Advances in dairy Research
Open Access
ISSN: 2329-888X
ISSN: 2329-888X
Research Article - (2017) Volume 5, Issue 2
Keywords: Green tea; Moringa oleifera ; Yoghurt; phenolic content; Antioxidants; Texture
Milk is the main complete nourishment on the planet. Right now researches have been aimed towards generation of functional foods that advance human health and prosperity [1]. Yogurt enhances the diet quality by boosting the chances of approaching the recommended nutritional guidelines and improves lactose tolerance in individuals suffer from hypolactasia. Consumption of yogurt can enhance immune modulation linked with a lowered incidence of diseases such as cancer, gastrointestinal disorders and allergic symptoms. This can improve cytokine and antibody production and phagocytic and natural killer cell activities [2]. Green tea is produced using leaves of Camellia sinensis that have experienced negligible oxidation during production. Many studies proposing that consistent tea consumption may have a lower risk of creating coronary heart diseases and cancer. Polyphenols found in tea are generally flavonoids which have anti-oxidative and anti-carcinogenic effects. Furthermore, green tea showed to protect skin from UV damage, control body weight and intestinal dysbiosis [3].
Moringa oleifera is a drumstick tree of Moringaceae family, known as sahinjan in Hindi; it is a local of India and now developed broadly in numerous south eastern Asian regions. It's commonly considered as wound healing, antitumor, antifertility, hypotensive, antipyretic, antihepatotoxic, antiepileptic, anti-inflammatory, antiulcers, diuretic, hypocholesterolemic , antifungal, antibacterial, anti-cardiovascular agent, and reducing hyperglycemia [4,5].
Medical advantages of medicinal herbs have attracted food researchers to use it in designing novel functional dairy products and evaluate their impact on product quality and consumer acceptability [6,7].
The present investigation was thus carried out to identify the major biologically active components in green tea and Moringa oleifera leaves extracts using GC-MS. Also, yogurt was fortified with either leaves extract and their effects on physicochemical, rheological, sensory, antioxidant characteristics of bio-yoghurts were determined.
Materials
The following ingredients used to manufacture yoghurt trails were pasteurized/ homogenized milk 3.7% fat (Labanita, Co, Egypt), full cream milk powder (FCMP; Arla, UK), skim milk powder (SMP; Fonterra, New Zealand), whey protein concentrate (WPC, Altroika, Turkey), stabilizer (Mefad, Egypt), gelatin (MashrQ, Brazil), green tea (Ahmed Tea, Sri Lanka) and moringa leaves powder (Agriculture Research center El Doki, Ministry of Agriculture, Egypt). Starter used for manufacture of yoghurt was ThermophilicYoFlex® which composed of Streptococcus thermophiles and Lactobacillus delbrueckii subsp. bulgaricus cultures (Chr. Hansen, Denmark).
GC-MS analysis of green tea and Moringa leaves extracts: The dried leaves were crushed in a blender and then extraction was performed in 1000 ml Erlenmeyer flasks by mixing (1 gm to 20 ml ethanol 80%). Extraction is done in a water bath at a boiling point of ethanol for 2 hr. The extract was evaporated in an oven at temperature of 50°C to obtain concentrate. The concentrate was freeze dried to form granules [8]. Qualitative analysis of compounds in plant extracts was performed with Thermo scientific™ TSQ™ 8000 EVO Triple quadrupole GC MS/MS. The mass spectrometer paird with the Thermoscientific™ TRACE™ 1300 GC Equipped with TG5MSGC column ; carrier gas was helium with flow 1 ml/ min; 1 ml was injected ( split ratio1: 24); injector temperature was 260°C; ion source was set at 200c transfer line temperature 205°C. The mass spectrometer was operated in the electron impact mode using 70ev electron energy. The mass rang m/z 33-500 was scanned. The identification of the peaks was based on Willy9 and minilab, further, the spectra was determined on NIST5®.
Determination of minerals: Minerals (Fe, Mg and Ca) in plant leaves were determined in ash samples using Atomic Absorption Spectroscopy (Thermo® M series) according to AOAC [9]. The determination was done by measuring the absorption of light by atoms of the element reminds in the fundamental state when they are illuminated by a suitable source of light. The measurement of light intensity is made at a specific wavelength of the element to be determined.
Preparation of green tea and Moringa oleifera leaves extracts: The plant derived aqueous extract used in this study was prepared in our laboratory according to Najgebauer-Lejko et al. [10] with some modifications. One gram of plant leaf powder was mixed with 10 mL of boiling dist. water and held for 5 min. The mixture was then filtered twice through cheese cloths then through Whatman® No. 40. Filtrated extracts transferred into a sterile tube. The aqueous extract stock solution (100 mg/mL) was freshly prepared for each set of experiments and stored at 4°C for up to 5 days.
