
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2021)
Heavy metal pollution is a serious problem for the environment due to their toxicity, persistency, bioaccumulation, and bio magnifications property. Heavy metal contamination in the environment can occur from different natural and anthropogenic sources. The natural sources of heavy metals are mainly volcanic eruption and weathering of metal-bearing rocks, while the anthropogenic sources of heavy metals include agricultural and industrial activities, combustion of fossil fuel and gasoline, waste incinerators, mining, etc. The mobilization of these heavy metals to the aquatic ecosystem alters the physicochemical property of water which is hazardous for aquatic organisms. Heavy metals mainly enter the fish body through gills, body surface and digestive tract during ingestion of metal accumulated food materials. Cadmium, chromium, nickel, arsenic, copper, mercury, lead and zinc are the most common heavy metal pollutants that cause severe toxicity in fishes. Development of oxidative stress is the fundamental molecular mechanism of metal toxicity. The stress weakens the immune system, causes tissue and organ damage, growth defect and reduces reproductive ability. The rich source of high-quality protein filled with vitamins and omega-3 fatty acids encourage the human being to uptake fish as a major food source. So, accumulated heavy metals in the fish tissues directly transfer to the human body and cause toxic effects to expedite various diseases. Therefore, it is necessary to discuss the sources of heavy metals and their toxic effect on fish health to enforce the law and legislations regarding their protection in the aquatic environment and also to save human life.
Heavy metal; Aquatic ecosystem; Bioaccumulation; Toxicity; Oxidative stress
Environmental pollution is one of the major challenges for human society nowadays [1]. Due to the fast-growing industries, increased energy demand and careless destruction of natural resources from the last few decades environmental pollution is increasing day by day [2]. Different organic and inorganic toxic materials are constantly releasing from various natural and anthropogenic sources in the soil and aquatic ecosystem. Among them, heavy metals are playing a major role in environmental pollution, not only for their toxic nature but also possessing the potentiality of bioaccumulation in the food chain [3]. Heavy metals are mostly releasing from domestic and agricultural waste products, industrial waste materials, combustion of fossil fuels, mining, waste water treatment plants to the natural ecosystem [4].
Since heavy metals are persistent in the natural ecosystem, once enter into the living organism, it can accumulate inside. The heavy metals that contaminate the soil are easily taken up by the plants and lead to different adversity e.g. chlorosis, growth inhibition, defect in water balance and photosynthesis, senescence, and finally death [5]. The soil contamination of heavy metals also affects the microbiological balance and reduced soil fertility [6]. The heavy metals can easily dissolved in the aquatic environment and subsequently enter into the body of aquatic organisms [7]. In the course of the food chain, those metals then enter into the body of higher animals. Bioaccumulation of toxic heavy metals in the different tissues may harm animal health and causes damage to their normal physiological processes [8]. Heavy metal toxicity drastically affects the rate of survivability and reproductive capacity of the organisms. Some of these have been reported to be highly carcinogenic, mutagenic and teratogenic depending on the species, dose and exposure time [9].
Aquatic biota directly exposed to the heavy metals that dissolved in water or present as sediment in the water body [10]. Being the top consumers of the aquatic ecosystem fishes are affected most [11]. Heavy metal toxicity sometimes damages the nervous system of fish that affects the interaction of fish with its environment [12]. Humans are omnivorous and exposed to toxic heavy metals by different food items such as fish, vegetables and cereals. Therefore, the heavy metal contamination in the body of aquatic organisms or plants can biomagnified and persist in the food chain, results in transfer to the human body [13]. Heavy metal toxicity has become an important global threat for fish consumers [14].
The present review aims to discuss the bioaccumulation and toxic effect of different heavy metals like Chromium, Cadmium, Copper, Lead, Nickel, Arsenic, Mercury and Zinc on fish health, so that necessary steps can be taken to minimize the impact of these metal elements in our ecosystem.
