternative for the consumption of LBC.
Keywords: Edible coating; Lactobacillus casei Shirota; Cracker cookies; Texture
Food quality is decisive in human health. Currently, there is a greater consumer preference for purchasing minimally processed products with added value and sustainable production [1,2], and functional foods stand out significantly, as these foods provide additional benefits to the consumer beyond their nutritional properties. Such is the case with foods with added probiotics. Multiple studies have demonstrated the relationship between the consumption of probiotic bacteria such as Lactobacillus casei and the prevention or treatment of various illnesses, mainly of a gastrointestinal nature [3-8]. The food industry has found a growing market in dairy products with added probiotics [9] However, the consumption of this type of bacteria is still limited among the population by various factors such as food preferences or ignorance of health benefits and the avoidance of the consumption of milk with added lactic acid bacteria [10]. However, the world market for functional foods is growing. The United States is the largest market segment, reaching US $18.25 billion, followed by the European Community with $15.4 billion and Japan with $11.8 billion [11,12]. Certainly, yogurt is a food widely marketed and has been the main representative of the content of probiotic bacteria, however, due to the great importance that it has for the human consumption, it is necessary also there is a greater alternative of foods that could include them, in order to meet the consumption needs of those who may have symptoms of lactose intolerance even with yogurt consumption, this intolerance has a strong ethnic imprint and geographical because people unaccustomed milk consumption are the most prevalent present: Swedish 1%, English 6%, Spanish 15%, Arabs 80%, Eskimos 83%, Mexican 83%, African 83% and Thai 98% [13]. On the other hand, research and food technology have allowed ways of finding different methods to add probiotic bacteria to other foods, such as juices, ice cream and baby food [1]. Such is the case in the development of coatings and edible coatings (FC - EC), which have demonstrated potential application not only as packaging and treatment to improve food quality but also as vehicles for antioxidants, support, additives, nutrients and antimicrobial agents such as vitamins and inorganic nutrients [13,14]. Several scientific studies relating the use of EC and FC in a variety of foods such as fruits, vegetables, cheeses and meats, among others, to prolong their storage time and life demonstrate that their use represents a promising alternative in the field of packaging and food preservation [15-18]. Some authors have developed EC with probiotic based on alginate and protein, with the addition of viable bifidobacterium applied in fresh cut fruits, given their structural properties, EC and FC are potentially excellent vehicles for addition of probiotic bacteria [19] to other foods, which will facilitate the consumption of this type of bacteria. García-Argueta et al. [20] reported the survival of Lactobacillus casei in ECbased serum of milk, unflavoured gelatine, inulin and glycerol for a period of 20 days of storage.
The habanera cracker cookie is a baking product formed from a mass with very little or no sugar, moderate fat and little water. Rising agents such as water vapour, baker’s yeast and leavening agents [21] are used for its production. Another type of cookie made in the same manner is the cocktail cracker. The addition of dietary fibre results in its preferential inclusion in weight control treatment meal plans. Among the cracker’s main features are its low humidity (5%), low calories (20 calories/cookie 3 g), accessibility and slightly neutral flavour (not sweet or not salty). In addition, Mexico is among the major consumer countries of cracker cookies in the world with a per capita consumption of approximately 4 kg per year. In the country, more than 50% of the total production is for domestic consumption. In addition, there is continuing interest by the food industry in being innovative in the creation of functional cracker cookies to suit the nutritional needs of the population and creating possible alternatives to encourage the consumption of probiotics among the population through different types of dairy foods. The added benefit that might be, is that it is a different food that conventionally provide probiotics, the cookie is a food commonly consumed in the population with various forms of combination with foods both sweet and savory or neutral and its added value is that it could promote an increased consumption of probiotics among the population, plus it is a food that does not require a cold chain to keep. Therefore, it is necessary to investigate whether the application of this type of edible coating formulation is viable in different dairy foods such the habanera cracker cookie. Thus, the objective of this work was to evaluate the feasibility and the effect of the application of an edible coating based on inulin, unflavoured gelatine, glycerol and whey with added Lactobacillus casei Shirota on the physicochemical and texture properties and acceptance of habanera cracker cookies, as an alternative consumption of probiotics to improve consumer health.