Manufacturing of yogurts: Milk for yogurt preparation was standardized using 500 g of fresh homogenized pasteurized milk, 64 g full cream milk powder (FCMP), 25 g skim milk powder (SMP), 10 g whey protein concentrate (WPC), 2.5 g stabilizer, 1 g gelatin and complete volume to 1 kg with sterile water (Figure 1). In fortified yoghurt trails stock plant extracts (10% w/w) were added to yoghurt mix to obtain 1% and 0.9% (w/w) of green tea and moringa extract in the final products, respectively. The yoghurt treatments were prepared using Vorwerk® Thermomix with a capacity of 1.5 liter (Vorwerk®, France). The mixture was heated at 95°C/10 min [11], cooled to 43°C and inoculated with starter culture as recommended by the manufacturer. Green tea and moringa extracts were added to inoculated milk and poured into100-mL sterile plastic cups. The incubation proceeded @ 43°C ± 0.5°C. After achieving a pH of 4.8 (4 hr), yogurts were cooled and stored @ 7°C for 15 days (Figure 1).
Chemical analysis: Total solids and fat percent were determined according to [9] AOAC (2003). The changes in pH values were measured using pH meter (model 8417N, HANNA instrument). Total protein was determined using Kjeldahl Semi-atomized (Foss model 8100 Dairy Analyzer, Denmark), total crud fibers were determined according to Joslyn [12].
Microbiological analysis: The count of coliforms, yeast and molds and monitoring of starter culture whether during production or at cooled storage was carried out on Violet Red agar (35°C), Dichloran Rose-Bengal chloramphenicol agar (25°C) and MRS agar (37°C) respectively [13,14]. L-MRS and M17 agar were used to enumerate lactobacilli and streptococci respectively. All media were prepared and used according to the manufacturer instructions (Oxoid, Hampshire, UK). The yeast and molds group were determined using surface plating technique while all other microbial groups were enumerated using the pour plating technique. Only L-MRS agar plats were incubated anaerobically using gas generating kit (Oxoid, Hampshire, UK).
Rheological evaluation: Viscosity of yoghurt was analyzed using viscometer VR 3000 (Viscotech Hispania, El Vendrell, Spain). Texture profile analysis (TPA) was carried out according to the method of Sanchez and Perez, [15] using the Texture Analyzer TA.XT plus (Stable Micro Systems, UK). The operating conditions consisted of: back extrusion Rig 35 mm, disc probe max recommended load 2 kg, temperature 5°C, contact force 1 gm, post-test speed 10.0 mm/s and distance 5 mm. The following parameters were calculated: viscosity (cp), firmness (g), consistency (g/s), cohesiveness (ratio). Firmness and cohesiveness were related with, respectively, maximum and minimum force obtained during extrusion of the yogurt sample through the annular gap between the probes and jar wall, whereas consistency and index of viscosity are defined as the, respectively, positive and negative area of the force vs. time curve. Cohesiveness indicates the resistance of the sample to withdrawal from the extrusion disc being lifted; consistency indicates the thickness of the sample. Measurement of syneresis in samples was carried out using the Siphon method as described by Amatayakul el al. [16]. Syneresis percent was calculated using the following formula
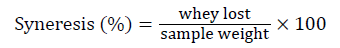
Color
The color reflectance measurement was determined using UltraScan  VIS (HunterLab, VA, USA) following the operating instructions of the manufacturer. The following parameters were defined: L * (lightness; ranging from black (0) to white (100)), a * (red saturation index; +a*=red, -a*=green) and b * (yellow saturation index; +b*=yellow, -b*=blue). The equipment was calibrated against a stander 2 (L=99.43, a=-0.11 and b=0.04) at the beginning of determination.
VIS (HunterLab, VA, USA) following the operating instructions of the manufacturer. The following parameters were defined: L * (lightness; ranging from black (0) to white (100)), a * (red saturation index; +a*=red, -a*=green) and b * (yellow saturation index; +b*=yellow, -b*=blue). The equipment was calibrated against a stander 2 (L=99.43, a=-0.11 and b=0.04) at the beginning of determination.
Total phenolics content and antioxidant activity: The total phenolic content in plant extracts or fortified yoghurts was determined by using the Folin-Ciocalteu assay (FCA) according to Lim et al. [17]. The total phenolic content was expressed as mg Gallic acid Equivalents (GAE mg/ 100 g sample).
Antioxidant activity was evaluated by DPPH according to Brand- Williams et al. [18]. A portion of sample (0.3 mL) was added to DPPH (2.7 mL; 0.1 mmoL). After 30 min of incubation in the dark, the absorbance was measured at 517 nm. Antioxidant inhibition percent was calculated from the following equation:
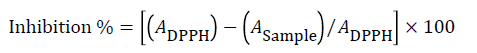
Where: ADPPH is sample control absorbance (DPPH solution); A Sample is sample absorbance. Radical scavenging capacity was expressed as mg ascorbic acid equivalents per 100 g sample (AAE mg/100 g sample). The IC50 value is define as the sample concentration (mg/ml) contained mg AAE give 50% inhibition of DPPH free radicals.
