Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2019)Volume 8, Issue 6
This study investigated the effect of ethanol leaf extract of Spilanthes filicaulis on liver indices and selected biochemicals on carbon tetrachloride induced hepatic damage in wistar rats. A total of thirty (30) rats comprising of five (5) rats per group (90-130 g), were used for the study. Groups 1-3, served as normal, negative (CCl4 induced 1 mL/kg, 1:1 i.p.) and positive (CCl4 induced and administered with silymarin 50 mg/kg) controls respectively. Groups 4-6 were the extract treated groups administered with 250 mg/Kg bw, 500 mg/Kg bw and 750 mg/Kg bw respectively for 14 days. The results of the liver marker enzymes showed a significant decrease (p<0.05) in Alanine Transaminase (ALT), Alkaline Phophatase (ALP) and γ-Glutamyl Transferase (GGT) levels in the positive control and extract treated groups, while aspartate transaminase (AST) levels recorded a significant decrease (p<0.05) in groups administered with 250 mg/kg (10.10 ± 0.17 U/L) and 750 mg/Kg bw (11.35 ± 2.01 U/L) when compared with the negative control group. Liver indices, showed no significant difference (p>0.05) in all treated groups when compared to the negative control group. Catalase (CAT) and Superoxide Dismutase (SOD) levels were significantly increased (p<0.05), while Nitric Oxide (NO) and Lipid Peroxidation (LPO) levels significantly decreased (p<0.05) in all groups administered with the extract when compared with the negative control group. Gluthahione levels, lipid profile, renal indices, serum electrolyte and hematological indices showed no significant difference (p>0.05) in all treated groups when compared to the negative control. The study showed that the ethanol leaf extract of Spilanthes filicaulis has potential of restoring hepatic liver damage.
Ethanol leaf extract; Spilanthes filicaulis; Carbon tetrachloride; Liver indices; Wistar rats
Cellular metabolism is a vital process in the lives of living organisms, which account for the general activities of the cells which includes ATP production, protein translation, DNA transcription and RNA replication, redox signaling, xenobiotic detoxification etc. These various processes are accompanied by some side reactions in some metabolic pathways which constantly generate Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) [1]. ROS and RNS production could mediate oxidative stress, a cellular state resulting from the equilibrium alteration between ROS production and the body defense system in favour of ROS generation [2]. Exogenous radicals such as CCl3 •, a product from the biotransformation of CCl4, mediate hepatic and nephrone damage in experimental models [3].
Antioxidants are the biological tools in which the cells utilize in checkmating free radical activities [4]. Plants are one of the major sources of exogenous antioxidants to man [5]. The use of plant in combating various pathological conditions is solely due to their rich abundances of various rich phytochemical classes of phenols, flavonoids, alkaloids, terpenes, saponin, glycosides etc. [6]. It is believed that these compounds possess medicinal properties whose modes of actions are via free radicals scavenging and inhibition of radical initiated reactions in the body [7]. The liver performs metabolic functions that involve the detoxification of xenobiotics including air pollutants. Consequently, the liver is susceptible to toxic effects associated with these air pollutants and thus, there is urgent need to investigate medicinal plants that may confer protective effects in order to compliment the metabolic functions of the liver.
Spilanthes filicaulis is a shrubby plant of the family of Asteraceae. The traditional application of the plant in herbal medicine according to folklore and some scientific study proved its efficacy as an antimicrobial agent, with diuretic potential as well as antimalarial properties against Plasmodium falciparum [8-11]. The invitro antioxidant activities of the ethanol leaf extract of Spilanthes filicaulis as reported by Ogunka-Nnoka et al., [12] revealed that the plant possesses antioxidants activities.
Collection of plant material and identification
Spilanthes filicaulis leaves were harvested from a swampy field site in Emonu-Orogun, Delta State, Nigeria in the month of April 2017. The studied plant was identified and authenticated by the Department of Botany, Delta State University, Abraka, Nigeria.
Plant extract preparation
The Fresh leaves of Spilanthes filicualis were washed with distilled water, debris were removed and air dried for two weeks and then reduced to coarse powder using a manual grinder. Then, 100 g of coarsely powdered leaves were extracted with 400 mL of 80% ethanol using cold maceration for 24 hours (A total of 1200 g of powdered form of the plant was used for the study). The extract was filtered through cheese cloth with fine pore, and the filtrate was filtered for the second time using Whatman No. 1 filter paper. The resulting extract was then concentrated at 50°C in a rotary evaporator for 2 hr and it was then transferred to a water bath maintained at 40°C and evaporated to dryness to yield a dark green mass. The extract obtained was put in a glass container and stored at 4°C until when required for use. The percentage yield of the extract was calculated using the formula:
% Yield=[Weight of extract/Weight of plant material] × 100 Experimental induction of liver toxicity
Liver damage (heptatoxicity) was induced by single dose of intraperitoneal injection (i.p.) of 50% v/v (1:1) carbon tetrachloride (CCl4) 1 mL/kg body weight, with paraffin serving as the vehicle [13]. The rats were fasted for 12 hrs, followed by the administration of CCl4. Each rat in the normal control group was injected with equivalent volume of normal saline solution.
Experimental design
In the experiment, a total of thirty (30) male Wistar albino rats were used. They were randomly divided into 6 groups containing 5 rats in each group.
Group 1: Normal rats that received distilled water orally (Normal control).
