Journal of Drug Metabolism & Toxicology
Open Access
ISSN: 2157-7609
ISSN: 2157-7609
Research Article - (2013) Volume 4, Issue 1
Keywords: Pharmacokinetics; Amikacin; Withania somnifera; Interaction; Buffalo calves
Synthetic antimicrobial drugs are often used for treating the diseases of the infectious origin in the human and animal clinical practices. The combinations of two or more than two antimicrobial drugs cause the drug interactions which may alter the pharmacokinetic and pharmacodynamic behavior of drugs. There are convincing evidences that inappropriate use of antimicrobial agents directly leads to the development of resistant organisms along with the undesired effects. According to World Health Organization (WHO) the microorganisms are becoming resistant to most of the antibiotics and by 2020 and antibiotics will lose their effectiveness and be no more in use to cure diseases in man and animals. With the increased incidence of resistance to antibiotics, natural products from plants could be interesting alternatives. Some plant extracts and phytochemicals are known to have antimicrobial properties which may be of great significance in therapeutics and also traditionally used in Indian medicine and chiefly denoted in the great medicinal literature, Ayurveda. Thus the investigation in the aims of the determination of the pharmacokinetic interaction of synthetic antibacterial drug, Amikacin and potent herbal antimicrobial W. somnifera in healthy buffalo calves and to evaluate kinetic parameter and dosage regimen after single administration to achieve their beneficial effects.
Amikacin, a recent aminoglycoside antibiotic derived from kanamycin by the process of acetylation, has proved its effective and greater clinical advantages over other aminoglycosides due to its high antibacterial spectrum against wide range of bacteria which are resistant to other aminoglycosides. It is most commonly used drug for the treatment of mixed bacterial infections which are unresponsive to other routine antibiotics, in medical and veterinary clinical practices. Accordance with a systemic review, an incidence of ototoxicity in Amikacin users is 5.4% [1].
Withania somnifera commonly known as “Ashwagandha” or Indian Ginseng is an important herb in indigenous medicinal systems (Ayurveda) for over 3000 years. Among all parts of this plant, Withania somnifera root is considered to be the most active for therapeutic purposes like strengthening the body and for helping to prevent disease. Withania somnifera is used in several indigenous drug preparations for maintaining health as well as treatment of several disease conditions. Under its therapeutic applications, the immunomodulator and antistress properties were chiefly described among medicinal literatures. The roots of W. somnifera contain several alkaloids, with anolides, and a few flavanoides a reducing sugars, Withania contains number of phytoconstituents, withanolides as the major constituent. It is one of the most commonly used herbs as natural antimicrobial agent [2].
The various evidences also promoted successful polypharmacy and combined therapy of combination of herbal and synthetic drugs for their antimicrobial property. Arora et al. [3] evaluated the methanol, hexane and diethyl ether extracts from both leaves and roots of Withania somnifera for the antibacterial and synergistic activity against Salmonella typhimurium and Escherichia coli and reported synergistic increase in the antibacterial effect of Tibrim, a combination of rifampicin and ionized. Henrique et al. [4] demonstrated synergistic effect of ethanol extract from Turnera ulmifolia on gentamicin and suggested that extracts from Turnera ulmifolia could be used as a source of plant-derived natural products with resistance-modifying activity, constituting a new weapon against the problem of bacterial resistance to antibiotics. However, the pharmacokinetic studies of W. somnifera and its interactions with Amikacin have not been reported in scientific literature so far.
The experimental protocol include the six clinically healthy male buffalo calves of non-descript breed between 4 to 6 months of age and 100-150 kg body weight. These buffalo calves were housed in animal shed and maintained on dry fodder and greens as well as routine grazing for at least 4-5 hours a day. Clean drinking water was supplied ad libitum. Amikacin was administered at the dose rate of 10 mg/kg body weight by i.v. route and W. somnifera was administered at dose rate of 500 mg /kg body wt. orally in each of six healthy calves to investigate the effect of W. somnifera on the disposition kinetics of Amikacin in buffalo calves. Amikacin (AKAYCI®) - an injectable commercial preparation containing Amikacin equivalent to 250 mg/ ml, marketed by Brihans lab Ltd., India was used and the roots of W. somnifera were obtained from the Department of Aromatic and Medicinal Plants, Agriculture College, J.N.K.V.V., Jabalpur. The roots of W. somnifera were shed dried and crushed in mixer and grinder to prepare fine powder. 100 g of W. somnifera powder was dissolved in 1 L of sterile triple distilled water for 24 h to make cold aqueous extract of W. somnifera. The cold aqueous extract of W. somnifera was administered at dose rate of 500 mg/kg body wt., orally by drenching tube in each of six healthy buffalo calves.