Sensory evaluation: Sensory evaluation was carried out by a panel consisting of 10-15 yoghurt graders, including staff members and assistants at the Faculty of Agriculture, Alexandria University and specialists in the field of dairy. Each panel member assessed the yoghurts separately; using the hedonic scale method as described by Bodyfelt et al. [19] taking into account the evaluation of color, appearance, flavor, smell and overall which were scored 1-10 point hedonic scale (1-2=extremely poor, 3-4=poor, 5-6=fair, 7-8=good, 9-10=excellent).
Statistical analysis
The obtained data were presented as mean ± standard deviation and subjected to ANOVA to evaluate the effect of plant extracts addition and storage time. The test of significance was determined on the basis of Duncan’s test at p<0.05 probability using the SPSS statistics® 13 software.
Chemical composition of green tea and moringa leaves powder
Table 1 represents the chemical analysis of green tea and Moringa oleifera leaves powder. Data indicated that green tea leaves powder contains less protein, higher total fiber, less calcium and ferric than Moringa oleifera leaves powders. Similar gross composition of green tea was reported by Adnan et al. [20]. Varied range of minerals concentrations were observed in five types of Japanese green tea [21]. The results of moringa chemical composition leaves agree with those of Moyo et al. [22] and Ogbe and Affiku [23].
| Plant leaves | Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | Total Fibers (%) | Fat (%) | Protein (%) | Moisture (%) | pH | Minerals (mg/100gm) | |||
| Fe | Mg | Ca | |||||||
| Green tea | 5.81 ± 0.1 | 22.0 ± 0.1 | 0 | 12 ± 0.02 | 3.57 ± 0.03 | 5.81 ± 0.1 | 3.5 ± 0.02 | 54 ± 0.1 | 360 ± 0 |
| Moringa | 4.8 ± 0.2 | 11.28 ± 0.05 | 1.9 ± 0.02 | 24.7 ± 0.02 | 8.81 ± 0.01 | 4.8 ± 0.2 | 24.5 ± 0.1 | 59 ± 0.01 | 450 ± 0 |
Table 1: Chemical analysis of green tea and Moringa oleifera leaves powder.
GC-MS analysis of green tea leaves
Table 2 illustrates the 21 identified phytochemical compounds by GC-MS in water-ethanol extract of green tea leaves powder. The most abundant compounds were 2,3-dihydroxysuccinic acid; DL-tartaric acid (50%), 2-bromotetradecanoic acid; myristic acid (8.7%), 5áandrostan- 16-one, storied (4%), 2,6-dichloro-1,5-napthoquinone; quinone (3.44%), acetyl-iso-codeine; alkaloids (2.72%) and octadecatrienoic acid; linolenic acid (2%). These compounds improve beverage and food taste and it has impact on heath as antioxidant, anti-inflammatory, anti-atherosclerosis and antimicrobial agents. Other different classes of organic compounds at low concentration included ester (#4), pyridine (#7), ketone (#12), and sugar (#17) were detected as shown in Table 2. Similar results were also reported by Ganesh and Vennila [24] and Afzal et al. [25]. Changes in extraction phase resulted in different spectrum. The GC-MS analysis of liquid layer of extracted Japanese green tea leaves using water-methanolchloroform (1:2.5:1) led to identify seventy-one compounds, including sugars, amino acids, and organic acids [26].
| # | Compound | MW | Area % |
|---|---|---|---|
| 1 | 2-Bromotetradecanoic acid | 306 | 8.7 |
| 2 | 2,3-Dihydroxysuccinic acid | 150 | 50.94 |
| 3 | 5á-Androstan- 16-one,cyclic ethylenemercaptole | 350 | 4.01 |
| 4 | Benzeneacetic acid,á,4- bis [(trimethylsilyl)oxy]-, trimethylsilyl ester | 384 | 1.54 |
| 5 | 3-Formyl-N-methyl-9- [phenylethynyl] dibenzo [2,3-a : 5,6-a] (1,4)-thiazine | 341 | 3.23 |
| 6 | 9,12,15-Octadecatrienoic acid,2-[(trimethylsilyl)oxy]-1- [[(trimethylsilyl) oxy]methyl ester, (Z,Z,Z)- | 496 | 2 |
| 7 | 3-Pyridinecarboxylic acid,2,7,10-tris(acetyloxy)-l,la, | 597 | 1.22 |
| 2,3,4,6,7,10,11,11a-decahydro-1,1,3,6,9-23 | |||