Chromium (Cr)
Chromium is one of the most common trace elements found in the earth's crust and seawater [15]. This element is not present in the environment as pure metal form, but present in divalent (Cr2+), trivalent (Cr3+) or hexavalent (Cr6+) oxidation states. Among these different forms, Cr3+ and Cr6+ are the most stable forms [16,17]. Cr3+ oxidation state is less toxic due to low membrane permeability, non-corrosiveness nature and minimum power of bio magnifications in the food chain. Cr6+ state is more toxic because of its strong oxidative potentiality and ability to cross the cell membrane [18]. In an aquatic ecosystem, chromium toxicity occurs from different anthropogenic sources such as leather tanneries, metal processing, petroleum refining, textile manufacturing, alloy preparation, wood preserving etc. [19,20].
The toxicity of chromium to aquatic organisms is dependent upon various biotic factors like age, developmental phase and type of species; and abiotic factors like pH, temperature and alkalinity of water. Initial exposure of fish to chromium showed different behavioural changes i.e. uneven swimming, mucous discharge, change in body colour, loss of appetite etc., [21]. Chronic exposure of chromium at a concentration of 2-200 μmol/L on Cyprinus carpio showed cytotoxicity, decreased mitogen-induced lymphocyte activation and phagocyte functions [22]. Blood coagulation time was decreased in the Tilapia sparrmanii exposed to chromium, which reflects by internal bleeding with an increase of pH value [23]. Accumulation of chromium in the tissue of Indian major carp Labeo rohita decrease total protein and lipid content in the muscle, liver and gill [24]. The depletion of liver glycogen content was observed in a freshwater teleost Colisa fasciatus, on chromium exposure [25]. In rainbow trout, Salmo gairdneri, Cr6+ toxicity showed osmoregulatory and respiratory dysfunction at pH 7.8 and 6.5 [26]. Chronic exposure of chromium to Chinook salmon caused DNA damage, microscopic lesions, physiological abnormalities, and reduction in growth and survival rate [27]. In rainbow trout Salmo gairdneri, embryo hatching and the growth of fish were affected after chromium exposure at a concentration of 2 mg/L [28].
Bioaccumulation of chromium varies differentially in various tissues of fish (Table 1). The highest accumulation of chromium is found in gills, liver and kidney and very low concentration is found in the muscle tissue [29].
| Heavy metal | Bioaccumulation in tissue or organ | Fish species | Reference |
|---|---|---|---|
| Chromium | Kidney>heart>muscle>gills Kidney>gills>muscle>heart Kidney>gills>heart>muscle Liver>kidney>gills>muscle Gills>muscle>kidney>liver Liver>kidney>gills>intestine>muscle |
Hydrocynusforskahlii Hydrocynusbebe occidentalis Clariasgariepinus Coregonus lavaretus Cyprinus carpio Pelteobagrusfulvidraco |
Murtala et al., 2012 Murtala et al., 2012 Murtala et al., 2012 Gashkinaet al., 2020 Rajeshkumaret al., 2018 Rajeshkumaret al., 2018 |
| Cadmium | Gills>liver>muscle Gills>intestine>liver Gills>liver>intestine Gills>muscle>heart>kidney Gills>heart>muscle Kidney>gills>heart Kidney>liver>gills>muscle Kidney>gills>muscle>intestine>liver Intestine>kidney>muscle>liver>gills |
Pleuronectes platessa Pleuronectes platessa Raja clavata Hydrocynusforskahlii Hydrocynusbebe occidentalis Clariasgariepinus Coregonus lavaretus Cyprinus carpio Pelteobagrusfulvidraco |
Westernhagenet al., 1978 Pentreath., 1977 Pentreath., 1977 Murtala et al., 2012 Murtala et al., 2012 Murtala et al., 2012 Gashkinaet al., 2020 Rajeshkumaret al., 2018 Rajeshkumaret al., 2018 |
| Copper | Kidney>Liver >gills>muscle Gills>intestine>kidney>liver>muscle Liver>kidney>muscle>gills>intestine |
Coregonus lavaretus Cyprinus carpio Pelteobagrusfulvidraco |
Gashkinaet al., 2020 Rajeshkumaret al., 2018 Rajeshkumaret al., 2018 |
| Lead | Gills>muscle>heart>kidney Gills>kidney>heart>muscle Gills>liver>kidney>muscle Gills>kidney>muscle>liver>intestine Kidney>liver>gills>intestine>muscle |