Before collection of blood, the sites around the jugular vein on either site of the neck of the animals were aseptically prepared. The sites were sterilized prior to each collection with rectified spirit. Blood samples were collected in sterilized centrifuge tubes containing appropriate amount of heparin by venepuncture with disposable syringe 18G at various above noted time intervals after drug administration. Blood samples (approx. 5 ml) were withdrawn from jugular vein into heparinized glass centrifuge tubes before and at 0, 1, 2.5, 5, 7.5, 10, 15, 20, 30, 45 min and 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12,16, 24 and 30 h after administration of the drug(s). Plasma was separated by centrifugation at 3,000 rpm for 15 min at room temperature and kept at -4°C until analysis. For preparation standards, normal plasma prior to drug administration was also collected.
The test organism used for the microbiological assay technique (Agar gel diffusion technique) of Amikacin was E. coli (ATCC 25922). The organism was grown on the slant of culture tube containing nutrient agar slants at 37°C for overnight. Then it was stored under refrigeration. The organism was transferred weekly to fresh media to maintain its normal activity. The concentration of Amikacin in plasma were estimated by a rapid, specific microbiological assay technique using Escherichia coli as the test organism [3,5,6]. A stock solution of 1 mg/ml of Amikacin was prepared. Pure base of 0.5 ml of Amikacin was taken and dissolved in 2 ml of triple distilled water under constant stirring to obtain a 1 mg/ml stock solution I of Amikacin. One ml of stock solution - I was dissolved in 11.5 ml of triple distilled water under constant stirring to obtain a 80 μg/ml of stock solution - II of Amikacin. From these stock solutions - II the working standards were prepared daily. Amikacin in concentration of 80 μg/ml was diluted in triple distilled water to make different strengths viz., 40, 20, 10, 5, 2.5, 1, 0.5, 0.25 and 0.1 μg/ml.
Pharmacokinetic analysis of Amikacin after a single i.v. administration was calculated from semi-logarithmic scale as a plot of plasma drug concentration versus time curve. The experimental data were analyzed using a three-compartment open model. On the basis of this data, the dosage regimen of Amikacin, for maintaining minimum therapeutic concentration of 1 and 2 μg/ml in plasma at dosage intervals of (γ) of 8 and 12 hr were calculated using the following equations [7].
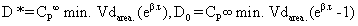
Where,
D*= Priming or Loading dose
D0= Maintenance dose
CP ∞ (min) =Desired minimum plasma concentration
τ = Dosage interval
e= Base of natural logarithm
β and Vdarea were obtained from kinetic study.
Plasma concentrations at various time intervals following combined administration of Amikacin (10 mg/kg) intravenously and W. somnifera (500 mg/kg) orally in healthy calves have been shown in figure 1. The drugs were present at 0.017 h with a mean of 17.05 ± 0.28 μg/ml and was detectable in plasma samples of all the calves up to 30 h with a mean value of 0.18 ± 0.009 μg/ml. The effective therapeutic concentration (≥ 1.0 μg/ml) was maintained up to 24 h with mean value of 1.86 ± 0.038 μg/ml. The mean peak drug concentration in plasma was recorded at 1 min.
The values of various kinetic parameters of Amikacin are presented in tables 1 and 2. The mean distribution half-life of phase 1(t1/2 α1), phase 2 (t1/2 α2) and elimination half-life (t1/2 β) were calculated to be 0.060 ± 0.004, 3.99 ± 0.27 and 6.15 ± 0.21 h, respectively. The value of area under curve in plasma (AUC) and area under first moment curve (AUMC) were found to be 145.55 ± 3.72 μg/ml.h and 1076.01 ± 60.94 μg/ml.h2 with a mean residence time (MRT) of 7.35 ± 0.24 h. The mean value of Vdarea was calculated to be 0.60 ± 0.01 L/kg. The total body clearance (ClB) ranged from 0.064 to 0.075 L/kg/h with a mean of 0.068 ± 0.002 L/kg/h.