| 8 | Acetyl-Iso-Codeine | 343 | 2.72 |
| 9 | 4,8-Bis(2-propylamino)-2,6-dichloro-1,5-naphthoquinone | 340 | 3.44 |
| 10 | Dimethoxy glycerol docosyl ether | 460 | 1.96 |
| 11 | 9-Acetoxy-1-methyl-8-propyl-3,6-diazahomoadamantane | 266 | 1.17 |
| 12 | Deoxyherqueinone | 356 | 2.11 |
| 13 | βN-acetylneuramic ME ester-2-ME-7,9-ME-boronate- 3,8-Ditms | 505 | 1.48 |
| 14 | 6-Amino-5-cyano-4-(5-cyano-2,4-dimethyl-IH-pyrrol-3-yl)-2-methyl-4H- pyran-3-carboxylic acid ethyl ester | 326 | 1.04 |
| 15 | Int-1-phenyl-1-(2-amino-4-methylphenyl)ethylene | 209 | 2.18 |
| 16 | Acetic acid, 3-acetoxy-6-(2-cyanovinyl)-3a,6-dimethyl-2,3,3a,4,5,5a,6,9,9a,9b-decahydro- 1 Hcyclopenta[a]naphthalene-7-ylmethyl ester | 385 | 2.32 |
| 17 | á-D-Galactopyranoside, methy12,3-bis-O-(trimethlsilyl)-,cyclic butylboronate | 404 | 1.77 |
| 18 | 2,4-Octadienoic acid, 9a-(acetyloxy)-1a,1b,4,4a,5,7a,7b,8,9,9a-decahydro-4a,7b-dihydroxy-3-(hydroxymethl)-1,1,6,8-tetramethyl-5-oxo-1H-cyclopropa[3,4]benz[1,2-e]azulen-9-ylester | 528 | 1.56 |
| 19 | Glycine,N-[(3á,5á, 7á, 12á)-24-oxo-3,7,12-tris[(trimethylsilyl)oxy]cholan- 24-yl]-, methylester | 695 | 1.82 |
| 20 | 1,2-Bis[1-(2-hydroxyethyl)-3,6- diazahomoadamantantydene-9]hydrazine | 416 | 1.59 |
| 21 | 4,6-Dimethyl-3’acetoxy-2-benzylidene coumaran 3-one | 308 | 1.08 |
Table 2: Identified major phytochemical compounds by GC/MS/MS in water/ ethanol extract of green tea leaves powder.
GC-MS analysis of Moringa oleifera leaves
The main constitutes of Moringa oleifera leaves extracts are shown in Table 3. The GC-MS results indicated the presence of 27 compounds with different concentration. The constituent compounds in the leave extract are steroidal compounds, long chain aliphatic carboxylic acids (saturated and unsaturated) and their derivatives including alcohols, aldehyde and in addition benzene carboxylic acid ester, flavone and carotenoid compounds. The major compounds found were dl- Glyceraldehyde; glycerol; trios monosaccharide (12%), Estra- 1,3,5(10)-trien-17á-ol; steroid (11%), and pregn-5-en-20-one; steroid (2.26%). Solvent system used in extraction process can affect the organic compound profile. A portion of 16 compounds were identified in methanolic extract. These compounds comprise mainly hydrocarbons, fatty acids, alcohols, esters and phenols [27]. Extraction in ethyl acetate identified 28 compounds. This analysis revealed to contain the presence of linalool oxide, upiol, adenine, palmitic acid [28]. In the present study, the presence of various classes of bioactive chemical constituents in ethanolic extract was confirmed. It is currently pertinent to recognize the possible role of these constituent substances in the therapeutic properties credited to the moringa. In clinical trial fatty acids included lauric acid (dodecanoic), palmitic acid (teradecanoic), myristic (hxadecanoic) and stearic (octadecanoic) acid showed potential antibacterial and antifungal effects [29]. DL glyceraldehyde showed potential as carcinogenic agent. It was found to be more active against Ehrlich ascites tumor [30]. Steroid compounds have a similar structure in basic and are extremely specific [31]. Alkaloids, such as Uleine, play an important role as AChE inhibitors [32]. Application of acetylcholinesterase (AChE) inhibitors is the primary treatment for Alzheimer's disease [33]. Moringa leaves contain different pyrrole formula (Table 3 #22). A pyrrole is a basic chemical structure that is used in the formation of hem, which makes bloodred. Several derivatives of carotenoids have detected. The antioxidant role of carotenoids is reviewed as a potent antioxidant, in protecting human body against oxidative stress that may result in degenerative diseases [34].