Hydrocynusforskahlii Hydrocynusbebe occidentalis Coregonus lavaretus Cyprinus carpio Pelteobagrusfulvidraco |
Murtala et al., 2012 Murtala et al., 2012 Gashkinaet al., 2020 Rajeshkumaret al., 2018 Rajeshkumaret al., 2018 |
| Nickel | Kidney>gills>muscle>heart Gills>heart>kidney Kidney>heart>muscle>gills Kidney>liver>gills>muscle |
Hydrocynusforskahlii Hydrocynusbebe occidentalis Clariasgariepinus Coregonus lavaretus |
Murtala et al., 2012 Murtala et al., 2012 Murtala et al., 2012 Gashkinaet al., 2020 |
| Arsenic | Liver>gills>blood>muscle>skin>brain Stomach>liver>gills>muscle |
Clariasbatrachus Oreochromis niloticus |
Kumar et al., 2012 Oliveira et al., 2017 |
| Mercury | Kidney>liver>muscle>gills Gills>kidney>muscle>liver>intestine Muscle>liver>kidney>head |
Coregonus lavaretus Cyprinus carpio Oreochromis niloticus |
Gashkinaet al., 2020 Rajeshkumaret al., 2018 Bradley et al., 2017 |
| Zinc | Gills>kidney>liver>gut Liver > kidney> intestine > gill > muscle |
Pleuronectes platessa Channa punctatus |
Pentreath 1973 Muruganet al., 2008 |
Table 1: Heavy metal bioaccumulation in different tissues or organ of fish-ranked in decreasing order.
Cadmium (Cd)
Cadmium is a trace element present in the earth's crust on an average concentration is about 0.1-0.5 ppm and is commonly found in association with zinc, copper and lead ores. In ocean water, the average concentration is between 5-110 mg/L and in surface water and ground water is usually <1 μg/L [30]. Element form of cadmium is not available in nature. Instead, compound forms e.g. cadmium chloride, cadmium oxide, cadmium sulphide, cadmium carbonate, cadmium nitrate and cadmium cyanide are commonly found [31]. Cadmium is released in the aquatic ecosystem from different natural and anthropogenic sources. Natural sources of cadmium are from the earth's crust and mantle by the volcanic eruption and weathering of rocks. Whereas anthropogenic sources include combustion of fossil fuels, fertilizers, agricultural waste and industrial use (plastic stabilizers, pigment, batteries, electroporating industries) which contaminate the water body [32,33]. The flora and fauna of water body uptake water soluble or sediment form of cadmium compounds, which indirectly enter into the fish body in course of the food chain [34]. Whereas fishes uptake water dissolved free ionic form of cadmium directly through gill, gastrointestinal tract and skin [35].
Cadmium is considered as a nonessential element and causes severe toxicity to fishes. It inhibits the electron transfer chain in mitochondria and stimulates Reactive Oxygen Species (ROS) production [36]. A low level of cadmium exposure induced DNA damage in Cyprinus carpio [37]. Trans-epithelial calcium influx in rainbow trout gill was found to be inhibited by Cd+2 [38]. Micro- nucleated and bi-nucleated cells formation in blood, gills and liver were observed in subchronic cadmium chloride exposure in fish [39,40]. Reported histopathological alteration like fatty vacuolation in the liver; necrosis in hepatocytes; congestion of sub mucosal blood vessels in the intestine and glomerular shrinkage and necrosis in kidney tissue of Tilapia (Oreochromis niloticus). Fish exposed to cadmium showed a differential haematological response. After 8 weeks of exposure to 150 μg/L of cadmium in American eel fish (Anguilla rostrata), lead to anaemia due to reduction in haemoglobin and erythrocyte counts. Significant increase in leukocyte and large lymphocytes count was also observed after cadmium exposure [41]. The level of glycogen reserve in muscle and liver was decreased significantly and blood glucose level increased in Cyprinus carpio, exposed to sublethal concentration of cadmium [42]. Cadmium is an endocrine disrupter and an inhibitor of vitellogenesis, observed in rainbow trout Oncorhynchus mykiss [43]. Exposure to cadmium chloride affected the gonad function and sexual maturity in common carp Cyprinus carpio [44]. Cadmium exposure to the larvae of ide Leuciscus idus showed body malformations and reduced embryonic survival rate due to death in newly hatched larvae [45].