Group 2: CCl4 induced rats (Negative control).
Group 3: CCl4 induced rats that received 50 mg/kg body weight of Silymarin orally (Positive control).
Group 4: CCl4 induced rats that received 250 mg/kg body weight of ethanol leaf extract of Spilanthes filicaulis orally.
Group 5: CCl4 induced rats that received 500 mg/kg body weight of ethanol leaf extract of Spilanthes filicaulis orally.
Group 6: CCl4 induced rats that received 750 mg/kg body weight of ethanol leaf extract of Spilanthes filicaulis orally.
Blood collection and preparation of tissue homogenate
The study period lasted 14 days after which the rats were fasted for 12 hrs before sacrificing via cervical decapitation. Each rat was placed on dorsal surface, and a laparotomy was carried out to expose the internal organs. Blood samples were collected in plain EDTA tubes and allowed to clot and the serum separated by centrifuging at 3000 rpm for 10 min. A portion (0.5 g) of the liver was collected, rinsed with cold saline and homogenized using mortar and pestle in 4.5 mL of 0.1 M cold phosphate buffer (pH 7.4). The homogenate was then placed on a plane tube and centrifuged and the resultant supernatant obtained was then subjected to various biochemical analyses. The sera collected were also subjected to various biochemical analyses. The samples of sera and tissues organelles were stored in the freezer at 4°C until they were required for use. A section of the liver was preserved in 10% formalin and used for histopathological studies.
Determination of liver and kidney indices
Determination of liver enzymes and other biochemical indices were done using Prietest Easylab Biochemistry Analyzer [14]. Kidney function indices were analyzed from the sample. Urea concentration was determined by the diacetyl monoxime method using assay kit from Randox laboratories UK, while creatinine concentration was determined by the alkaline picrate method [15].
Determination of serum electrolytes
Determination of serum sodium and potassium concentration was done using reagent kit [14]. Serum bicarbonate concentration was determined titrimetrically while mercuric nitrate method was used to determinethe concentration of chloride as modified by Teco diagnostics1268N Lakeview Avenue Anahein; CA 92807, USA.
Determination of lipid profile
Plasma total cholesterol, triglycerides, and HDL were determined enzymatically using commercially available kits (Randox kits). From the results, LDL cholesterol was calculated using the formula of Friedel-wald et al. [16].
Determination of hematological indices
The Packed Cell Volumes (PCV), White Blood Cell (WBC) counts, Red Blood Cell (RBC) counts, Hemoglobin (Hb) Concentrations, and platelets counts, were obtained using an Automated Hematology Analyzer–MC-2800 manufactured by Mindray Company, China.
Determination of hepatic antioxidant markers
Catalase (CAT) activity: Fifty microliter (50 μL) of the sample was added to a cuvette containing 2 mL of phosphate buffer 0.05 M (pH 7.4) and 1 mL of 30 mM H2O2 and the absorbance was read at 240 nm for 3 min at 30 sec interval using spectrophotometer [17].
Super oxide dismutase (SOD) activity: A 0.2 mL of sample was added to 2.5 mL 0.05M of bicarbonate buffer (pH 10.2) for test; while for reference 0.2 mL of distilled water was used in place of the sample [18]. Then 0.3 mL of 0.3 mM adrenaline solution was added and the absorbance was read immediately at 420 nm for 3 min at 1 min interval. The SOD activity was estimated using the formula below:
% Inhibition=[O.D.Ref-(O.D.Test/O.D.Ref)]
SOD activity (Units/g tissue)=[% inhibition/50 × tissue (g)]
Total reduced glutathione (GSH): A 0.5 mL of sample was precipitated with 2.0 mL of 5% TCA. 1.0 mL of supernatant was taken after centrifugation and 0.5 mL of Ellman’s reagent and 3.0 mL of phosphate buffer was added to all the tubes. The absorbance was read at 412 nm within 2 min against the reagent blank. The amount of glutathione was expressed as μg/g tissue [19].
Estimation of nitric oxide: Sample and Griess reagent of 0.4 mL each were mixed well and left at room temperature for 10 min in dark. The absorbance was measured at 540 nm against a reagent blank. The concentration of nitrite in the sample was determined from sodium nitrite standard curve (0- 40 μM). Nitrite levels were expressed as μM/g tissue [20].
Estimation of lipid peroxidation (LPO): One milliliter tissue homogenate was added into a test tube labeled test and 1.0 mL of water into another tube labeled as blank. The TBA-TCA-HCl reagent mixture of 2.0 mL was added to all test tubes. All the tubes were mixed well and placed in boiling water bath for 15 min, cooled and centrifuged at 2000 rpm for 10 mins. The absorbance of the supernatant was measured at 535 nm. The malondialdehyde concentration of the sample was calculated using the extinction coefficient of 1.56 × 105m-1cm-1 [21].
All data were subjected to statistical analysis. Values were reported as Mean ± Standard deviation while one way ANOVA was used to test for differences between treatment groups. The results were considered significant at 95% confidence level (p<0.05). Supplementary analysis was carried using post hoc test. SPSS 20.0 software was used for all analysis.