Sample preparation
The process for the production of the edible coating (EC) was performed under sterile conditions in a laminar flow hood (Class II Biosafety Cabinet Purifier, Labconco, Kansas City, Missouri, USA). Sterile distilled water was used with 5 components in the proportions listed in Table 1. Whey milk (extra dry grade whey made from pasteurised sweet whey, Darigold Inc., Seattle, WA, USA), glycerol (J.T. Baker Analyzed™, ACS Mexico), gelatine (Duche 12/1), inulin (Frutafit IQ, VA Mexico SA CV), and Lactobacillus casei Shirota were used. The Lactobacillus was used as a probiotic and obtained by centrifuging a fermented drink called Yakult® (1-2%) at 3500 rpm (5810 R Eppendorf AG 22331 centrifuge, Hamburg, Germany) and 4°C for 30 minutes.
| Formulas | % Whey* | % Glycerol* | % Inulin | % Grenetin | % Lactobacillus casei |
|---|---|---|---|---|---|
| t1 | 8 | 6 | 4 | 5 | 1 |
| t4 | 8 | 6 | 4 | 2 | 1 |
| t6 | 8 | 6 | 2 | 5 | 2 |
| t10 | 8 | 6 | 2 | 3.5 | 1 |
| t15 | 8 | 6 | 4 | 3.5 | 2 |
| OFZγ | 8 | 6 | 3.96 | 2 | 1.3 |
| OMYγ | 8 | 6 | 4 | 1.98 | 1.98 |
γ Eminently good formulations derived from preliminary research (García-Argueta et al.)
*Constant concentration for all treatments.
Table 1: Concentration of components in edible coating.
The edible coating was created according to Ref. [20]. Distilled water was heated to 70°C, and the following components were added one-by-one in order under constant agitation for 30 minutes until completely dissolved: gelatine, inulin, whey and glycerol. The mixture was allowed to stand until it cooled to 25°C, and then, the LAB was added to the mixture. Constant stirring was maintained until the bacteria were completely incorporated into the solution. This mixture was designated the coating-forming solution (FFS). The FFS was placed on cracker cookies (Gamesa) that were 6 cm in diameter. A total of 1 and 2 mL of FFS was applied, and the cookies were FFS-added cookies were kept in a controlled atmosphere in an incubator (Model 131 Felisa, Mexico) at 25°C for 20 days.
Texture tests of cookies with edible coating
A model TA-XT2 texture analyser (Stable Micro Systems Texture Technologies, Corp.) was used. Samples of cookies with edible coating were tested at 3, 10, 15 and 20 days of storage. We used the “threepoints break method” and obtained the maximum force to rupture (N).
Humidity
A gram of each of the sample was weighed and subjected to warming to 130°C in a vacuum oven until constant weight [22,23]. The weight of the samples was assessed at 4 different time points (3, 10, 15, and 20 days of storage). The change in weight was considered to the thousandth of a gram. Desiccator glass with CaCl2 as a desiccant was employed. Determinations were performed in triplicate, and the average of the results obtained was expressed as a percentage.
Colourimetry
A Konica Minolta colourimeter model CR-400 Chroma Meter (Sesing, Inc., Japan) was used. To calibrate the colourimeter, calibration plate number 126633047 was used. Cookies with EC samples were used after 3, 10, 15 and 20 days of storage. The previously calibrated colourimeter was placed on the cookies, and a shot was fired to record values according to the Hunter L scale, a* b*, in which L 100%=white, L 0%=black; +a> 0=red, −a <0=green, +b> 0=yellow, and −b <0=blue. The tonality and chromaticity values were calculated using the following formulas:
Tonality=Tan-1(b/a)
Chromaticity=(a2+b2)1/2
The net change of colour was calculated with respect to the initial time or zero using the following formula:

where L1, b1, and a1 were the values for samples after different storage times, and L0, a0 and b0 were the respective values for the control sample [24].
Survival of lactic acid bacteria (LBC)
The technique for producing a casting plate [25], was used on 1 g of cookies with EC samples after 3, 10, 15 and 20 days of storage. The samples were placed in Man, Rogosa and Sharpe (MRS) broth and incubated for 48 hours at 35°C. Then, the mixtures were diluted. Aliquots (1 mL) of the last three dilutions were placed in sterile petri dishes. MRS agar was added and allowed to incubate for 48 hours at 30°C. Colony counting was then performed (Solbat® scientific apparatus S de RL, Mexico).