Cadmium accumulation is a serious environmental concern because of its slow rate of excretion. The highest level of cadmium bioaccumulation is found in the liver, kidney and gill and lowest level in the skin. Gill is the most efficient organ for cadmium detoxification [46]. Cadmium is considered is one of the most toxic heavy metals for aquatic organisms because of its high rate of bioaccumulation.
Copper (Cu)
Copper pollution in the freshwater ecosystem occurs due to extensive use of fungicides, algaecides and insecticides in the agricultural field and then discharge of the waste materials to the water body. Other than that, copper toxicity also occurs from the electroplating industry, metal refining industry, plastic industry, mining, sewage sludge, atmospheric deposition etc. [47,48].
Copper is an essential trace element and micronutrient, important for the growth and metabolism of living organisms. In fish and other vertebrates, copper is the key constituent of many metabolic enzymes and glycoprotein. It is also essential for haemoglobin synthesis and nervous system function [49,50]. But, at higher concentration, copper causes toxic effect on living organisms [51]. Copper causes toxicity to freshwater fish at a concentration ranging from 10-20 ppb [52]. Toxicity of copper to aquatic life is dependent on several factors, i.e. water hardness, pH, anions and Dissolved Organic Carbon (DOC). Fish uptake copper mainly through the dietary route or ambient exposure [53]. Exposure to waterborne copper on freshwater fish induced oxidative stress response [54]. Chronic toxicity of copper in fish causes poor growth, shortening of life span, decreased immune response, and fertility problems [55]. Copper toxicity in the gill of teleost fish (Oreochromis niloticus), showed induction in apoptosis [56]. In Cyprinus carpio, copper sulfate exposure showed biochemical and morphological changes in the liver tissue [57]. Micronuclei and binuclei formation was induced in blood erythrocytes, gill epithelial cells and liver cells of fish, after subchronic exposure to copper sulphate. Copper impaired complex fish behaviours such as, social interaction, avoidance of predators and reproductive behaviour that are important for survival. Copper toxicity to Mytilus edulis lead to a decrease in heart rate and cardiac function [58]. Copper exposed Oreochromis mossambicus showed an increase in RBC count, haemoglobin content and hematocrit value [59]. This element is neurotoxic to fish and interferes with the function of olfactory neurons [60]. Copper exposed zebrafish larva became greater sensitive than embryonic and adult stage and showed lateral line dysfunction [61]. The larvae of goldfish Carassius auratus showed a high rate of body deformities and mortality on copper exposure [62]. Copper accumulates at the highest concentration in the liver and less concentration in the gill and body flesh of fish [63]. Bioaccumulation of this trace element influenced the oxidative metabolism, lipid peroxidation and protein content in carp tissue [64].
Lead (Pb)
Lead is considered as one of the most hazardous heavy metals, which is naturally present in the environment, in combination with other elements i.e. PbS, PbSO4 and PbCO3.The concentration of lead in the environment is very much increased by different anthropogenic sources such as metal mining, combustion of coal, oil and gasoline, battery manufacturing, lead-arsenate pesticides, lead-based paint, pigments, food cans etc., [65]. Lead discharge from various industries, agricultural fields, street runoff, lead dust and municipal wastewater that directly come to the aquatic environment and cause toxicity for the aquatic life [66]. The solubility of lead in water is depending upon pH, salinity, hardness etc. Highest solubility of lead is observed in soft and acidic water.
