Entomology, Ornithology & Herpetology: Current Research
Open Access
ISSN: 2161-0983
ISSN: 2161-0983
Research Article - (2017) Volume 6, Issue 3
Mulberry being a C3 crop responsive to carbon, climate change in the form of elevated CO2 coupled with increased temperature would be helpful for crop but it gets altered in presence of herbivore. The eCO2 and temperature favoured growth and development of mulberry only in terms of quantity which was evidenced by accelerated growth of more plant height, leaves, leaf area index, leaf yield and plant biomass. Biochemical analysis of mulberry showed a lot of changes in it, wherein, the chlorophyll, carbon and carbon based compounds viz., tannins, phenols, total sugars, carbohydrates significantly increased in eCO2 as compared to aCO2 treatments. On the contrary, nitrogen (N) and N-based compounds viz., proteins decreased in eCO2 compounds which in turn altered C:N ratio.
<Keywords: C3; Elevated carbon dioxide; Ambient carbon dioxide; C:N-carbon to nitrogen ratio
Mulberry belongs to the family “Moraceae” under the genus “Morus” with several species. It thrives under varied climate ranging from temperate to tropical conditions and major mulberry growing areas of the globe is located north of the equator between 28 °N and 55 °N latitude. The ideal range of temperature is from 24 to 28°C. Mulberry grows well in places with an annual rainfall ranging from 600 to 2500 mm. In areas with low rainfall, growth is limited through moisture stress, resulting in low yields. On an average, mulberry requires 340 m3 per ha of water every ten days in case of loamy soils and 15 days in clayey soils. Atmospheric humidity range of 65-80% is ideal for luxuriant mulberry growth. Sunshine is one of the important factors controlling growth and leaf quality. In the tropics, mulberry grows with a sunshine range of nine to 13 hours a day. Mulberry can be ideally cultivated up to an elevation of 1000 m above mean sea level. Mulberry leaves are highly palatable and digestible to mulberry silkworms and is the only satisfactory food plant. Protein content in the leaves and young stems, with a good essential amino acid profile, varying from 15 to 28% depending on the variety. Mineral content is high with no anti-nutritional factors or toxic compounds have been identified. India is the second largest producer of silk in the world. Production of raw silk in India during the year 2013-14 has been 26,480 MT. Now, as a result of growing realization, sericulture is gaining ground in non-traditional areas too. The existence and prosperity of sericulture industry depends upon the production of quality silk. For production of quality cocoon and silk, silkworm larva should be fed with quality mulberry leaves, which is the exclusive food plant of the Bombyx mori L.
An investigation was carried out to study the effect of elevated CO2 and temperature on growth, yield and quality parameters of mulberry under Open Top Chamber (OTC’s) conditions and subsequent effect on mulberry silkworm at Main Agricultural Research Station (MARS), University of Agricultural Sciences, Raichur, Karnataka. The present study was carried out during kharif and rabi 2014-15 at MARS, Raichur, which is situated in the North Eastern Dry Zone (Zone-II) of Karnataka between 16° 15' N latitude and 77° 20' E longitude with an altitude of 389 m above the mean sea level. The cement pots (2½ feet height with 2 feet diameter) were procured and filled with loamy soil and added with Farm Yard Manure (FYM). Mulberry saplings which have been grown for a period of four months were uprooted from mulberry nursery and planted two saplings per cement pots allowed for establishment with proper watering and care as per treatment in OTC as well as in open plot. Plant growth parameters viz., time taken for sprouting, number of leaves per plant, number of shoots and plant height were recorded in one crop growth cycle. Also, leaf samples were collected on 65th days of pruning for biochemical analysis. Organic carbon, leaf nitrogen, C:N ratio, chlorophyll, flavonoids, tannins, phenols, morin were analysed as per the standard procedure. The leaves from each plant were collected from each treatment and weighed at 65 DAP ad also shoot and root biomass was weighed by uprooting plants from the pot at last in order to know plant biomass. Leaf chlorophyll and flavonoids were measured using a Dualex Scientific sensor a hand tool leaf clip combining the use of fluorescence as well as light transmission of a leaf to determine its physiological status.
Nitrogen content in the plant sample was determined by using Micro-Kjeldahl technique, discovered by McKenzie [1] and the per cent nitrogen was calculated by using the formula,
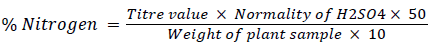 (1)
(1)
Total organic carbon was estimated by dry combustion method/ash method using Muffle furnace.