From this study, the serum liver marker enzymes activities were observed to be significantly decreased (p<0.05) in all groups administered with the extract in a dose-dependent manner when compared to the untreated group (negative control). AST levels was significantly reduced (p<0.05) in groups administered with 250 mg/Kg bw (10.10 ± 0.17 U/L) and 750 mg/Kg bw (11.35 ± 2.01 U/L) of the extract when compared to the negative control group as shown in Table 1. Liver indices of serum total protein, albumin, total bilirubin and direct bilirubin levels showed no significant difference in all treated groups when compared to the negative control group as presented in Table 2.
| Groups | ALT (U/L) | AST (U/L) | ALP (U/L) | GGT (U/L) |
|---|---|---|---|---|
| 1 | 30.32 ± 2.42a | 10.17 ± 0.29a | 40.14 ± 0.25a | 45.80 ± 1.21a |
| 2 | 51.35 ± 2.01b | 13.47 ± 0.32b | 52.88 ± 5.26b | 59.53 ± 0.84b |
| 3 | 36.81 ± 2.39c | 11.47 ± 1.73a | 41.41 ± 0.66a | 50.88 ± 1.27c |
| 4 | 42.19 ± 2.66c | 10.10 ± 0.17a | 48.31 ± 3.47b | 53.18 ± 0.78c |
| 5 | 40.13 ± 3.02c | 14.40 ± 2.14b | 43.97 ± 0.48a | 52.13 ± 1.9c |
| 6 | 37.88 ± 0.54c | 11.35 ± 2.01a | 41.90 ± 0.25a | 51.50 ± 2.02c |
Values are means ± standard deviations of triplicate determinations. Values with different superscript (a-c) on the same column differ significantly (p<0.05). ALT=Alanine transaminase, AST=Aspartate transaminase, ALP=Alkaline Phosphatase GGT=γ-Glutamyl transferase.
Table 1: Serum liver marker enzyme activities of male wistar rats in CCl4 induced liver damage administered with ethanol leaf extract of Spilanthes filicaulis.
| Groups | Total Protein | Albumin | Total Bilirubin | Direct bilirubin |
|---|---|---|---|---|
| 1 | 9.50 ± 0.27a | 4.13 ± 0.15a | 1.11 ± 0.10a | 0.26 ± 0.00a |
| 2 | 7.93 ± 0.78b | 4.07 ± 0.06a | 1.24 ± 0.27a | 0.35 ± 0.16a |
| 3 | 8.63 ± 1.36b | 4.17 ± 0.15a | 1.29 ± 0.21a | 0.35 ± 0.02a |
| 4 | 7.74 ± 0.51b | 4.40 ± 0.01a | 1.10 ± 0.15a | 0.21 ± 0.07a |
| 5 | 8.00 ± 0.10b | 4.47 ± 0.42a | 1.42 ± 0.37a | 0.26 ± 0.01a |
| 6 | 8.30 ± 0.63b | 3.80 ± 0.35a | 1.32 ± 0.16a | 0.36 ± 0.09a |
Values are means ± standard deviations of triplicate determinations. Values with different superscript (a-b) on the same column differ significantly (p<0.05).
Table 2: Serum protein (mg/dl) and bilirubin (mg/dl) levels in CCl4 induced liver damage on male wistar rats administered with ethanol leaf extract of Spilanthes filicaulis.
The results of the in-vivo study of the effect of ethanol leaf extract of Spilanthes filicaulis on renal indices in CCl4 induced liver damage of male wistar rats are presented in Table 3. There was no significant difference in creatinine, urea and uric acid levels in all extract treated groups when compared to the negative control group. The results of the in-vivo study of the effect of ethanol leaf extract of Spilanthes filicaulis on serum electrolytes in CCl4 induced liver damage of male wistar rats are embodied in Table 4. Serum sodium, calcium, chloride, phosphorus and bicarbonate levels showed no significant difference in all extract treated groups when compared to the negative control group.
| Groups | Creatinine (mg/dl) | Urea(mg/dl) | Uric acid(mg/dl) |
|---|---|---|---|
| 1 | 1.17 ± 0.06a | 31.84 ± 2.66a | 5.40 ± 0.46a |
| 2 | 1.77 ± 0.15b | 43.87 ± 5.70a | 5.30 ± 0.36a |
| 3 | 1.70 ± 0.36b | 34.23 ± 4.18a | 4.53 ± 0.64a |
| 4 | 1.53 ± 0.40b | 36.59 ± 5.74a | 5.67 ± 0.49a |
| 5 | 1.73 ± 0.15b | 33.11 ± 2.35a | 5.53 ± 0.81a |
| 6 | 1.83 ± 0.25b | 37.25 ± 6.09a | 5.27 ± 0.35a |
Values are means ± standard deviations of triplicate determinations. Values with different superscript (a-b) on the same column differ significantly (p<0.05).
Table 3: Serum renal indices in CCl4 induced liver damage of male wistar rats administered with ethanol leaf extract of Spilanthes filicaulis.
| Groups | Sodium | Calcium | Chloride | Phosphorus | Bicarbonate |
|---|---|---|---|---|---|
| 1 | 141.33 ± 1.16a | 7.17 ± 0.06a | 94.67 ± 5.03a | 4.23 ± 0.25a | 20.00 ± 1.41a |
| 2 | 137.33 ± 1.53a | 7.2 ± 0.72a | 87.00 ± 14.73a | 4.57 ± 0.45a | 17.45 ± 5.51a |
| 3 | 130.67± 3.06a | 7.20 ± 0.72a | 86.00 ± 12.17a | 4.37 ± 0.60a | 21.97 ± 0.76a |
| 4 | 135.00 ±14.14a | 7.00 ± 0.28a | 86.50 ± 9.19a | 4.45 ± 0.78a | 17.13 ± 0.12a |
| 5 | 134.33 ± 6.80a | 7.07 ± 0.91a | 85.67 ± 5.13a | 4.03 ± 0.40a | 19.17 ± 0.06a |
| 6 | 128.33 ± 6.03a | 6.37 ± 0.50a | 82.67 ± 11.02a | 4.67 ± 0.76a | 20.18 ± 0.21a |
Values are means ± standard deviations of triplicate determinations. Values with different superscript on the same column differ significantly (p<0.05).