| # | Compound | MW | Area % |
|---|---|---|---|
| 1 | Estra- 1,3,5(10)-trien-17á-ol | 256 | 11 |
| 2 | dl-Glyceraldehyde | 90 | 12 |
| 3 | Torosaflavone B | 430 | 1.55 |
| 4 | Azafrin | 426 | 1.48 |
| 5 | 9,12,15-Octadecatrienoic acid , 2-[(trimethylsilyl)oxy]-1-[ [(trimethylsilyl) oxy] methyl]ethyl ester, (Z,Z,Z)- | 496 | 1.4 |
| 6 | .psi.,.-Carotene, 3,3’,4’,4’,tetradehydro- 1,1’- dimethoxy- 2,2’-dioxo- | 624 | 1.1 |
| 7 | Pregn-5-en-20-one,3-(acetyloxy)-16,17-epoxy-6-methyl-, (3á,16á)- | 368 | 2.26 |
| 8 | 2,7-Diphenly-1,6-dioxopyridazino[4,5-2’,3’] pyrrolo[4’5’-D]pyridazine | 355 | 1.7 |
| 9 | Acetic acid, 3-acetoxy-6-(2-cyanovinyl)-3a,6-dimethyl-2,3,3a,4,5,5a,6,9,9a,9b-decahydro-1Hcyclopenta[a]naphthalen-7-lmethyl ester | 385 | 1.1 |
| 10 | 5,7,9(11)-Androstatriene,3-hydroxy-17-oxo- | 284 | 1.24 |
| 11 | á-D-Glucopyranoside, methy12- (acetylamino)-2-deoxy-3 -O-(trimethylsilyl)-, cyclic methylboronate | 331 | 1.6 |
| 12 | (5á)Pregnane-3,20á-diol,14á,18á-[4-methyl-3-oxo-(1-oxa-4-azabutane-1,4-diyl)]-, diacetate | 489 | 1.2 |
| 13 | Benzoic acid,2,4- bis[(trimethylsilyl)oxy]-,trimethylsilyl ester | 370 | 0.7 |
| 14 | 1,1,3,3,5,5,7,7,9,9,11,11,13,13,-tetradecamethyl heptasiloxane | 504 | 1 |
| 15 | 9,12,15-Octadecatrienoic acid, 2,3-bis [(trimethylsilyl)oxy]propyl ester, (Z,Z,Z)- | 496 | 1.3 |
| 16 | 5-(4-Chlorophenyl)-3-(3-phenylsydnon-4yl)-IH-[1,2,4]triazole | 339 | 0.7 |
| 17 | 1-Pentene, 1,3-diphenyl-1-(trimethylsilyloxy)- | 310 | 1.6 |
| 18 | Uleine, 1,13-dihydro-13-hydroxy- | 266 | 1.6 |
| 19 | Dihydrositsirikine | 356 | 1.33 |
| 20 | Benzoicacid, 4-methyl-, [methoxycarycarbonyl)phenyl] methyl ester | 284 | 1.4 |
| 21 | Stearic acid, 3-(octadecyloxy)propyl ester (CAS) | 594 | 1.1 |
| 22 | 3,5-Diphenyl-2-(3’,4’-dimethoxyphenyl)-pyrrole | 355 | 1.2 |
| 23 | 1h-pyrrol-3,4-diacetic acid | 341 | 1.2 |
| 24 | 2,4,8,10-Tetradecapenteanoic acid | 606 | 2.8 |
| 25 | 2-Morpholinoethanesulfonic acid | 437 | 1.5 |
| 26 | Benzoic acid, 4(1-hydroxy-3-phenl1-2propynyl)-, ethyl ester | 341 | 1.1 |
| 27 | 9,12,15-Octadecatrienoic acid,2,3-bis[(trimethylsilyl)oxy] propyl ester, (Z,Z,Z)- | 496 | 1.3 |
Table 3: Identified major phytochemical compounds by GC/MS/ MS in water/ ethanol extract of Moringa oleifera leaves powder.
Chemical analysis of yoghurt
The chemical analysis of milk yoghurt mixture used throughout the study revealed the presence of 14.45 ± 0.15% total solid, 3.75 ± 0.15% protein, and 3.65 ± 0.03% fat. Mean values of pH, acidity and freezing point of the mixture were 6.66 ± 0.01, 0.19 ± 0.01% and 0.693 ± 0.01, respectively. There were no significant differences found among trails (P<0.5). No changes in the total solid, protein and fat were observed in yoghurt treatments during the storage (15 d @ 7°C). The pH was decreased during incubation reached 4.60 ± 0.05 after incubation while it changed but not significantly during storage. The titratable acidity of yoghurt showed inverse relationship to pH as it was increased to be 0.70 ± 0.02% at day one and continued to increase to level to 1.1 ± 0.05% at the end of storage.
Microbiological quality of yoghurt
The counts of coliforms and yeast and molds in yoghurt trails were found to be <10 cfu/g and reminded so even after 15 days of storage.
