Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Review Article - (2016) Volume 4, Issue 2
The concept of modulating gut health through diet is not new and dates back to at least the beginning of the 20th century. However, it is only recently that sound scientific rationales have been proposed and investigated. Three microflora modulation tools have emerged, the addition of exogenous living microorganisms to foods (i.e., probiotics), the selective stimulation of the growth and activity of beneficial microorganisms indigenous to the gut (i.e., prebiotics), and a combination of both approaches (i.e., synbiotics). Fruit wastes which are highly perishable and seasonal, is a problem to the processing industries and pollution monitoring agencies. A valuable byproduct that can be obtained from fruit wastes is pectin. An effort was taken to extract pectin from different fruit waste (Musa sp. and Citrus limetta and rind of Citrullus lanatus and putrefied fruits of Solanum lycopersicum and Psidium guajava). An attempt was made to observe the enhancement of growth of Lactic Acid Bacteria (LAB-Lactobacillus casei, L. acidophilus, Bifidobacterium bifidum) by introducing the pectin samples from the above fruit waste. It was observed that pectin was able to enhance the growth of the bacteria and the titrable acidity considerably. Hence it can be concluded that pectin that is extracted from fruit waste can be used to enhance the growth of LAB. The current study aims to prove pectin as a potential prebiotic.
Keywords: Pectin; Musa acuminata ; Citrus limetta ; Citrullus lanatus ; Solanum lycopersicum ; Psidium guajava ; Lactobacillus casei ; L. acidophilus ; Bifidobacterium bifidum
The food industry produces large volumes of wastes, both solids and liquids resulting from the production, preparation and consumption of food. These wastes pose increasing disposal and potentially severe pollution problems and represent a loss of valuable biomass and nutrients. Thus new methods and policies are needed for waste handling and treatment in order to prevent pollution of the environment [1]. By-product recovery from fruit wastes can improve the overall economics of processing units and can reduce the environmental pollution effectively. A valuable byproduct that can be obtained from fruit wastes is pectin. Pectin designates that water soluble pectinic acid (colloidal polygalacturonic acids) of varying methyl ester content and degree of neutralization, which are capable of forming gels with sugar, and acids, under suitable condition (GITCO). It is used in pharmaceutical preparation as filler, as an agglutinated in blood therapy and also to glaze candied fruits [2]. Pectins are mainly used as gelling agents but can also be used as thickner and water binder. The classical application is giving the jelly-like consistency to jams or marmalades which would otherwise be sweet juices. For house hold use, pectin is an ingredient in jelling sugar where it is diluted to the right concentration with sugar and some citric acid to adjust the pH. In some countries, pectin is also available as a solution or extract or as a blended powder for home jam making. Pectins can also be used to stabilize acidic protein drinks, such as drinking yogurt and as a fat substitute in baked foods. Typical levels of pectin used as a food additive is about 0.5-1.0% this are about the same amount of pectin as in fresh fruit.
Pectin can also be a potential prebiotic. For a food ingredient to be classified as a prebiotic, it must neither be hydrolyzed nor absorbed in the upper part of the gastrointestinal tract; be a selective substrate for one or a limited number of potentially beneficial commensal bacteria in the colon, thus stimulating the bacteria to grow, become metabolically activated, or both; and be able to alter the colonic microflora toward a more healthier composition [3]. Although any food ingredient that enters the large intestine is a candidate prebiotic, it is the selectivity of the fermentation in the mixed culture environment that is critical. At present, most searches for prebiotics are directed toward the growth of lactic acid–producing microorganisms. This is due to their purported health-promoting properties. However, it may be that future developments in the study of prebiotics may include aspects of their effect on pathogenic flora components [4]. The term synbiotic is used when a product contains both probiotics and prebiotics. A synergy between probiotics and prebiotics is termed as a synbiotic system. The primary intention of using a synbiotic is to give a layer of protection for the bacteria during their travel through the gastrointestinal tract [3]. Hence the present study aims at extraction of pectin from peels of Citrus limetta , Citrullus lanatus , Musa acuminata , and the putrefied fractions of Solanaum lycopersicum and Psidium guajava followed by the determination of the prebiotic potential of the different extracted pectin.