SEM
Coating morphology: Scanning electron microscopy (SEM) was used to investigate the morphology of the coatings using a JSM-5510 microscope (JEOL Ltd., Tokyo, Japan) at 5.0 kV. Coating samples were cut into appropriate-sized samples and mounted on stubs using double-sided adhesive tape. Prior to analysis, the coatings were coated with gold to make the samples conductive. Subsequently, the samples were observed at 500 × magnification.
Water vapour permeability: The edible coating samples’ water vapour permeability (WPV) was determined using a modification of the standard gravimetric method, ASTM E 96-80 (ASTM, 1989), known as the “Cup method” or “test cell” method [26], with modifications. A Thermo-brand desiccator and a 23 mL glass vial were used. A saturated solution of NaCl was added to the desiccator to generate a constant relative humidity of 72%, and a supersaturated solution of KNO3 was added to the vial to achieve a constant relative humidity of 92.5%. A 0.07-mm thick edible coating sample was affixed to the vial. The device remained in a controlled atmosphere at 30°C for 36 hours, and this allowed the generation of a pressure gradient resulting in water vapour disseminating through the edible coating, causing a loss of weight in the higher humidity salt. The vial was weighed using an analytical balance (Voyager® Pro Modelo VPZ14, Switzerland) at the beginning of the test and consecutively for 36 hours until achieving a constant weight.
The speed measure (WVTRm) of water vapour transmission was calculated using the following equation:
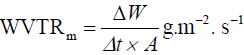
where Δw is the change in weight (g) of the permeation cell during time Δt in a constant area.
Sensorial analysis: An effective method was applied involving a triangular test and a test of acceptance with a hedonic scale. There were 53 untrained judges. The data analysis is expressed in terms of percentages [27].
Statistical analysis: Was used a factorial design with 7 treatments and control. All textural, humidity and colourimetry determinations were performed in triplicate. Tukey’s test was used to identify significant differences between the applied treatments. In all cases, we used a 95% confidence level. The readings were recorded and analysed using the Statgraphics Plus version 4.1 database (Statistical Graphics Corp. 1994-1999, USA). Table 1 shows the compositions of the different formulations.
When the coating was applied, cookies with a shiny surface appearance were obtained. The cookie colour averages were 64.8% L, 3.95 a*, and 26 b*. The humidity of the cookies with the edible coating was 6.5%, which was 1.5% higher than the humidity of the control cookies (p<0.05). The sample control required a force of 14.1 N to break, whereas the edible coating-added cookie samples needed 13.5 N for breakage. The difference was significant (p<0.05) (Table 2).
| Volume | L (%) | a* | b* | Humidity (%) | Hardness (N) |
|---|---|---|---|---|---|
| v1 | 65.1 ± 1.7ª | 4.0 ± 0.7ª | 26.5 ± 1.6ª | 6 ± 1ª | 13.3 ± 3.3ª |
| v2 | 64.5 ± 1.9ª | 3.9 ± 0.6ª | 25.7 ± 1.4ª | 7 ± 1b | 13.7 ± 3.2b |
| Control o/EC | 65.9 ± 1.3ª | 3.8 ± 0.5ª | 26.9 ± 1.7ª | 5 ± 1c | 14.1 ± 3.5c |
v1: volume 1(1 mL); v2: volume 2 (2 mL); ± standard deviation; o/EC: out edible coating. p<0.05
Table 2: General characteristics of different samples by volume for applied edible coating.