Effect of green tea and moringa leaves on starter culture
Figure 2a and 2b show that fortification of yoghurt either with green tea or moringa extract had a little stimulated effect, but not significant on the growth of starter cultures. On the other hand, the pH was gradually decreased from 6.5 to 4.80 during the fermentation period (4 hr) in all trails. Moreover, no significant differences were detected among treatments. At the end of storage the pH values were gradually decrease up to 4.3, 4.31 and 4.28 in control, green tea and moringa yoghurts, respectively. Also, no significant differences were observed among treatments. Starter numbers (log10cfu/g) were increased during fermentation and it were gradually decreased during storage period at the same trend among all trails. At the end of fermentation the starter count in the green tea treatment (log10 8.98) was higher than the moringa (log10 8.91) and plain yoghurts (log10 8.9). At the end of storage, the starter count in the treatments were higher than log10 7 with a highest level in green tea yogurts followed by moringa then plain yoghurts. In agreement with our findings Najgebauer-Lejko et al. [10] observed that the green tea infusion in yoghurt showed a positive effect on the count of L. bulgaricus and S. thermophiles in compared with the plain yoghurts, with no significant differences between the types and levels of tea used. Moreover, the addition of green tea powder (0.5%) did not significantly influence the growth and survival of starter during production and refrigerated storage of yoghurts [35]. This indicates the effect of the added plant formula which might enhance beneficial consequence to the functional products. In case of moringa in yoghurt, it is found that the addition of moringa leaves powder (0.5%) during yoghurt manufacture did not alter the acidification profile of yoghurt this reflects that the starter was not affected by the fortification [36].
Effect on syneresis
Generally in all the treatments, syneresis values (%) showed to increase up to 15 days of storage (Table 4). These results are in agreement with those reported by Ramirez-Santiago et al. [37] and Rao [38] who found that the syneresis increased with the extent of refrigerated storage yoghurt supplemented with either plant extracts gave a contradiction pattern of syneresis. The green tea yoghurts had a lower syneresis values compared to plain yoghurt whereas the addition of moringa leaves extract resulting in increasing syneresis (p<0.05). Amirdivani and Baba [39] observed the same effect of green tea extract (2%) on yoghurt syneresis which they explained by the shorter time for green tea yogurts than plain yogurts to reach pH 4.5 and this may result in the reduction in whey separation as compared to plain yogurt. Moreover, Donmez et al. [40] suggested that the effect of green tea extract on the syneresis rate was concentration dependent. The extract decreased syneresis rate when it was added at 0.02%, but it caused an increase when added at 2%. These differences between results might reflect the effect of plant source and species, extraction procedure or differences in total solids of the yogurts. In the present study moringa extract was more acidic with a higher calcium and fiber content than green tea extract (Table 1) these factors my influence the yoghurt curd tension caused higher syneresis.
| Time (days) | Treatments | ||
|---|---|---|---|
| Plain yoghurt | Green tea yoghurt | Moringa yoghurt | |
| 1 | 1.99 ± 0.14b,B | 1.805 ± 0.007b,B | 2.87 ± 0.024c,A |
| 5 | 2.365 ± 0.049b,B | 2.215 ± 0.021c,B | 3.25 ± 0.070b,A |
| 15 | 2.98 ± 0.028a,B | 2.8 ± 0.014a,C | 3.795 ± 0.021a,A |
| Mean values of n=3 ± Standard Deviation. a-cMeans followed by different superscript letters in each column indicate significant differences at p<0.05. A-CMeans followed by different superscript letters in each row indicate significant differences at p<0.05. | |||
Table 4: Effect of the addition of green tea or moringa leaves extract to yoghurt on the syneresis during storage.
The addition of fiber increased syneresis in low-fat yoghurts [41] while calcium concentration is considered one of the main factors could affect syneresis [42]. There is no information on the literature concerning the effect of moringa extract on syneresis in yoghurts and soft-cheese.
Texture analysis
The textural features of yoghurt samples are presented in Table 5. The apparent viscosity of yogurt treatments did not show a significant difference (p<0.05) at the beginning or at the end of storage as compared with plain yoghurt (control). In except of green tea yoghurt, refrigerated storage has not affected the initial viscosity of plain and moringa yoghurts. It is reported the addition of 2% green tea extract in yoghurt decreased apparent viscosity [39]. These observations indicate that the effect of plant extracts is plant type and concentration dependent. Table 5 revealed that in fresh yoghurt firmness values did not also alter by the addition of either plant extract. No significant change in the firmness was observed during storage up to 15 days in plain and moringa yoghurt while it decreased in green tea yoghurt. Izadi et al. [43] found that yoghurt added phytosterol (β-sitosterol) had a higher firmness compared to control. The results in Table 5 show that the consistency (g.s) of plain and moringa yoghurts were lower than those of green tea yoghurt. However, the consistency had not changed in green tea yoghurts during storage it is increased in plain and moringa yoghurts.