| Parameter | Experimental Calves | Mean ± S.E.M. | |||||
| C1 | C2 | C3 | C4 | C5 | C6 | ||
| A1 (mg/ml) | 80.16 | 69.02 | 79.91 | 69.30 | 80.11 | 80.24 | 76.45 ± 2.30 |
| A2 (mg/ml) | 8.91 | 8.48 | 8.75 | 8.49 | 8.82 | 8.80 | 8.7 ± 0.073 |
| B (mg/ml) | 10.44 | 10.38 | 10.25 | 10.40 | 10.45 | 10.50 | 10.40 ± 0.035 |
| β (h-1) | 0.11 | 0.11 | 0.10 | 0.12 | 0.11 | 0.13 | 0.11 ± 0.004 |
| α1 (h-1) | 11.39 | 11.39 | 11.10 | 11.21 | 11.4 | 11.11 | 11.26 ± 0.058 |
| α2 (h-1) | 0.18 | 0.19 | 0.20 | 0.19 | 0.17 | 0.20 | 0.18 ± 0.004 |
| t1/2 α1 (h) | 0.060 | 0.060 | 0.062 | 0.061 | 0.060 | 0.062 | 0.060 ± 0.0004 |
| t1/2 α2 (h) | 3.85 | 3.64 | 3.46 | 3.64 | 4.07 | 3.45 | 3.99 ± 0.27 |
| t1/2 β (h) | 6.3 | 6.3 | 6.9 | 5.77 | 6.30 | 5.33 | 6.15 ± 0.21 |
| AUC(mg/ml.h) | 151.43 | 145.04 | 153.44 | 137.52 | 153.9 | 131.98 | 145.55 ± 3.72 |
| AUMC (mg/ml.h2) | 1149.04 | 1093.3 | 1244.39 | 957.95 | 1169.43 | 841.95 | 1076.1 ± 60.94 |
| MRT (h) | 7.58 | 7.53 | 8.10 | 6.96 | 7.59 | 6.37 | 7.35 ± 0.24 |
| Kel (h-1) | 0.65 | 0.60 | 0.64 | 0.64 | 0.64 | 0.75 | 0.65 ± 0.020 |
| T ≈ P | 5.25 | 4.55 | 4.66 | 4.55 | 4.88 | 4.88 | 4.96 ± 0.17 |
| Vdarea (L/kg) | 0.60 | 0.62 | 0.65 | 0.60 | 0.59 | 0.58 | 0.60 ± 0.010 |
| ClB (L/kg/h) | 0.066 | 0.068 | 0.065 | 0.072 | 0.064 | 0.075 | 0.068 ± 0.0017 |
Table 1: Pharmacokinetic parameters of Amikacin (10 mg/kg, i.v.) and W. somnifera (500 mg/kg,orally) after combined administration in Buffalo calves (n=6).
| CP∞ min (mg/ml) | τ (h) | Dose (mg/kg) | Experimental calves | Mean ± S.E.M. | |||||
|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | ||||
| 1.0 | 8 | D* | 1.44 | 1.49 | 1.44 | 1.56 | 1.42 | 1.64 | 1.49 ± 0.035 |
| D0 | 0.84 | 0.87 | 0.79 | 0.96 | 0.83 | 1.05 | 0.89 ± 0.039 | ||
| 12 | D* | 2.24 | 2.31 | 2.15 | 2.53 | 2.20 | 2.76 | 2.36 ± 0.095 | |
| D0 | 1.64 | 1.69 | 1.50 | 1.93 | 1.61 | 2.17 | 1.75 ± 0.10 | ||
| 2.0 | 8 | D* | 2.88 | 2.98 | 2.88 | 3.12 | 2.84 | 3.28 | 2.99 ± 0.070 |
| D0 | 1.68 | 1.74 | 1.58 | 1.92 | 1.66 | 2.1 | 1.78 ± 0.079 | ||
| 12 | D* | 4.48 | 4.62 | 4.3 | 4.06 | 4.4 | 5.52 | 4.733 ± 0.19 | |
| D0 | 3.28 | 3.38 | 3.0 | 3.86 | 3.22 | 4.34 | 3.51 ± 0.20 | ||
Table 2: Dosage regimens for combined administration of Amikacin (10 mg/kg) and W. somnifera (500 mg/kg) in healthy calves.
Following administration of Amikacin at (10 mg/kg i.v.) and W. somnifera (500 mg/kg oral) the peak plasma concentration of Amikacin was recorded as 17.05 ± 0.28 g/ml at 1 min in the present study this value was lower as compared to 116.9 ± 3.16 μg/ml in bovine calves [8] in Amikacin alone administration. Similarly Sumano et al. (2005) determined the pharmacokinetic variables of amikacin in cows after administration of amikacin sulphate either intravenously (i.v.) or intramuscularly (i.m.) at a dose of 25 mg/kg per day for three days and Amikacin concentrations at time zero and maximum serum concentrations were found as 240.8 μg/mL and 122.53 μg/ mL, respectively. According to Edward et al. (1993), Amikacin administration resulted in peak values of 27.3 ± 6.9 μg/ml in study of the pharmacokinetic properties of Gentamicin and Amikacin in the Cockatiel. In case of Wistar Rats, Hugo et al. [9] analysed the effect of mannitol on the pharmacokinetics (PK) of amikacin and Cmax were evaluated as 62.26 ± 15.75 mg/ml, 72.63 ± 24.80 mg/ml and 68.61 ± 27.40 mg/ml for three different groups.