Colour
It is expected that a product with an applied edible coating will have minimal modifications to its original appearance, and thus, these modifications should not affect the consumer’s perception. The changes in the parameters L*, a*, and b* in the different samples were not significant (Table 2). The maximum value for net change of colour (ΔE) observed during the storage period was 3.06 for the t6 formula with volume of 2 mL, and the lowest recorded was 0.45 for the OFZ formula with volume of 1 mL. In general, there minimal change in the original colour of the samples, and these changes were not significant (Table 3).
| ΔE | ||
|---|---|---|
| Formulas | v1 | v2 |
| t1 | 0.90 | 2.83 |
| t4 | 1.44 | 1.83 |
| t6 | 1.08 | 3.06 |
| t10 | 1.76 | 2.52 |
| t15 | 2.32 | 2.28 |
| OFZ | 0.45 | 0.78 |
| OMY | 1.31 | 1.14 |
| Average | 1.32 | 2.06 |
| max | 2.32 | 3.06 |
| min | 0.45 | 0.78 |
v1: volume 1 (1 mL); v2: volume 2 (2 mL); ΔE: colour change p>0.05; on treatments p=0.71; on volume p=0.17
Table 3: Average Values of colour change during storage by formulation and volume applied to habanera cookies with edible coating.
The type of edible coating formula applied had a significant effect (p<0.05) on the values of L, whereas the volume did not influence this feature. In the control cookies, there was an average of L of 65.9 ± 1,3%, whereas in the edible coating samples, this value was 64,8 ± 1, 8%, i.e., a difference of 1.1% (Table 2). Formulations with greater L* including t1 and t4 group v1 and Group t4 and OFZ v2.
The type of applied formula of edible coating had a significant effect (p<0.05) on the values of a* and b*, but the applied volume (v1, v2) did not influence these feature. There was an average a*=4.0 for both volumes and an average of b*=26.5 for the v1 groups and 25.8 for the v2 groups, whereas the sample control had an a* of 3.8 and a b* of 26.9. The formula with the highest a* was t4, and the sample with the lowest value was OMY. The highest values b* were found for the t4 formula of the v1 group, and the lowest values were found with formula t1 of the v2 group. Both a* and b* increased with longer storage. Significant differences were detected in hue angle and chroma (p<0.05) (Table 4), which is in agreement with what was observed in the sensory evaluation in terms of observed differences in the parameters L*, a* and b*, and it is possible that variations in the colour of the samples with edible coating could be due to the baking characteristics, as more or less toasting can occur in the same batch of cookies.
| Hue angle¥ | Chroma¥ | |||
|---|---|---|---|---|
| Formulas | v1 | v2 | v1 | v2 |
| to | 7.10 | 7.20 | 27.72 | 26.55 |
| t1 | 6.87 | 6.63 | 26.41 | 25.32 |
| t4 | 6.16 | 6.38 | 27.63 | 26.90 |
| t6 | 6.25 | 5.70 | 27.13 | 26.21 |
| t10 | 6.26 | 6.47 | 26.75 | 25.43 |
| t15 | 6.06 | 6.54 | 26.86 | 25.54 |
| OFZ | 7.75 | 7.48 | 27.25 | 26.54 |
| OMY | 7.63 | 7.57 | 25.87 | 26.09 |
Hue angle=(b*/a*); ¥Chroma= [(a*2+b*2)]1/2; p>0.05 on volume; p<0.05 on formulas
Table 4: Hue angle and chroma of samples of habanera cookies with edible coating.
Moisture
The applied volume of edible coating had a significant effect (p<0.05) on sample moisture; however, the composition of the formula did not significantly affect this feature, as the cracker cookies with the higher humidity were with a 2 mL applied edible coating. The average humidity was maintained during the storage period in both groups (v1 and v2). Variations in moisture were observed because the edible coating was applied as an emulsion and gradual loss of moisture from the coating during storage. However, the average moisture was 1.5% above the average humidity of the sample control (5%), and this difference was significant (p<0.05) (Figure 1). The formulations with humidity of 5% at the end of the storage period were group v1 t10 and t15. OFZ and OMY in the v2 group had 6 ± 0% and 6 ± 1% humidity, respectively, i.e., only one percentage point above the sample control. Although there was an increase in moisture, there were changes in the structure of the cracker cookie, which is important, as it is expected that the application of the edible coating has minimal effects on its characteristics, and this may be because of the amount of emulsion applied was not sufficient to alter the cracker cookie structural characteristics, coupled with the process of drying, was the reason for the fast edible coating moisture loss during storage.