The lethal concentration of lead for fish is 10-100 mg/L [67]. Sublethal concentration of lead exposure causes behavioural change, impotency and growth retardation of fish [68]. Katti reported a change in lipid and cholesterol content in the liver, brain and gonad of Clariasbatrachus, in prolonged exposure to a low concentration of lead nitrate [69]. Histological distortion of gill and liver tissue was observed in African catfish Clariasgariepinus, exposed to lead. Freshwater teleost (Mastacembelus pancalus) showed histological alterations in the ovarian tissue in lead exposure [70]. Necrosis of parenchyma cells, fibrosis of hepatic cords and connective tissue, reduction in growth and body weight, collapsing of blood vessels were also observed in lead-exposed fish [71]. Lead exposure in Nile tilapia (Oreochromis niloticus) showed decreased haemoglobin content, red blood cell count and hematocrit value [72]. Oxidative stress is induced by lead toxicity, which caused synaptic damage and neurotransmitter malfunction in fish [73]. Alteration of the immunological parameters was observed in Tench (Tinca tinca) lethal and sublethal exposure to lead [74].
Lead bioaccumulation in fish mainly occurs in the liver, spleen, kidney and gills [75]. Lead bioaccumulation also affected free locomotion and induced morphological deformities in Chinese sturgeon, Acipenser sinensis [76].
Nickel (Ni)
Nickel is a very abundant trace element found in the environment, present in combination with oxygen or sulphur. Nickel is released into the environment from both natural and anthropogenic sources. The element is discharged from industries during nickel mining and transformation of new nickel into alloys or nickel compounds. Nickel is also released from coal-burning power plants, oil-burning power plants and trash incinerators [77].
Nickel is an essential element for many organisms at low concentration, but at high concentration, it causes toxicity [78]. Nickel toxicity in fishes is dependent upon different physiochemical properties of water like pH, ionic strength, temperature, hardness, Dissolved Organic Carbon (DOC) etc. [79]. Exposure to nickel chloride in Nile tilapia showed abnormal swimming behaviour, rapid opercular movement, respiratory disorder and lesions in the skin. Nile tilapia exposed to nickel also showed a change in blood parameters like, increase of RBC count and a decrease of haemoglobin and WBC counts [80]. Histopathological changes in different tissues like gill, kidney, liver and intestine were observed in nickel exposed freshwater fish Hypophthalmichthys molitrix. The fusion of gill lamellae, necrosis of hepatocytes, blood vessels degeneration, hypertrophy, vacuolation, pyknotic nuclei and lesion were observed in the liver tissue. Hyperplasia and degeneration of tubular cells in kidney tissue were also observed on nickel exposure [81]. In chronic and acute exposure of nickel to freshwater fish Oreochromic niloticus, reduced ATPase activity in the brain [82]. Nickel exposure in freshwater fish Prochilodus lineatus, affected the antioxidant defence system in the liver and induced DNA damage in both blood cells and gills [83]. Short term exposure of a high concentration of nickel resulted in stress reaction of common carp Cyprinus carpio. Alteration of haematological parameters and behavioural changes were also found in Cyprinus carpio, to sublethal concentration of nickel exposure [84]. Nickel toxicity showed some adverse effect on protein metabolism of freshwater fish, Cyprinus carpio. The observed alterations were decrease of structural, soluble and total proteins, increase of free amino acids and protease activity and ammonia in gill and kidney after exposure to a lethal concentration of nickel [85]. Nickel poisoning in fish showed loss of body equilibrium and behavioural changes like surfacing, rapid mouth and operculum movement before death [86].
Nickel accumulates in the blood, kidney, muscle and liver of fish but highest accumulation is observed in the kidney [87]. Bioaccumulation expressed a general decrease of glycogen level in both liver and muscle of Tilapia nilotica. High level of nickel bioaccumulation in Tilapia nilotica, elevated blood cell count, packed cell volume and haemoglobin content and caused lymphopenia and leukopenia.
Arsenic (As)
Arsenic is a ubiquitous element, release in the aquatic environment from various anthropogenic sources including manufacturing companies, smelting operations, power plants etc. Another major source of arsenic from the agricultural field is the use of arsenic pesticides, herbicides and fungicides [88].