Table 4: Serum electrolyte (mEq/L) in CCl4 induced liver damage on male wistar rats administered with ethanol leaf extract of Spilanthes filicaulis.
The results of the in-vivo study of the effect of ethanol leaf extract of Spilanthes filicaulis on serum lipid profile in CCl4 induced liver damage of male Wistar rats are shown in Table 5. Total Cholesterol (TC), triglyceride, HDL-C and LDL-C values showed no significant difference in all the groups administered with the extract when compared to the negative control group.
| Groups | TC(mg/dl) | Triglyceride (mg/dl) | HDL-C (mg/dl) | LDL-C (mg/dl) |
|---|---|---|---|---|
| 1 | 196.67 ± 5.77a | 187.67 ± 6.81a | 52.67 ± 0.58a | 106.47 ± 6.52a |
| 2 | 207.33 ± 4.61b | 181.00 ± 5.57a | 51.00 ± 4.36a | 120.13 ± 1.60a |
| 3 | 205.00 ± 5.00b | 191.33 ± 16.29a | 52.00 ± 3.46a | 114.73 ± 8.53a |
| 4 | 200.00 ± 0.00b | 185.00 ± 25.52a | 50.00 ± 0.00a | 112.88 ± 5.11a |
| 5 | 208.67 ± 1.16b | 182.67 ± 15.54a | 52.67 ± 5.69a | 119.47 ± 3.83a |
| 6 | 208.00 ± 10.58b | 196.67 ± 5.77a | 54.00 ± 5.29a | 114.67 ± 16.17a |
Values are means ± standard deviations of triplicate determinations. Values with different superscript (a-b) on the same column differ significantly (p<0.05).TC=Total Cholesterol, HDL-C=High density lipoprotein cholesterol, LDL-C=Low density lipoprotein cholesterol.
Table 5: Serum lipid profile in CCl4 induced liver damage on male wistar rats administered with ethanol leaf extract of Spilanthes filicaulis.
The in-vivo study on the effect of ethanol leaf extract of Spilanthes filicaulis on heamatological indices are shown in Table 6. There was no significant difference in PCV, Hb, RBC, WBC, MCH, MCHC and MCV levels in all extract treated groups when compared to the negative control.
| Groups | PCV(%) | Hb(g/l) | RBC (X1012/L) | WBC (x109/L) | MCH(pg/cell) | MCHC(%) | MCV(fL) |
|---|---|---|---|---|---|---|---|
| 1 | 44.00 ± 0.00a | 13.56 ± 0.17a | 6.50 ± 0.06a | 4.80 ± 0.06a | 25.88 ± 0.92a | 32.44 ± 0.40a | 84.39 ± 6.10a |
| 2 | 47.00 ± 2.12a | 14.71 ± 0.26a | 6.52 ± 0.50a | 5.32 ± 0.21a | 27.72 ± 2.22a | 32.28 ± 0.88a | 86.32 ± 0.89a |
| 3 | 40.00 ± 1.44a | 12.24 ± 1.73a | 5.99 ± 0.37a | 4.18 ± 0.23a | 24.05 ± 2.85a | 32.91 ± 4.07a | 79.99 ± 13.92a |
| 4 | 44.00 ± 5.67a | 13.73 ± 1.50a | 6.18 ± 1.14a | 4.48 ± 1.15a | 25.39 ± 2.54a | 32.01 ± 0.62a | 81.63 ± 10.09a |
| 5 | 37.33 ± 7.51a | 12.24 ± 2.27a | 5.69 ± 0.76a | 4.21 ± 0.70a | 22.43 ± 3.09a | 30.46 ± 1.39a | 68.75 ± 11.52a |
| 6 | 43.00 ± 3.61a | 13.61 ± 1.00a | 6.50 ± 0.17a | 4.47 ± 0.60a | 24.28 ± 4.32a | 31.57 ± 0.43a | 79.49 ± 16.88a |
Values are mean ± standard deviations of triplicate determinations. Values with different superscript on the same column differ significantly (p<0.05). PCV=Packed cell volume, Hb=Heamoglobin, RBC=Red blood cells, TWBC=Total white blood cells, MCH=Mean corpuscle heamoglobin, MCHC = Mean corpuscle heamoglobin concentrations, MCV=Mean corpuscle volume.
Table 6: Heamatological indices in CCl4 induced liver damage on male wistar rats administered with ethanol leaf extract of Spilanthes filicaulis.