Three-point break test
The volume of edible coating applied to the sample had a significant effect (p<0.05) on the maximum force (MF) necessary to achieve the breakdown of the sample in the three-point break test. The average sample MF control value was 14.1 ± 3.5 N, whereas the values for the samples with an edible coating were 13.3 ± 3 N for the v1 group and 13.7 ± 3 N for the v2 group, a difference of 0.8 N and 0.4 N, respectively. During the period of storage, for the v1 group, the MF increased (Figure 2), which could suggest that the edible coating increases the hardness of the samples, most likely due to the texture characteristics that the edible coating acquired subsequent to the loss of moisture during storage, which contributed to the protection of the structural integrity of the cookie during exposure to the environment. The group v2 MF was not significantly increased at the end of the storage period, most likely due to the higher humidity in the samples of this group (Figure 2).
Different formulations did not differ significantly in this parameter. The formulations with higher hardness were t15 and OMY in the v1 group and t10 in v2 group. The cookie hardness results were related to the moisture results, and the above agrees with the results of Piga et al. [28] that reported the relationship of moisture to this feature; however, the difference in the MF needed to achieve the breakdown of the experimental units in both groups was less than 0.8 N. This could indicate that the changes in the hardness of the cookies were minimal.
Water vapour permeability
Edible coatings of 3 cm in diameter were used, with an average weight of 1.1 g of weight and an average thickness of 0.11 mm. The determination of water vapour permeability (WVP) was performed with coatings OMY and OFZ because they presented good results in terms of the survival of the LBC once applied to the food model (cracker cookie). As shown in Table 5, the permeability registered for the OMY formulation was higher than that of OFZ, with a difference of 0.2807756 g.mm/kpam2 day.
| EC | WVP g.mm/kpam2 day |
|---|---|
| OMY | 0.8037596 |
| OFZ | 0.522984 |
| Difference | 0.2807756 |
EC: Edible coating; OMY and OFZ formulas derived of preliminary research
Table 5: Edible coating’s water vapour permeability (WVP).
Compared with the results of [26,29], these edible coatings presented low WVP even though it has been proven that EC-based proteins and polysaccharides can act as barriers to the O2 and CO2 and present high WVP [29]. On the other hand, considering that the edible coating had a highly hydrophilic character and very thin samples were used, there were fewer molecules hydrophilic available to absorb the same unit mass of water molecules, which thus reduced the WVP, which is directly related to the hydrophilic structure and mainly to the concentration and distribution of its components within the structural matrix, as reported by [26]. It is likely that the presence of unflavoured gelatine as a structural component of the edible coating matrix contributes significantly to the reduction of permeability due to its gelling capacity. In addition to the above, the concentration of glycerol was low (6%) in the different edible coating formulations employed in this study, which could also be another reason explaining the low permeability, as [29] notes that with higher concentrations of glycerol (up to 30%), the structure becomes less dense and, as a result, is more permeable. Thus, it is advisable to consider further studies with the objective of applying this type of edible coating formulation with probiotic bacteria to perishable foods, as in this study, the coating was applied to a food with a low amount of moisture, and the results may not be comparable as when applied to fresh food.
Survival of Lactobacillus casei Shirota®
Using scanning electron microscopy (SEM), the presence of Lactobacillus casei Shirota® cells were observed within the structure of the various samples of edible coating (Figure 3). In addition, biochemical tests indicating the presence of its features. These results indicate the growth of these bacteria countless CFU/g in the formulations t15, OFZ and OMY after 20 days of storage. It is noteworthy that these formulations contained 2, 1.3 and 1.9% (2.8 × 109, 18.2 × 108, 26.6 × 108 CFU/g), respectively, of Lactobacillus. In the t6 and t10 formulations, growth decreased by the 15th day, and there was no growth on day 20. For formulation t1, growth of Lactobacillus had stopped by the 15th day of storage, and this could be due to the proportion of the components of the EC, as with the exception of the t6 formula, all formulations contained above 1% Lactobacillus (Table 6). Better survival of Lactobacillus was achieved with edible coating formulations where the probiotic level was above 1%. It was noted that the formulas with countless CFU/g contained approximately 4% inulin, which could have contributed to the survival of probiotic because, as noted in various investigations, inulin is a prebiotic key substance for the human intestinal flora as it acts by stimulating the selective growth and/or metabolic activity of a limited number of strains of bacteria such as bifidobacteria and Lactobacillus, generating a healthy biomass and an optimal pH, and the recommendation for consumption of inulin ranges currently between 1 and 11 g/day [29-31].