Fish are continuously exposed to arsenic-contaminated water through their gill and skin and also by arsenic-contaminated food. Arsenic is present in various forms, i.e. element, trivalent and pentavalent oxidative form. Inorganic arsenic in trivalent oxidation state (arsenites) is very rapidly absorbed into the fish tissue and is more toxic than the pentavalent state (arsenates). The toxic effect of arsenic is dependent upon different abiotic factors of a water body such as pH, temperature, salinity, organic matters, phosphate content, suspended solids as well as other toxic substances [89]. Continuous exposure of freshwater fish to the low concentration of arsenic results in bioaccumulation mostly in the liver and kidney tissue [90]. Arsenic exposure showed histopathological alteration in gills and liver tissue of freshwater fish, tilapia (Oreochromis mossambicus). The alterations in gills were epithelial hyperplasis, lamellar fusion, epithelial lifting and oedema, desquamation and necrosis. The liver histology showed macrophage infiltration, vascularisation, hepatocytes shrinkage, dilation of sinusoids, vascular degeneration, nuclear hypertrophy and focal necrosis [91]. A range of histological alterations was found in the heart of freshwater teleost, Channa punctata including necrosis in the heart tissue [92]. Acute exposure of common Indian catfish Clariasbatrachus to sodium arsenite elicited disturbed haemopoiesis, disruption of the erythrocyte membrane, impaired iron uptake by erythrocytes and haemolysis [93]. Arsenic exposure in the catfish Clariasbatrachus showed a time-dependent change in total leucocyte count and reduction of organo-somatic indices in kidney and spleen. Arsenic also induced alteration in T-cell and B-cell functioning and interfere bacterial phagocytosis function of catfish [94]. Developmental arrest of Japanese medaka (Oryzias latipes) embryo was observed in the sublethal concentration of arsenic toxicity [95]. The induction of stress response proteins were found in rainbow trout Salmo gairdnerii, on arsenic exposure [96]. Arsenic toxicity in zebrafish embryos significantly inhibits genes involved in innate immune responses, which function against viral and bacterial infection [97]. Wanget treated two fish cell lines, JF (fin cells of Therapon jarbua) and TO-2 cells (ovary cells of Tilapia), with sodium arsenite [98]. They observed apoptosis in JF cells probably due to induction of oxidative stress and distortion of the cell cycle in TO-2 cells. In long term exposure of freshwater fish Colisa fasciatus, to arsenic oxide caused impaired ovarian function and reduction in the development of 2nd and 3rd stage oocyte [99]. Bioaccumulation of arsenic affects various physiological systems of fish such as growth, reproduction, gene expression, ion regulation, immune system and histopathology.
Mercury (Hg)
Mercury is considered as one of the most toxic heavy metal found in the environment. Mercury contamination in the environment increased rapidly from the 20th century due to huge industrialization [100]. Mercury ranked third in the list of the hazardous substance of the environment after lead and arsenic by United State Environmental Protection Agency (EPA) and the Agency for Toxic Substances and Disease Registry (ATSDR) [101]. The natural sources of this element are forest fire and volcanic eruption and anthropogenic sources include fungicides, electronic equipment, batteries, paint etc. Burning of fossil fuels and mining also contribute a major role in mercury pollution of our environment [102].