Endogenous antioxidants markers such as Catalase (CAT) and Superoxide Dismutase (SOD) activities were significantly increased (p<0.05) in a dose-dependent manner in all groups administered with the extract when compared to the negative control (Table 7). Groups administered with the extract at 500 mg/kg (2.58 ± 0.54 Units/g tissue) and 750 mg/kg (2.77 ± 0.52 Units/gtissue) showed a significant increase (p<0.05) when compared to the normal control group and positive control group for the SOD activities. NO (Nitric oxide) and Lipid peroxidation (LPO) levels showed a significant decrease (p<0.05) in all groups administered with the extract in a dose-dependent manner when compared to the negative control. However, group administered with 750 mg/kg (67.61 ± 2.36 μM/g tissue) of the extract showed a significant decrease (p<0.05) in NO levels when compared to the positive control group (75.25 ± 2.73 μM/g tissue). Glutathione (GSH) levels showed no significant difference (p>0.05) in all extract treated groups when compared to the negative control group.
| Groups | CAT (Units/g tissue) | SOD (Units/g tissue) | NO (µM/g tissue) | GSH (µg/g tissue) | LPO (Units/g tissue) X 10-3 |
|---|---|---|---|---|---|
| 1 | 45.79 ± 4.68a | 1.34 ± 0.14a | 67.29 ± 1.43a | 220.96 ± 2.58a | 6.42± 0.23a |
| 2 | 29.72 ± 8.67b | 0.35 ± 0.26b | 92.72 ± 4.15b | 198.79 ± 3.92b | 11.93 ± 0.19b |
| 3 | 47.15± 5.16a | 1.77 ± 0.15a | 75.25 ± 2.73 d | 194.51 ± 1.10b | 4.70 ± 0.16a |
| 4 | 43.79 ± 1.86a | 1.53 ± 0.99a | 82.13 ± 1.19c | 198.11 ± 8.97b | 8.92 ± 0.20a |
| 5 | 47.92 ± 8.88a | 2.58± 0.54c | 74.61 ± 1.06 d | 194.61 ± 2.23b | 6.73 ± 0.18a |
| 6 | 53.93 ± 9.33a | 2.77 ± 0.52c | 67.61 ± 2.36a | 197.76 ± 5.35b | 5.21± 0.22a |
Values are means ± standard deviations of triplicate determinations. Values with different superscript (a-d) on the same column differ significantly (p<0.05). CAT=Catalase, SOD=Superoxide dismutase, NO=Nitric oxide, GSH=Glutathione and LPO=Lipid peroxidation.
Table 7: Liver antioxidant markers in CCl4 induced liver damage on male Wistar rats administered with ethanol leaf extract of Spilanthes filicaulis.
The histological findings as seen from the micrographs revealed that the hepatic tissue appear essentially normal for group 1 (Plate 1). Periportal inflammatory cells and increased vascular congestion were observed in group 2 (Plate 2), while in group 3 (Plate 3) there was absence of vascular congestion and inflammatory cells. Also in group 4 (Plate 4) periportal inflammations was seen and in group 5 (Plate 5) mild hepatitis and vascular congestion was observed. Group 6 (Plate 6) showed absence of vascular congestion and inflammatory cells.
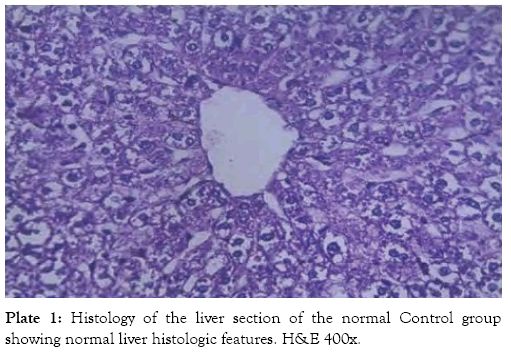
Plate 1: Histology of the liver section of the normal Control group showing normal liver histologic features. H&E 400x.
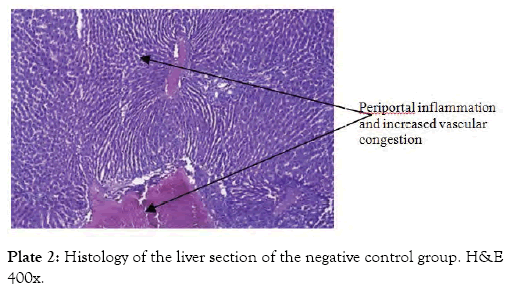
Plate 2: Histology of the liver section of the negative control group. H&E 400x.
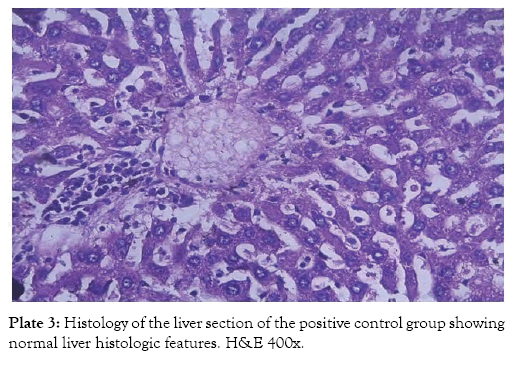
Plate 3: Histology of the liver section of the positive control group showing normal liver histologic features. H&E 400x.
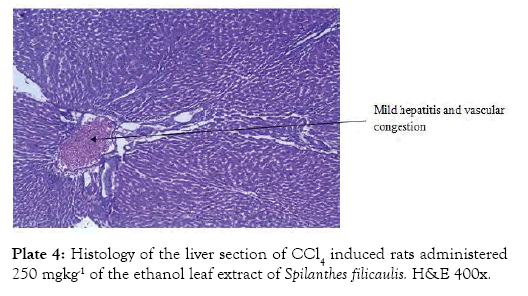
Plate 4: Histology of the liver section of CCl4 induced rats administered 250 mgkg-1 of the ethanol leaf extract of Spilanthes filicaulis. H&E 400x.