Extraction of pectin
Fruit extracts from rind of Musa acuminata c.v. banana, Citrus limetta c.v. sweet lime, Citrullus lanatus c.v. watermelon, Malus domestica c.v. apple and putrefied fruits of Solanum lycopersicum c.v. tomato and Psidium guajava c.v. guava were homogenized using deionized water (1:1.5 w/v). Lemon juice was added to the homogenate to adjust the pH to 2-2.5. The homogenate was then boiled at 80°C for 15 minutes. The boiled homogenate was filtered through a cloth filter followed by Whatman filter of under vacuum. The filtrate was then treated with isopropanol (1:1) to precipitate the pectin. The precipitated pectin was then recovered from the filtrate and dried for further use.
Probiotic culture maintenance
The Starter culture of Lactobacillus acidophilus was procured from Microbial Type Culture Collection and Gene BankInstitute of Microbial Technology, Chandigarh (MTCC number 10307) and the culture of Bifidobacterium bifidum and Lactobacillus casei was procured from the Department of Dairy Microbiology, Dairy Science College UAS, Bangalore. Probiotic strains were maintained individually in sterile MRS (Himedia) (De Man, Rogosa and Sharpe) broth at 37°C for 4 hours, and then refrigerated at 4°C. Subcuturing was done in MRS media every 7 days.
Growth of LAB
Probiotic cultures were grown in MRS broth and were incubated at 37°C for 48 hrs. Growth was measured at 48 hrs and 60 hrs by optical density (660 nm) measurements. The effect of pectin on the growth of LAB was assessed by the addition of pectin (0.4%) from different fruit waste to the MRS broth maintained at different dilutions. The dilutions were plated on the MRS agar medium. All the experiments were done in triplicates.
Study of the titrable acidity
The titrable acidity of the samples was determined according to AOAC method. The milk samples (pasteurized skim milk) were curdled (6 hrs) using above LAB strains with pectin (0.4%). The curd was diluted and titrated against 0.1N sodium hydroxide, using phenolphthalein as indicator to a faint pink end point.
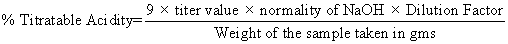
Extraction of pectin
Pectins are structural polysaccharides present within all dicotyledonous plant cell walls. The primary structural feature of pectin is a linear, 4 α linked D-Galacturonic acid chain with varying degrees of methylation. The main raw materials used to produce commercial pectin are apple pomace and citrus peel [5]. The maximum amount of pectin (dry weight) was obtained from Citrus limetta (14.49%) and least quantity of pectin obtained from Citrullus lanatus (3.41%), whereas Musa sp. and Psidium guajava has similar pectin concentration (5.57% and 5.19%) (Table 1). Simmaky and Jaanaki [6] reported the amount of pectin extracted from orange peels was 31.5 g which was equivalent to 15.25% of total orange peel weight, and the amount of pectin extracted from lemon peels was 41.5 g which was equivalent to 20.75% as total lemon peels weight which is similar to the current finding.
| Fruit Wastes | Wet Weight (gms) | Dry weight (gms) | Percentage of pectin |
|---|---|---|---|
| Solanum lycopersicum | 105 | 7.5 | 7.14 |
| Citrus limetta | 182.26 | 26.79 | 14.69 |
| Musa sp | 1409.33 | 78.6 | 5.57 |
| Citrullus lanatus | 454 | 15.5 | 3.41 |
| Psidium guajava | 1200 | 62.3 | 5.19 |
Table 1: Quantity of pectin obtained from fruit wastes.
Growth of LAB
The MRS broth culture was maintained at pH 6.0 (normal pH of the broth ranges between 5.7-6.3) and the colony forming units/ml was counted and was found to be almost similar for all the LAB samples (Table 2a).
| Sample | Colony forming unit/mL (10-4) Mean ±SD | Colony forming unit/mL (10-5) Mean ±SD | Colony forming unit/mL (10-7) Mean ±SD |
|---|---|---|---|
| L. acidophilus | 226 ±8 | 34 ±6.24 | 2 ±1 |
| B. bifidum | 281.66 ±8.08 | 20.33 ±8.50 | 2 ±1 |
| L. casei | TNTC* | 45 ±7.00 | 2 ±1 |
Table 2a: Growth of LAB at pH 6.0. *TNTC=Too numerous to count.