| Parameter (s) | Time (days) | Treatments | ||
|---|---|---|---|---|
| Plain yoghurt | Green tea yoghurt | Moringa yoghurt | ||
| Viscosity (cp) | 1 | 20236 ± 189.50a,A | 20201 ± 19.79b,A | 19809 ± 132.93a,A |
| 5 | 20533 ± 128.69a,A | 20400 ± 33.94b,A | 20224 ± 108.89a,A | |
| 15 | 20712 ± 678.82a,A | 20699 ± 106.06a,A | 20319 ± 165.46a,A | |
| Firmness (g) | 1 | 329 ± 11.31a,A | 319 ± 1.41b,A | 313 ± 19.79a,A |
| 5 | 338 ± 7.07a,A | 329 ± 4.24ab,A | 326 ± 1.41a,A | |
| 15 | 347 ± 4.24a,A | 340 ± 7.07a,A | 339 ± 5.65a,A | |
| Consistency (g.s) | 1 | 8687 ± 9.89c,B | 8995 ± 98.99a,A | 8914 ± 35.35c,AB |
| 5 | 9210 ± 42.42b,B | 9266 ± 247.48a,A | 9210 ± 42.42b,B | |
| 15 | 9629 ± 85.55a,A | 9632 ± 43.84a,A | 9558 ± 69.29a,B | |
| Mean values of n=3 ± Standard Deviation. a-cMeans followed by different superscript letters in each column indicate significant differences at p<0.05. A-BMeans followed by different superscript letters in each row indicate significant differences at p<0.05. | ||||
Table 5: Rheological consistent of yoghurt fortified with green tea or moringa leaves extract during storage period.
Color analysis
Table 6 illustrates color reflectance values of plain (control), green tea and moringa yoghurts. Color measurement showed a significant difference of color between different kinds of yoghurts. Supplementation with plant extracts, L* values decreased while b* values increased, demonstrating that fortified yoghurts were darker and yellowish compared to plain yoghurts. The analysis of a* values showed contradictory effect between green tea and moringa yoghurts whereas the former was redder and the latter was greenish compare to plain yoghurt.
| Treatments | Color values | ||
|---|---|---|---|
| L* | a* | b* | |
| Plain yoghurt | 88.24 ± 0.05a | -0.30 ± 0.01b | 11.29 ± 0.04b |
| Green tea yoghurt | 83.40 ± 0.10c | -0.05 ± 0.02c | 13.99 ± 0.11a |
| Moringa yoghurt | 86.19 ± 0.04b | -0.37 ± 0.01a | 13.82 ± 0.02a |
| a*- negative and positive values indicate, respectively, green and red. b*- negative and positive values indicate, respectively, blue and yellow color. L*=Lightness on a scale from o (blank) to 100 white. Mean values of n=3 ± Standard Deviation. a-cMeans followed by different superscript letters in each column indicate significant differences at p<0.05. |
|||
Table 6: Color values of the fortified yoghurt supplemented with green tea or moringa leave extracts at day one.
In agreement Najgebauer-Lejko et al. [44] reported that the addition of tea infusion to yoghurt significantly and in a concentration dependent manner lowered the lightness while a* shifted to positive range with higher values of b* color parameter. In raw and cooked patties added of green tea powder increased intensity of red color [45]. The yoghurt produce with the addition of moringa in powder were darker, reddish and yellowish compared to present results [36].
Phenolic content and antioxidant activity
Data in Table 7 represent the phenolic content and antioxidant properties of green tea and moringa leave extracts and their yoghurt. The total phenols (mg gallic acid equivalents GAE /100 g) was higher in green tea extract (712.12 GAE /100 g) followed by moringa extract (280.65 GAE/ 100 g). In a study on 51 kinds of herbal and tea infusions made in China were measured by the Folin-Ciocalteu method it is found that the total phenolic contents varied from 0.32 to 13.95 g gallic acid equivalent /100 g [46]. It is reported that decoction of dried leaves by boiling with distilled water resulted in total phenols amounted to 2.61 g chlorogenic acid equivalent/ 100 g dry weight [47] while lower results were also found [48].
| Sample | Total phenols (mg gallic acid equivalents; GAE/ 100g) | DPPH Radical scavenging activity (Inhibition %) | IC 50 (µg/ml) |
|---|---|---|---|
| Green tea extracts (10%) | 712.12 ± 4.10a | 95.50 ± 0.98a | 0.064 ± 0.00d |
| Moringa extract (9%) | 280.65 ± 3.05b | 81.30 ± 1.60b | 0.065 ± 0.00d |
| Plain yoghurt (control) | 9.00 ± 0.16e | 54.70 ± 0.565d | 1732.99 ± 0.47a |
| Green tea Yoghurt | 31.86 ± 0.34c | 80.60 ± 0.99b,c | 554.20 ± 7.07c |
| Moringa yoghurt | 18.31 ± 0.32d | 78.00 ± 0.85c | 937 ± 6.38b |
| Mean values of n=3 ± Standard Deviation. a-dMeans followed by different superscript letters in each column indicate significant differences at p<0.05. | |||
Table 7: Phenolic content and antioxidant properties of moringa and green tea water leave extracts, plain yoghurt and yoghurts supplemented with plant leave extracts day one.
The plain yoghurt contained only 9.00 GAE/ 100 g while, fortification with green tea or moringa extracts had increased significantly the total phenolics by 254% and 103% respectively. In comparable with the present results, Najgebauer-Lejko et al. [10] found that the level of tea polyphenols in green tea yoghurts ranged from 36.7 to 111.7 mg GAE in 100 g and was concentration dependent.