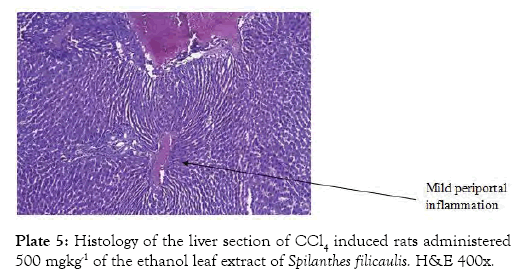
Plate 5: Histology of the liver section of CCl4 induced rats administered 500 mgkg-1 of the ethanol leaf extract of Spilanthes filicaulis. H&E 400x.
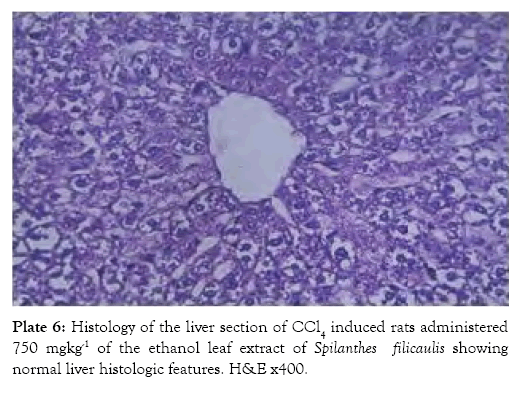
Plate 6: Histology of the liver section of CCl4 induced rats administered 750 mgkg-1 of the ethanol leaf extract of Spilanthes filicaulis showing normal liver histologic features. H&E x400
Group 7 (Plate 7) showed the micrograph of the kidney of normal control group with the interstitium been free from inflammatory cells and congestion. Group 8 (Plate 8) showed the micrograph of the kidney of the negative control group with few foci of vascular congestion and mild glomerular scleriosis been seen. Group 9 (Plate 9) showed the micrograph of the kidney of the positive control group with the renal tissue free of inflammatory cells. Group 10 (Plate 10) showed the micrograph of the kidney of CCl4 induced group that was administered 250 mg/Kg bw of ethanol leaf extract of Spilanthes filicualis, presence of mild vascular congestion been seen. Group 11 (Plate 11) Showed the micrograph of the kidney of CCl4 induced group that was administered 500 mg/Kg bw of ethanol leaf extract of Spilanthes filicualis. Renal tissue composing of the renal corpuscle made up of the glomeruli and Bowman’s capsule with renal tubules dispose within a loose connective tissue stroma. Group 12 (Plate 12) showed the micrograph of the kidney of CCl4 induced group that was administered 750 mg/Kg bw of ethanol leaf extract of Spilanthes filicualis with the interstitium free from inflammatory cells, and congestion.
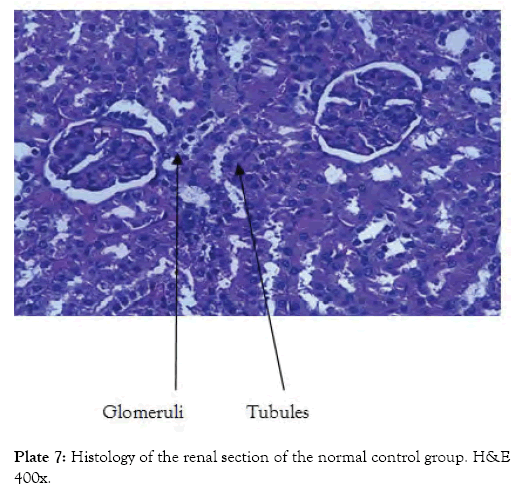
Plate 7: Histology of the renal section of the normal control group. H&E 400x.
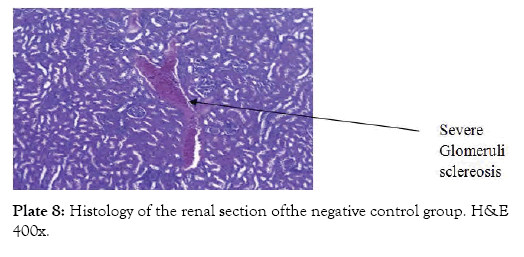
Plate 8: Histology of the renal section ofthe negative control group. H&E 400x.
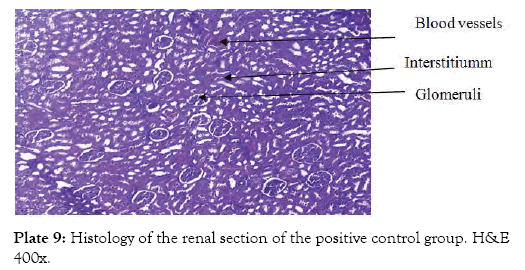
Plate 9: Histology of the renal section of the positive control group. H&E 400x.
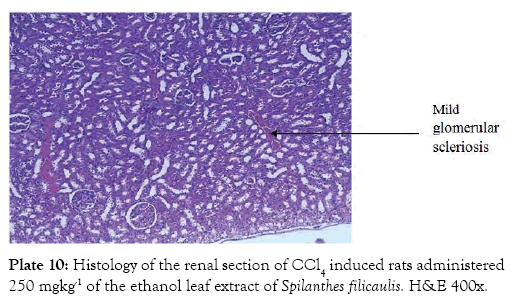
Plate 10: Histology of the renal section of CCl4 induced rats administered 250 mgkg-1 of the ethanol leaf extract of Spilanthes filicaulis. H&E 400x.