The optical density was measured at 660 nm, for L. acidophilus culture it was found to be 0.8 at 48 hrs of incubation and 0.77 by 60 hrs of incubation at 37°C. Whereas B. bifidum O.D. value was 0.6 at 48 hrs and 0.56 at 60 hrs of incubation. There was no significant change in the growth of LAB on incubation for 60 hrs, L. casei the OD was 1.004 at 48 hrs and 0.985 at 60 hrs. There was a reduction in the absorbance when the cultures were incubated more than 48 hrs (Table 2b).
| Sample | OD at 660nm at 48hrs of incubation | OD at 660nm at 60hrs of incubation |
|---|---|---|
| L. acidophilus | 0.8 | 0.77 |
| B. bifidum | 0.6 | 0.56 |
| L. casei | 1.004 | 0.985 |
Table 2b: Growth of LAB at 48 and 60 hrs of incubation.
The various growth responses of the bacteria to the pectin samples at various pH was also observed by Palframan et al. [7] stated that the pH at which the fermentation is carried out has a direct effect on the substrate metabolism due to the change in enzyme activity at different pH. It was found that pectin samples increased the growth of Bifidobacteria. Similar results were also reported by Olano-Martin et al. [8], they investigated the effect of various pectin and pectinoligosaccharides on Bifidobacteria which is accordance to the current study which indicates the enhancement of growth of LAB in the presence of the prebiotic pectin (Table 2c). It was observed that L. casei showed the maximum growth (2.4 OD at 660 nm) with pectin from S. lycopersicum as compared to L. acidophilus and B. bifidum . Yeo also indicated that prebiotics in the form of soy products are capable of significantly enhancing the growth of L. acidophilus.
| Sample Pectin | Probiotic Culture | O.D. at 660nm after 48 hours of incubation |
|---|---|---|
| Nil | Commercial curd | 0.6 |
| L. acidophilus | 1.8 | |
| Musasp | L. casei | 2.2 |
| B. bifidum | 0.9 | |
| C. lanatus | L. acidophilus | 1.9 |
| L. casei | 1.8 | |
| B. bifidum | 0.7 | |
| L. acidophilus | 1.9 | |
| S. lycopersicum | L. casei | 2.4 |
| B. bifidum | 1.2 | |
| L. acidophilus | 1.7 | |
| Psidiumsp | L. casei | 2 |
| B. bifidum | 0.9 |
Table 2c: Effect of pectin on the growth of LAB.
Titrable acidity of synbiotic
Lactic acid is one of major products of lactose degradation in milk and milk products due to the bacterial fermentation. Depending on the microorganisms involved, fermentation of milk proceeds via glycolysis pathway will produce lactic acid while via pentose phosphate pathway with formation of lactic and acetic acids [9]. The titrable acidity (as percentage lactic acid) of the synbiotic product ranged between 0.45 to 0.9% depending upon the type of culture (Table 3). High titrable acidity (0.94–0.95%) was observed when all three stains of LAB was cultured together with the pectin samples. There was an increase in titrable acidity of individual LAB culture when compared to the control (without pectin). The titrable acidity of individual LAB culture varied between 0.52–0.57% with the pectin. The findings corresponded to Gomes and Malcata [10] study on acid tolerance of L. acidophilus , which varied from 0.3% to 1.9% titrable acidity, with an optimum pH lying at 5.5 ± 6.0.
| Prebiotic sample | Probiotic Culture | % Titrable acidity |
|---|---|---|
| Nil | Commercial curd | 0.1 |
| Musa sp. | L. acidophilus | 0.5 |
| B. bifidum | 0.57 | |
| L. casei | 0.55 | |
| L. acidophilus+B. bifidum+L. casei | 0.95 | |
| C. lanatus | L. acidophilus | 0.54 |
| B. bifidum | 0.58 | |
| L. casei | 0.56 | |
| L. acidophilus+B. bifidum+L. casei | 0.94 | |
| S. lycopersicum | L. acidophilus | 0.54 |
| B. bifidum | 0.55 | |
| L. casei | 0.56 | |
| L. acidophilus+B. bifidum+L. casei | 0.94 | |
| C. limetta | L. acidophilus | 0.5 |
| B. bifidum | 0.56 | |
| L. casei | 0.57 | |
| L.acidophilus+B. bifidum+L. casei | 0.95 | |
| P. guajava | L. acidophilus | 0.52 |
| B. bifidum | 0.57 | |
| L. casei | 0.55 | |
| L. acidophilus+B. bifidum+L. casei | 0.95 |
Table 3: Titrable acidity of the Synbiotic.
Pectin is already being used in microencapsulation of probiotics [11] in order to create more effective delivery systems for the probiotic bacteria. Further understanding of the protective mechanisms conferred by pectin will further enhance the numerous beneficial effects of probiotics. The results of the current study suggests that natural pectin from fruit sources can be used as an efficient prebiotic.