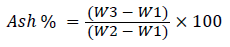 (2)
(2)
Organic carbon %=(100-Ash %) × 0.58 (3)
Where, W1 is weight of crucible, W2 is weight of oven-dried sample along with crucibles, and W3 is the weight of ash with crucibles and 0.58 is the conversion factor. Carbon to nitrogen ratio was analysed by taking the ratio of carbon and nitrogen content readings previously obtained by estimation of the same. Tannins are polyphenolic compounds present in all types of plants. They were estimated by Folin-Dennis method Malick and Singh [2] whereas; Phenols were estimated by Folin-Ciocalteau (FCR) method as suggested by Malick and Singh [2]. The method used for estimation of sugars was suggested by Nelson Somogyi Marais et al. [3] and for the estimation; alcohol was evaporated from the sample and was diluted appropriately. The aromatic amino acids present in a protein like tyrosine, tryptophan react with phosphomolybdic-phosphor tungstate (FCR) reagent to produce a blue coloured complex at 660 nm. The procedure for carbohydrates estimation was carried out as per AOAC [4].
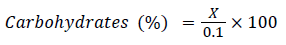 (4)
(4)
Where, X=Concentration of D-glucose from standard graph. The crude fibre content in mulberry leaves was determined by sequential acid and alkali hydrolysis method (AOAC [4] using Fibra-Plus apparatus.
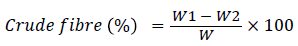 (5)
(5)
Carbon dioxide is the most studied greenhouse gas in climate change research and the impact of elevated greenhouse gas concentrations on the performance of plants and herbivores has been well documented Lindroth [5] The effect of elevated CO2 and temperature on mulberry has direct effect on growth, development, nutritional status and yield of mulberry. The leaves and plant height showed significant increase in elevated CO2 conditions over the cropping period. At 60 DAP, thee CO2 (550 ± 25 ppm) alone treatment recorded maximum plant height (70.33 and 93.74 cm) and more number of leaves (247.33 and 350.00 leaves/plant). The growth parameters of mulberry are well supported by earlier studies by Nereu [6] Mirwais and David [7], David and Christopher [8], Attipalli et al. [9] according to them plants/crops exhibited increased growth rates in elevated CO2 treatment conditions. And also the leaf yield and total plant biomass were highest in elevated CO2 (550 ± 25 ppm) treatment 701.17 g/plant and 1026.28 g/plant, respectively. The results of the present study are in line with earlier findings of Thomas and Luit [10] and they reported 56% increased total shoot biomass in Arabidopsis thaliana L. Similarly the leaf are index, nitrogen biossed index and chlorophyll was also found to be significantly highest in eCO2 alone (2.09 μ/cm2, 28.51 μ/cm2 and 36.61 μ/cm2) respectively. The eCO2 has positively influenced the growth indices viz., leaf area index, nitrogen balanced index and the earlier studies of Nereu [6] reported increased growth rates by C3 plants under eCO2 conditions and mulberry being C3 plant in the present study responded positively to CO2. Further, the direct effects of eCO2 on individual plant species have been well documented Curtis and Wang [11] wherein, eCO2 generally resulted in increased leaf area index and increased growth rate and the rise in chlorophyll content in the elevated climate change treatments was because of that the plant chlorophyll content increased with increased CO2 concentration exposure, especially in C3 plants, Hamid et al. [12] Whereas, the flavonoid content was significantly highest in reference plot compared to elevated conditions (1.53 μ/cm2) (Table 1).
| Treatment | Plant height(cm) | No. of leaves(No.) | Leaf yield(g) | Plant biomass(g) | LAI(µ/cm2) | NBI(µ/cm2) | Chlorophyll(µ/cm2) | Flavonoid(µ/cm2) |
|---|---|---|---|---|---|---|---|---|
| eCO2 (550ppm) | 93.74 | 350 | 701.17 | 1026.28 | 2.09 | 26.21 | 36.61 | 1.24 |
| eCO2+eTemp. (550ppm+2°C) | 95.11 | 331.66 | 657.83 | 935.5 | 1.98 | 28.51 | 35.38 | 1.07 |
| aCO2+eTemp. (390ppm+2°C) | 91.02 | 312 | 663 | 900.77 | 1.62 | 25.23 | 35.6 | 1.05 |
| aCO2 (390 ppm) | 92.98 | 324.94 | 630 | 947.614 | 1.9 | 22.65 | 35.13 | 1.46 |
| Reference plot | 91.55 | 319 | 627.33 | 947.38 | 1.81 | 20.65 | 34.7 | 1.53 |
| CV (%) | 0.85 | 0.93 | 4.12 | 2.18 | 3.3 | 1.54 | 0.58 | 1.35 |
| S.Em+ | 0.39 | 1.5 | 13.52 | 10.4 | 0.03 | 0.19 | 0.1 | 0.01 |
| CD@0.01 | 1.21 | 6.6 | 58.4 | 44.95 | 0.13 | 0.82 | 0.44 | 0.04 |
Table 1: Effect of eCO2 and temperature on growth and yield parameters of mulberry.