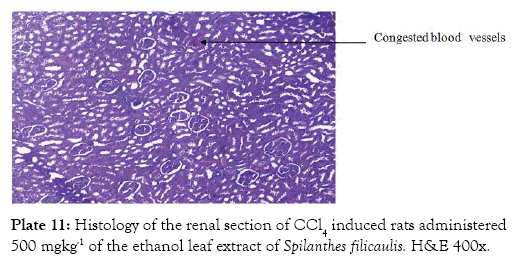
Plate 11: Histology of the renal section of CCl4 induced rats administered 500 mgkg-1 of the ethanol leaf extract of Spilanthes filicaulis. H&E 400x.
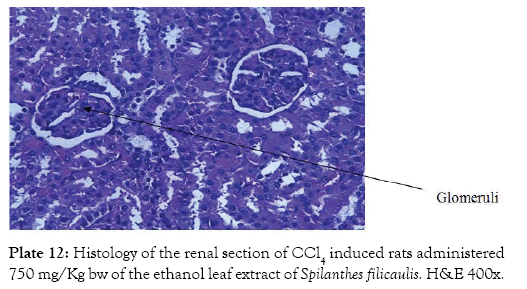
Plate 12: Histology of the renal section of CCl4 induced rats administered 750 mg/Kg bw of the ethanol leaf extract of Spilanthes filicaulis. H&E 400x.
The study investigated the effect of ethanol leaf extracts of Spilanthes filicaulis on hepatic markers, renal indicators, lipid profile, haematological indices and oxidative stress makers using carbon tetrachloride as the agent for inducing hepatic damage in male wistar rats. The present study revealed a significant increase (p<0.05) in the levels of serum AST, ALT, ALP, and GGT upon administration of CCl4. The hepatotoxicity induced by CCl4 is due to its metabolite CCl3•, a free radical that alkylates cellular proteins and other macromolecules with a simultaneous attack on polyunsaturated fatty acids, in the presence of oxygen, to produce lipid peroxides, leading to liver damage which result in leakage of hepatic enzymes [22]. Administration of ethanol leaf extract of Spilanthes filicaulis attenuated the increased activities of the serum livers enzymes, produced by CCl4 and caused a subsequent recovery towards normalization comparable to the normal control groups. The results of the findings in this present study with respect to the significant (p<0.05) changes recorded in the extract treated group is similar to the reports of Gupta et al. [3] and Saba et al. [23] in CCl4 induced hepatoxicity administered with Solanum xanthocarpum leaf extract and aqueous leaf extract of Cnidoscolus aconitifolius respectively.
Total protein level recorded a significant decrease (p<0.05) which also infers loss of liver integrity on exposure to CCl4, while levels of serum albumin and bilirubin showed significant changes. Changes in albumin levels are not observed in this study; this could be attributed to the half-life of serum albumin which last for 20 days [24]. Administration of ethanol leaf extract of Spilanthes filicaulis showed restoration to the alteration observed in total protein levels especially at dose level of 750 mg/Kg bw while serum albumin and bilirubin levels recorded no significant changes (p<0.05). This also implies that the treatment has no effect on them. Urea is the major nitrogen containing metabolic product of protein catabolism. The biochemical indices monitored in the kidney are useful ‘markers’ for the assessment of tissue damage. The significant reduction in serum urea concentration following the administration of ethanol leaf extract of Spilanthes filicaulis at various doses may be attributed to impairment on the urea cycle leading to reduced production of the metabolic product [25]. This is an indication of abnormality in the physiological excretion of urea caused by non-renal factor which is the plant extract in this case.
The consistency of endogenous creatinine production and its release into the body fluids at a constant rate is an indication of nephrotoxicity. However, clearance was observed following the administration of ethanol leaf extract of Spilanthes filicaulis at dose level of 250 mg/kg bw. Inorganic electrolytes occur in large quantities in both extracellular and intracellular fluids. Due to their ability to dissociate readily into their constituent ions or radicals, they comprise the single most important factor in the transfer and movement of water and electrolyte between three divisions of extracellular and intramuscular components [26] Serum phosphate released during cell breakdown can be used in building nucleic acids of cells. The increase in serum phosphate as observed in group 6, might suggest that the extract may help in building nucleic acids in cell [27]. The significant decrease in serum Sodium ion concentration following oral administration of the ethanol leaf extract of Spilanthes filicaulis may be due to excessive loss of heat from the body fluid. It may also be attributed to decreased production of aldosterone to other mineral corticoids which will in turns decrease the reabsorption of sodium ion concentration. Aldosterone can achieve this since its action on the membrane aldosterone receptors has been linked to stimulating Na+ /Hexchanger [28]. Serum chloride and bicarbonate ions are group of electrolytes that can be used to asses renal functions therefore, the significant increase in serum chloride and bicarbonate ions at various doses may be an indication of tubular glomerular function. It might also be that the extract induces a pathological condition resulting in impairment on renal function.