Apart from elementary form, mercury is present in an ionic form which forms a compound with sulphide, chloride or organic acid and organic form, especially methyl mercury [103]. Literature suggests methyl mercury is the most chemically toxic form of mercury and 70-100% of mercury present in the fish body is of methylated form. Methylation of inorganic mercury occurs by microorganisms such as anaerobic sulphate-reducing bacteria, iron reducers, and methanogens [104,105]. Increase in water temperatures attributed to climate change which stimulates the methylation of mercury. Mercury can enter into the fish body by food through the alimentary canal, skin and gills. The acute lethal concentration of inorganic mercury is 0.3-1.0 mg/L for salmonids and 0.2-4 mg/L for cyprinids depending upon the physical and chemical property of water. The acute lethal concentrations of commonly found organic mercury compounds are 0.025-0.125 mg/L for salmonids and 0.20- 0.70 mg/L for cyprinids. The maximum admissible concentration of the inorganic form of mercury for salmonids is 0.001 mg/L and for cyprinids is 0.002 mg/L [106]. Mercury is very toxic for fish and at sublethal concentration and causes structural, physiological and biochemical alteration on the fish nervous system. Methyl mercury is considered as the most neurotoxic compound because it can cross the blood-brain barrier due to its lipophilic nature and can accumulate in the nervous system of fish. Mercury can also interfere with the physical property and structural integrity of cell membrane by affecting the configuration of purines, pyrimidines and nucleic acids [107]. Chronic exposure of mercurial compound to the kidney of Clarias batrachus expressed damage and necrosis of kidney tubules [108]. Mercury oxide toxicity on African catfish Clarias gariepinus showed a significant increase of serum cortical, cholesterol, aspartate aminotransferase, alanine aminotransferase, alkaline phosphorous, urea and creatinine levels and a significant decrease in haemoglobin and haematocrit value [109]. The freshwater fish Channa punctatus exposed to 0.3 mg/L of HgCl2 for 7 days showed oxidative damage and up regulation of pro- inflammatory cytokines [110]. Inorganic mercury exposure in zebra fish showed histological alteration and oxidative stress in gonads. Mercury toxicity also disrupted the transcription of Hypothalamic- Pituitary-Gonadal (HPG) axis genes and altered the sex hormone levels of adult zebra fish [111]. The male reproductive system of tropical fish Gymnotus caropo showed sensitivity to Hg toxicity. HgCl2 induced seminiferous tubule disorganization, congestion of blood vessels, interstitial tissue proliferation, and reduction in germ cells and sperm’s number of Gymnotus caropo [112].
Mercury has a high affinity to proteins, therefore more than 90% of total mercury accumulates in fish muscle [113]. Rate of methyl mercury excretion from fish body is extremely slow therefore in addition to muscle, high concentration of mercury also found in blood [114]. Additionally, liver also function as the site of storage, detoxification or redistribution of mercury [115].
Zinc (Zn)
Zinc contamination in the environment is increasing because of different anthropogenic sources such as industrial activities, mining, combustion of coal and waste materials, steel processing etc., [116].
Zinc is a ubiquitous trace element and one of the essential micronutrients for living organisms. Zinc is involved in various metabolic pathways such as nucleic acids and protein synthesis, immunity, energy metabolism, cell division and body growth. It acts as a cofactor for many enzymes that aid in metabolism, digestion, nerve function and other processes [117,118]. Deficiency of zinc causes several physiological disorders such as poor pregnancy rate, cardiovascular diseases and cancer; but it becomes toxic in excess amount [119]. Zinc toxicity is also species-specific and varies with different developmental stages of fish. The toxic effect of zinc on aquatic animals depends upon several environmental factors, especially temperature, water hardness, and dissolved oxygen concentration. At an acute toxic concentration of zinc, it kills fish by destroying gill tissue and at the chronic toxic level, it induces stress which results in the death of fish [120].
Fish take zinc through the gastrointestinal tract and gills. The major mechanism of zinc toxicity occurs as the divalent cationic form which disrupts the absorption of calcium ion in the tissue, results in hypocalcaemia and eventually fish death [121]. Zinc sulphate exposed Tilapia nilotica showed slow swimming activity and loss of body equilibrium. The hepatocytes of the liver became vacuolated with frequent necrosis [122]. Zebrafish embryos exposed to different concentrations of ZnCl2 showed a delay in hatching capacity, growth defect and skeletal malformations due to defective calcification [123]. Zinc exposed fish Phoxinus phoxinus showed alteration in movement pattern and behavioural change. These fish become less active, easily frightened and formed denser shoals which mostly stayed close to the bottom [124]. Zinc exposed killifish (Fundulus heteroclitus) led to oxidative stress response by increasing hepatic lipid peroxidation level, which is an oxidative stress biomarker and decrease of liver catalase (CAT) activity [125].
Zinc accumulates in fish through gills and digestive track, however the role of water as a source of zinc is not fully elucidated [126]. Murugan examined the accumulation of zinc in different tissue of Channa punctatus and concluded that zinc deposit at the order of liver>kidney> intestine>gill>muscle [127].
Some heavy metals have an essential role in the normal biological processes, and the insufficiency or excess amount can cause a disturbance in the metabolic pathways and serious illness [128]. Essential heavy metals are which have known biological functions [129]. Other group of heavy metals have no biological role and at higher concentrations cause a toxic effect to the tissues [130].