The values of elimination rate constant (β) in Amikacin and W. somnifera combined administration in healthy buffalo calves was 0.11 ± 0.004. This indicates that low rate of elimination of Amikacin had occurred in combination with W. somnifera. This is further supported by decreased value of rate constant of drug elimination from central compartment (Kel) was noted as 0.65 ± 0.02 h-1 in healthy buffalo calves. The elimination half-life was calculated as 6.15 ± 0.21 in combined administration of Amikacin and W. somnifera, this value was longer as compared to elimination half-life of Amikacin as 3.09 ± 0.27 h in bovine calves [8], in lactating sheep 1.64 ± 0.06 h [10] and 2.16 h for goats. This is clearly indicated that the slow rate of elimination of Amikacin from body in the presence of W. somnifera. Distribution half life (t1/2 α1) was found lower in healthy buffalo calves 0.060 ± 0.0004 but higher values were reported in goat as 0.24 h [11], 0.36 h in calves and 0.43 h in sheep [12] in Amikacin alone administration this result showed the alteration in the distribution of Amikacin by W. somnifera in combined administration.
The rapid distribution of the drugs was further confirmed by high ratio of T/P which was recorded 4.96 ± 0.17 in healthy calves. The findings also indicate a relatively less wide distribution of Amikacin after administration with W. somnifera. The high values of AUC and AUMC reflect that most of the body area is covered with the drug concentrations. The AUC value in combined administration of Amikacin and W. somnifera was 145.55 ± 3.72 μg/ml.h which was higher as compared to value in alone administration of Amikacin in goats 73.18 μg/ml.h [13], in lactating sheep 94.09 ± 6.95 (mg.h)/ml [10] and in Greyhounds dogs 79.97 h·μg/ml [14].
Similarly, the mean residence time (MRT) in combined administration of Amikacin and W. somnifera was 7.35 ± 0.24 h in healthy buffalo calves which was significantly higher than Amikacin alone administration as in goat 4.67 ± 0.19 h [13] and lower in oryx 2.27 h. In healthy buffalo calves the Vdarea (0.60 ± 0.010 L/kg) is significantly higher than in alone i.v. administration of Amikacin in bovine calves 0.40 ± 0.03 L/kg [8], in human i.e. 0.27 ± 0.04 L/kg [15] and in Beagles dog as 234.0 ml/kg [14]. This is reflecting good penetration of Amikacin into various body fluids and tissues after intravenous administration of Amikacin with oral administration of W. somnifera. The total body clearance (ClB) was noted in the present investigation as 0.068 ± 0.001, where higher values of the total body clearance (ClB) were noted down for goats as 2.34 ± 0.17 ml/kg/min [13], in camel as 0.97 ml/kg/min after i.m. administration, in dogs 2.66 ml/kg/min [16] and in cow calves 0.09 ± 0.002 L/kg/h [17] after i.v. administration of Amikacin alone. That clearly showed the slower rate of drug clearance from the body due to the combined administration of Amikacin with W. somnifera.
An average plasma concentration of 1.0- 4.0 μg/ml has been reported to be the minimum therapeutic concentration (MIC90) of Amikacin against most gram positive, gram negative and atypical bacteria [13,18]. In the present study dosage regimen was derived at MIC of 1.0 and 2.0 μg/ml for Amikacin at dosage interval of 8 and 12 h. Significantly lower loading (D*s) and maintenance (D0s) dose were observed for Amikacin at all dosage intervals when Amikacin (10 mg/kg b.wt. i.v.) administered together with W. somnifera (500 mg/ kg orally). For maintaining Cp°min of 1.0 μg/ml, the loading doses (D*s) were calculated to be 1.49 ± 0.03 and 2.36 ± 0.09 mg/kg while maintenance doses (D0s) were calculated to be 0.89 ± 0.03 and 1.75 ± 0.10 mg/kg at the dosage intervals (τ) of 8 and 12 h, respectively. This calculated dose were significantly lower as compared to dosage regimen for Amikacin alone administration in goats calculated by Agrawal et al. [13] as 14.75 mg/kg. Likewise, the loading dose D*s were calculated to be 2.99 ± 0.070 and 4.73 ± 0.19 mg/kg while maintenance doses (D0s) were calculated to be 1.78 ± 0.079 and 3.51 ± 0.20 mg/kg respectively for maintaining Cp°min of 2.0 μg/ml at the dosage intervals (τ) of 8 and 12 h interval in combined administration of Amikacin and W. somnifera may be recommended for buffalo calves.
Administration of W. somnifera markedly alters the plasma levels and pharmacokinetics of Amikacin resulting in the modification of the dosage regimen of Amikacin in healthy buffalo calves which clearly indicated their safe and effective therapeutic use with promising antimicrobial polypharmacy.