The lipid profile indicator of total cholesterol significantly increased (p<0.05) prior to treatment. CCl4 induced hepatic damage interfere with lipid metabolism by increasing the availability of acetate in the liver [29]. Acetate is a precursor for cholesterol synthesis; hence the increase in cholesterol levels associated with CCl4 toxicity [30]. However, these effects could not be reversed by the administration of the extract. Changes not observe in lipid profile (Triglyceride, HDL and LDL), renal indices (Urea and uric acid), and serum electrolytes, suggest that induction and treatment didn’t have effect on these parameters.
The slight reduction (p<0.05) in WBC following the administration of the ethanol leaf extract of Spilanthes filicaulis suggests that the extract contains some bioactive agents (like Saponin, Tannin, Alkaloids and Flavonoids) that could cause such destructed or imposed production of WBCs. It has also been reported that granulocytes, macrophage colony stimulating factor, interleukins, IL-2, IL-4 and IL-5 regulate the proliferation, differentiation and maturation of committed stem cells responsible for the production of WBC [25,28]. It may be suggested that some components of the extract reduced the production of these regulatory factors or interfered with the sensitivity of the committed stem cells (responsible for the production of WBCs.). Moreso, the reduction (p<0.05) in RBC following the administration of Spilanthes filicaulis on wistar rats is an indication that the extract might prevent RBC synthesis through the inhibition of erythropoesis in the bone marrow. This also suggests that the extract can induce anaemia possibly by causing bone marrow supression resulting in inadequate production of RBC and ultimately cell death.
Prior to treatment, there was decrease in the activities of catalase (CAT) and superoxide dismutase (SOD) levels of the negative control group as compared with the normal control group. There was also an increase in lipid peroxidation (LPO) levels and NO levels of the negative control group. Similarly, there was a significant decrease (p<0.05) in the levels of GSH of the negative control group when compared with those of the normal control as shown in Table 7. These conditions, also confirms damage to the liver [31]. Upon the administration of the ethanol leaf extracts of Spilanthes filicaulis, there were significant increases (p<0.05) in the activities of CAT and SOD of the treated groups when compared with the negative control group. This study is in line with the reports of Upadhyay et al. [32] and Ganie et al. [33]. The mode of action of ethanol leaf extracts of Spilanthes filicaulis in attenuating the damage of CCl4 induced toxicity may be due to the cell membrane stabilization and activation of antioxidant enzymes via de novo synthesis of SOD and CAT. LPO levels of the extract treated groups were significantly decreased (p<0.05) upon the administration of the extracts as compared with those of the negative control group. This shows a decrease in triacylglyceride oxidation and an eventually, proper utilization. GSH serves as a substrate for GPx and GST, primarily involved in the detoxification of toxic electrophilic metabolites via formation of GSH-conjugate [34]. During the enzymatic reaction catalyzed by GPx, GSH is oxidized to GSSG which is reduced back to GSH by Gluthathione Reductase (GR). GR therefore plays a central role in maintaining the cellular GSH level. Upon the administration of the ethanol leaf extracts of Spilanthes filicaulis, there was no significant difference (p>0.05) in the levels of GSH of the treated groups when compared with the negative control group. This could be as a result of the tripeptide conformation of the enzyme. There was significant decrease (p<0.05) in the levels of Nitric Oxide (NO) of the treated groups when compared with the negative control group. This may be due to the antioxidant property of the extract to enhance the reactive nitrogen species (RNS) scavenging activity in the liver. Spilanthes filicualis leaves contain spilanthol, which possesses anti-inflammatory activities attributed to its dual inhibition of cyclooxygenase (COX) and lipoxygenase (LOX) owing to the mimicking of arachidonic acid a precursor for prostaglandin and leukotriene synthases by spilanthol [35].
The result of the histological studies revealed that ethanol leaf extract of Spilanthes filicualis showed restoration to damage caused by CCl4 with increasing dose concentration. Remarkable restoration was seen in the group administered with 750 mg/kg of the extract and the silymarin treated group. The result of this study is in agreement with the studies carried out by Gupta et al. [3] and Ozturk et al. [36].
The study showed that the administration of ethanol leaf extract of Spilanthes filicaulis ameliorated the damage induced by CCl4 in male wistar rats on the levels of serum hepatic markers of ALT, AST, ALP and GGT. Also hepatic antioxidants markers such as CAT, SOD and NO and LPO were restored by the extract to some remarkable extent [37,38].
Catalase (CAT) and Superoxide Dismutase (SOD) levels were significantly increased (p<0.05), while Nitric Oxide (NO) and Lipid Peroxidation (LPO) levels significantly decreased (p<0.05) in all groups administered with the extract when compared with the negative control group. Gluthahione levels, lipid profile, renal indices, serum electrolyte and hematological indices showed no significant difference (p>0.05) in all treated groups when compared to the negative control. The study showed that the ethanol leaf extract of Spilanthes filicaulis has potential of restoring hepatic liver damage.
Citation: Ohwokevwo OA, Ogunka- Nnoka CU (2019) Effect of Ethanol Leaf Extract of Spilanthes filicaulis on Liver Indices and Selected Biochemicals on Carbon Tetrachloride Induced Hepatic Damage in Wistar Rats. Med Aromat Plants (Los Angeles) 8: 342. doi: 10.35248/2167-0412.19.8.342
Received: 18-Nov-2019 Accepted: 18-Dec-2019 Published: 16-Dec-2019 , DOI: 10.35248/2167-0412.19.8.342
Copyright: © 2019 Ohwokevwo OA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.