Beyond tolerance level, metal ions induce Reactive Oxygen Species (ROS) production, which causes an oxidative stress response in fishes [131]. Redox-active metals e.g. copper and chromium generate reactive oxygen species through redox cycling. Whereas redox inactive metals e.g. mercury, nickel, lead, arsenic and cadmium bind to the Sulfhydryl groups (SH) of proteins involved in antioxidant defences, thereby impair the defence mechanism [132]. Elevated ROS production in fish causes DNA lesions, oxidation of lipids and proteins and alterations of cellular redox status [133,134].
Antioxidant defences mechanism in fish includes the antioxidant enzyme system and low molecular weight scavengers (Figure 1). Super Oxide Dismutase (SOD), Glutathione Peroxidase (GPX), Catalase (CAT), and Glutathione-S-Transferase (GST) enzymes protect cells from oxidative damage by detoxification of ROS [135]. Whereas low molecular weight protein i.e. Metallothioneins (MTs) reached the cysteine residues that sequester the metals. Different isoforms of MTs bound to various metals with different affinities in fishes [136].
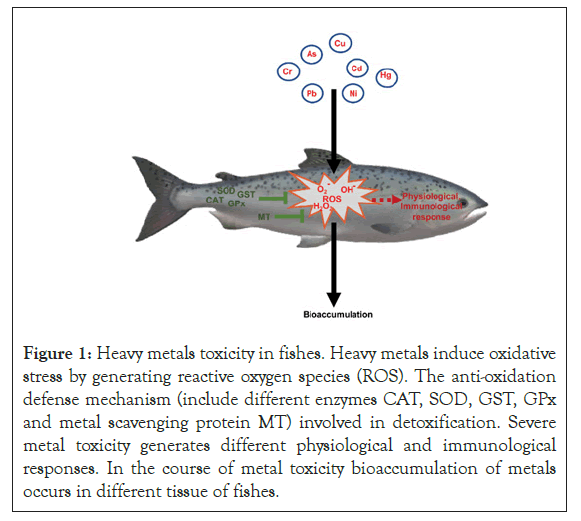
Figure 1: Heavy metals toxicity in fishes. Heavy metals induce oxidative stress by generating reactive oxygen species (ROS). The anti-oxidation defense mechanism (include different enzymes CAT, SOD, GST, GPx and metal scavenging protein MT) involved in detoxification. Severe metal toxicity generates different physiological and immunological responses. In the course of metal toxicity bioaccumulation of metals occurs in different tissue of fishes.
In addition, to detoxify the metals, metallothioneins are the major cause of bioaccumulation of heavy metals in different tissue of fishes [137]. The accumulated heavy metals not only affect the fish population in the aquatic ecosystem but also transfer through the food chain/web to the next tropic level. Trophic transfer of these elements from aquatic to the terrestrial ecosystem has serious implications for human health by promoting different diseases including cancer, neurodegenerative disease, etc. [138,139].
Therefore, this comprehensive study about the heavy metal toxicity on fish health suggests that essential steps should be taken to minimize the toxic impact of heavy metals on human health and the environment. Here, some recommendation is made-
• The level of heavy metal on soil, water and sediment should be monitored regularly. Such data should be used for the assessment of health risk in the human population.
• Agricultural and industrial waste should be decontaminated effectively before discharge into the water body.
• Proper awareness should be provided to the public about the harmful effect of heavy metal toxicity in our environment.
• More scientific research should be encouraged and promoted about the toxicity of heavy metals, their trophic level transfer and their effect on the environment.
Pramita Garai, Priyajit Banerjee are contributed equally.
Citation: Garai P, Banerjee P, Mondal P, Saha NC (2021) Effect of Heavy Metals on Fishes: Toxicity and Bioaccumulation. J Clin Toxicol. S18:001.
Received: 12-May-2021 Accepted: 28-May-2021 Published: 05-Jun-2021 , DOI: 10.35248/2161-0495.21.s18.001
Copyright: © 2021 Garai P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.