Ambient CO2 treatments recorded higher nitrogen percentage wherein, the leaves from reference plot recorded higher values (3.93 mg/g) at 60 DAP. Further, carbon percentage recorded in the eCO2 treatments was higher (25.67 mg/g) over aCO2 treatments at 60 DAP. Furthermore, with increased carbon and decreased nitrogen, the C:N ratio increased (8.89 mg/g) in the eCO2 treatments as compared to aCO2 treatments at 60 days after pruning. It is well known that the direct effect of enriched atmospheric CO2 on plant growth, physiology and community structure is depicted by increased plant growth by accelerating rates of photosynthesis, which reduced tissue quality and increased the carbon to nitrogen (C:N) ratio Robinson et al. [13] Elevated CO2 conditions has promoted increased biomass production with reduced foliar nitrogen and increased C:N ratio for most plants, especially C3 crops.
Tannins and phenols content were significantly higher in eCO2 treatments over a CO2 treatment. Highest tannins (1.91 mg/g) and phenols (4.19 mg/g) were recorded in eCO2 (550 ppm ± 25 ppm). The present findings with respect to tannins and phenols revealed that elevated conditions favoured plant defensive chemicals wherein, highest values of tannins and phenols were found in the elevated treatments as compared to ambient levels. Since, tannins and phenols are carbon based compounds, along with increased carbon; these compounds have also increased in the elevated climate change treatments. The current findings are in line with earlier studies of Xiaowei et al. [14] who reported that some kinds of defensive secondary compounds such as phenolics tend to increase in the eCO2 conditions. The total sugars were significantly highest in eCO2 (550 ppm) treatment (13.08 mg/g) at 60 DAP. The present findings are in line with earlier studies by Xin et al. [15] who reported that eCO2 conditions favoured increased carbohydrates accumulation in tomato plants. Further, the leaf carbohydrate determinations showed that the starch, total sugars and sucrose concentration increased significantly in plants exposed to eCO2 conditions. Elevated CO2 treatments recorded lower protein levels (4.82 mg/g) at 60 DAP whereas, the carbohydrate content was significantly maximum in eCO2 (550 ± 25 ppm) treatment (5.26 mg/g) at 60 DAP. The carbohydrate content was lower in aCO2 treatments in comparison with eCO2 treatment. Contrary to this, protein production in mulberry was higher in aCO2 treatment is in line with the reports of Yin et al. [16] according to them atmospheric CO2 enrichment induced changes in phytochemistry of maize plant with decreased protein. Further, the present investigations showed that soluble proteins and carbohydrates had a significant negative effect of eCO2 and temperature (Table 2).
| Treatment | Leaf nitrogen(mg/g) | Leaf carbon(mg/g) | C:N(mg/g) | Tannin(mg/g) | Phenol(mg/g) | Total sugars(mg/g) | CHO(mg/g) | Proteins(mg/g) | Crude fibre(mg/g) |
|---|---|---|---|---|---|---|---|---|---|
| eCO2 (550ppm) | 3.08 | 23.54 | 7.66 | 1.91 | 4.19 | 13.08 | 5.26 | 5.39 | 7.87 |
| eCO2+eTemp. (550ppm+2°C) | 2.94 | 25.67 | 8.89 | 1.52 | 4.09 | 12.9 | 5.18 | 4.82 | 7.5 |
| aCO2+eTemp. (390ppm+2°C) | 3.27 | 22.7 | 6.96 | 1.17 | 3.09 | 12.13 | 4.84 | 5.44 | 6.9 |
| aCO2 (390 ppm) | 3.83 | 22.5 | 5.91 | 1.07 | 3.26 | 12 | 4.91 | 6.12 | 6.33 |
| Reference plot | 3.93 | 21.25 | 5.45 | 0.98 | 3.39 | 11.89 | 5.15 | 6.24 | 6.18 |
| CV (%) | 1.01 | 0.32 | 1.27 | 2.73 | 0.92 | 2.52 | 0.58 | 2.07 | 2.47 |
| S.Em+ | 0.02 | 0.04 | 0.04 | 0.02 | 0.02 | 0.16 | 0.02 | 0.06 | 0.09 |
| CD @ 0.01 | 0.07 | 0.16 | 0.19 | 0.08 | 0.07 | 0.68 | 0.08 | 0.25 | 0.37 |
Table 2: Effect of elevated CO2 and temperature on phytochemistry of mulberry.