| Formulas | Day 3 | Day 10 | Day 15 | Day 20 |
|---|---|---|---|---|
| t0 | NG | NG | NG | NG |
| t1 | cl CFU/g | cl CFU /g | NG | NG |
| t4 | cl CFU /g | cl CFU /g | cl CFU/g | 357 × 105/73-91 × 106 |
| t6 | cl CFU/g | cl CFU/g | 265 × 106 CFU/g | 9 × 106 |
| t10 | cl CFU/g | cl CFU /g | 312 × 106 CFU/g | 11 × 104/27 × 105 |
| t15 | cl CFU/g | cl CFU/g | cl CFU/g | cl CFU/g |
| OFZ | cl CFU/g | cl CFU/g | cl CFU/g | cl CFU/g |
| OMY | cl CFU/g | cl CFU/g | cl CFU/g | cl CFU/g |
cl CFU/g: countless colony forming unit per gram; NG: No growth, t0 control sample without edible coating; t1, t4, t6, t10, t15, OFZ and OMY samples with edible coating with Lactobacillus casei. EC: Edible oating
Table 6: Survival of Lactobacillus casei Shirota applied via EC on habanera cookies during 20 days storage.
Sensory analysis
An affective test was used to assess consumer response to products. A total of 53 people between 18 and 34 years of age (36.5% men and 63.4% women) were surveyed. A total of 86% of judges identified samples with the edible coating as a different sample. It was possible to observe that the cookies with an edible coating were brighter than those without a coating. In assessing the appearance, 34.6% reported the cookies were different (p<0.05) with respect to the controls. In addition, 30.7% reported they were a little different (p<0.05), and 20% reported no difference. A total of 48% reported little or nothing different about the appearance or aroma, 61% little or no differences in flavour and 52% little or no differences in texture. In terms of acceptance, 62% reported moderately to greatly liking the cracker cookie, whereas only 4% mentioned disliking it (Figures 3-5). During this study, a cookie beside the other were placed, in order to allow the edible coating added with the probiotic, could dry on this, once past the process no downside to stacking was observed and be presented as any other commercial product. One advantage with these packaging materials is that they allow properly isolate moisture environment, allowing in the case of the cookie maintain its sensorial properties. It is important to mention that habanera cracker cookie are commonly preferred by consumers as a snack accompanied by other foods such as cheese spread, jam, ham, ate, pate or as food suggested by meal plans involving low energy intake control as a source of carbohydrates and fibre, so their consumption is lower compared with traditional sweet cookies. However, the cracker cookie could be a viable alternative as a functional food whose added value would be to provide probiotic bacteria, thereby promoting a higher consumption of these type of bacteria among the population.
Based on the previous observations, the best formulations of edible coating applied to the experimental units were t15, OFZ and OMY, which were the best formulations of previous experiments. It should be noted that both t15 and OMY produced similar results; therefore, either could be used either in a foodstuff with low humidity, such as cookies, for the purpose of adding functional food value via supplementation with probiotic bacteria.
The application of edible coating to cracker cookies produced no significant difference in the texture and moisture or in the colour. Considering Lactobacillus casei survived 20 days of storage and there was a good acceptance, it is possible that this food could be a good alternative for increasing consumption of probiotic bacteria among consumers seeking value in the selection of healthy foods. Although, the results are encouraging, it is necessary to carry out the survival probiotic study probiotics for at least one year of storage at different storage temperatures.
Considering the nutritional value of this type of bacteria, the consumption of probiotics via edible coating as a dietary supplement may have important implications in preventing various health problems and could provide a LAB alternative in non-dairy foods. In addition, the edible coating added with LAB could provide products with increased shelf lives.
The economic cost of adding probiotics by an edible film to a food like cookie, does not exceed significantly the cost of it and the benefit is related to what is scientifically been proven as profit after consumption of probiotics to the consumer.
Imelda García-Argueta thanks the Mexiquense Council of Science and Technology (Consejo Mexiquense de Ciencia y Tecnología) for a graduate scholarship.