In DPPH assay green tea and Moringa oleifera leaves extracts reduced DPPH radicals significantly as compared to the control (p<0.05) while the effect of green tea was superior than moringa (Table 7). In regard to IC50, the green tea and moringa extracts showed very closer value of 64 and 65 μg/ml, respectively. Addition of extracts in yoghurt elevated the inhibition% to be 80.6% for green tea yoghurts and 78% for moringa yoghourts in compared to 54% of plain yoghurts. Higher values of inhibition% were reported when yoghurt was produced by direct acidification without starter inoculation and 2% of green tea extract was added [48]. In similar manner the IC50 of green tea yoghurts was lower than moringa yoghurts with a biggest value noticed in plain yoghurts.
Sensory evaluation
In the preliminary study yoghurts were made using plant extract ranged from 0.5-1.5% (w/w) intervals of 0.1% using a stock extract (10%) and evaluated by panelists to choose the best concertation this resulted in applying of 1 and 0.9% of green tea and moringa extract respectively. The preliminary results revealed that the threshold choice was to apply 1% green tea and 0.9% moringa. Sensory evaluation of the yoghurt samples are presented in Table 8 and Figure 3.
| Parameter (s) | Time (days) | Treatments | ||
|---|---|---|---|---|
| Plain yoghurt | Green tea yoghurt | Moringa yoghurt | ||
| Appearance | 1 | 9.20 ± 0.36a,A | 7.17 ± 0.68a,B | 8.45 ± 0.41a,A |
| 15 | 8.20 ± 0.60a,A | 6.80 ± 0.7a,B | 7.41 ± 1.14a,A | |
| Color | 1 | 9.60 ± 0.40a,A | 6.67 ± 0.41a,B | 6.67 ± 0.42a,B |
| 15 | 8.30 ± 0.54a,A | 5.86 ± 1.04a,B | 5.91 ± 0.54a,B | |
| Flavor | 1 | 9.00 ± 0.51a,A | 6.73 ± 0.86a,B | 6.62 ± 0.51a,B |
| 15 | 8.45 ± 0.41a,A | 5.80 ± 0.79a,B | 5.89 ± 0.42a,B | |
| Smell | 1 | 9.15 ± 0.44a,A | 7.1 ± 0.91a,B | 8.02 ± 1.44a,A |
| 15 | 8.10 ± 0.65a,A | 6.61 ± 0.82a,B | 6.27 ± 0.65b,B | |
| Overall acceptability | 1 | 9.15 ± 0.4a,A | 7.23 ± 1.16a,B | 6.88 ± 1.01a,B |
| 15 | 8.19 ± 0.67a,A | 6.00 ± 0.97a,B | 5.91 ± 0.48a,B | |
| Mean values of n=3 ± Standard Deviation.a-bMeans followed by different superscript letters in each column indicate significant differences at p<0.05. A-BMeans followed by different superscript letters in each row indicate significant differences at p<0.05 | ||||
Table 8: Sensory evaluation of fortified yoghurt with green tea or moringa leaves extracts.
In general, sensory attributes grouped the yoghurt treatments into two groups, plain yoghurts with highest scores placed in group one while green tea and moringa yoghurts came in the second group. However, appearance and smell of moringa yoghurts received excellent marks equal to plain yoghurts at day one. Meanwhile, both fortified yoghurts gained a good score level. It was clear that appearance, color, overall acceptability were affected by the brightness of the product as control yoghurts had a highest score than the rest treatments either fresh or after storage. Sensory scores for all parameters after 15 days of storage of all yoghurts were equal as first day while there were no significant differences (p>0.05). Varied results have been reported dependent on the culture type and green tea concentration. The sensory results of bio-yoghurts mix with green tea infusion at different concentrations (5%, 10% and 15%) showed no significant differences between the sensory notes received bio-yoghurts while acidophilus milk scored lower values [49]. Higher sensory scores reported from Malaysian tasters’ who preferred green tea yoghurts more than control [39]. In regard to moringa and in agreement with our results Madukwe et al. [50] reported that color of the control was preferred over Moringa beverage. Addition of banana with moringa leaves powder in yoghurt was resulted in comparable score with control while moringa yoghurt alone was not appreciated [51]. On the contrary Hassan et al. [36] found flavor and taste had a highest score in moringa powder yoghurts than control.
Application of green tea and moringa extracts in yoghurts is of great interest because it is dramatically enhanced the antioxidant status of this bio-product. GCMS/MS analysis of both plants revealed the presence of several compounds with known health potent effects. Present results indicate that green tea extract provide higher antioxidant activities in yoghurt followed by moringa extract. Both fortified yoghurts had good sensory scores and supported the growth and survival of starter culture during fermentation and storage. Moringa showed to affect yoghurt syneresis more than green tea in compared to plain yoghurts further, mechanical characteristics did not altered.