Journal of Horticulture
Open Access
ISSN: 2376-0354
ISSN: 2376-0354
Research Article - (2019)Volume 6, Issue 3
Green roofs have positive effects on urban settings, leading to improvements in aesthetics and energy consumption of buildings. However, green roofs are extremely difficult environments for plant growth and survival because water availability fluctuates dramatically and is often limited between dry periods and rain events. We analyzed the effects of different concentrations of a water-retention additive, hydrogel, in green-roof substrate mixtures on the growth and ornamental quality of Mentha suaveolens. Coir and perlite were mixed in the ratio of 80:20 (coconut coir dust to perlite, v/v; referred to as C4 P1 ), 50:50 (C1 P1 ), or 20:80 (C1 P4 ) at a substrate depth of 20 cm. Hydrogel (hydrophilic polymer: medium, w/v; dry weight basis) was added to the substrate mixtures at 0 (control), 0.25, 0.5, 1.0, or 2.0 kgm-3. All plants were watered every two days in the first week until they were well established, and then were not irrigated. The substrate with high coir substrate content increased the growth of Mentha suaveolens under drought conditions, but decreased the ornamental quality in rainy conditions. This study revealed that substrate C4 P1 , which has high concentration of coir, is not recommended for Mentha suaveolens in the rainy season. The addition of hydrogel increased the growth and ornamental quality of Mentha suaveolens in substrates C1 P1 and C1 P4 under drought condition. Moreover, substrate C1 P4 with 1.0 kg m-3 added hydrogel was optimal for Mentha suaveolens growth on green roofs, regardless of drought and rainy conditions.
Apple mint; Green roofs; Hydrogels; Ornamental quality; Plant growth; Water retention ability
Green roofs, vegetative layers grown on rooftops, are an important environmental technology that is becoming popular in many cities around the world, because they have numerous environmental benefits and provide green, open-air spaces without requiring additional land. In recent years, accompanied by the rapid development of urbanization and the rise of urban agriculture, more attention has turned to the combination of green roofs and urban agriculture [1]. More and more edible plants are grown on green roofs, not only because of their ecological effects, but also because of the social benefits of supplying a safe and adequate food supply [2]. Mentha suaveolens (known as apple mint or woolly mint), a common wild plant, is native to Africa, temperate Asia, and Europe [3]. It is a popular and attractive tall-growing (to 1 m) plant used for culinary and ornamental purposes, with rounded, gray-green, hairy leaves, lavender flowers in midsummer, a slight apple scent, and a mildly fruity flavor [4,5]. Most mints prefer moist, sunny to partly sunny conditions [6]. However, the ecological environments on roofs and the ground are quite different. Extreme environmental conditions are often found on rooftops, including intense solar radiation, a high rate of heating, large temperature gap between day and night, strong winds, shallow substrate depths and drought. These factors present great challenges in sustaining plant material on green roofs, especially during a drought. Hydrogels can retain water several hundred times greater than their weight and thus are widely used to improve the moisture content of soil in the fields of agriculture, horticulture and forestry [7-9]. The effects of hydrogel on plant growth are evidenced by prolonged survival time, increased plant height, wider leaves, greater leaf area, greater biomass, greater vegetable yield, increased succulence and a higher concentration of chloroplasts [10-13]. However, such reduction might result if hydrogels lose effectiveness with time [14]. According to Al-Harbi et al. the maximum water holding capacity of hydrogel decreased by 17.3% and 27.8% in soil fortified with hydrophilic polymer at a rate of 0.1% and 0.4%, respectively, in two years [15]. Jobin et al. observed no difference in available water or porosity in three substrates containing hydrogel after nine weeks [16]. Bai et al. studied soil moisture of sandy soils mixed with four types of super-absorbent polymer under alternating dry and weight conditions [17]. They found that the water-retention capacity of the polymer decreased sharply when soil moisture was lower than a critical threshold. In addition, the water-holding capacity of hydrogel could be affected by the extreme environmental conditions on a rooftop, such as intense solar radiation, high temperatures, water deficit, freeze-thaw cycles caused by seasonal changes and wetting-drying cycles caused by weather changes [18-20]. Those result indicated that the effect of hydrogel depended on substrate type and plant species. Therefore, we proposed to find out if addition of appropriate amounts of hydrogel to Mentha suaveolens growth substrates can increase its growth and ornamental quality of on green roofs.
Experimental design and substrate
This experiment had a factorial design with three different substrates and five hydrogel treatment rates (0, 0.25, 0.5, 1.0, and 2.0 kgm-3). There were three replicates for each treatment. A total of 45 square containers (50 cm L × 50 cm W × 25 cm H) were installed on a roof platform at the Complex Practice Building of Konkuk University, Chungju, Chungcheongbuk-do, located at latitude 35°49' N and longitude 127°08' E. From top to bottom, each container was filled with the following four layers: vegetation, growth medium, geotextile filter, and plastic drainage. Experimental substrates were formulated with 80%, 20%, or 50% (by volume) coconut coir dust and 20%, 80%, or 50% (by volume) per liter, respectively. Hydrogel (K-SAM, Kolon Chemical Co., Ltd., Korea) was incorporated into substrates at concentrations of 0 (control), 0.25, 0.5, 1.0, or 2.0 kgm- 3 (polymer: medium w/v; dry weight basis) with three replicates per treatment. The depth of all substrates was 20 cm. The monthly precipitation was 88.2, 110.6, 277.7, 122.7 and 153.8 mm in June, July, August, September and October, respectively.
Plant material
Seedlings of Mentha suaveolens were grown in 10-cm diameter pots obtained from a commercial nursery. This species was selected based on their growth habits, high sensitivity to moisture stress and need for regular irrigation, and also because they are commonly used in mint tea, fruit drinks, salads [21]. Three seedlings of Mentha suaveolens with heights of about 8 cm were transplanted into green roof containers on May 14th, 2014. The initial height of transplants differed by <1 cm. All plants were watered every two days for the first week and no longer watered when they were well established.
Measurement of plant growth and ornamental quality
Plant height (H) above the stem base, width at the widest vegetative point of the plant passing through the center (W1) and widest width perpendicular to W1 (W2) were measured in June during the peak growth period and the drought period. The data on height and width were used to calculate the growth index (GI), ([W1 + W2]/2 +H)/2, which is commonly used as an indicator of plant size [22]. The length and width of leaves, number of leaves and relative appearance of the plants were also assessed in June. The relative appearance of the plants was evaluated based on visually. The visual rating evaluations were divided into five grades; grade 1, severely stressed and completely dried out; grade 2, stressed, with less than 50% of the leaves retaining green pigmentation; grade 3, mildly stressed, with 50% of the leaves retaining green pigmentation; grade 4, minor stress, with over 50% of the leaves appearing to be healthy; and grade 5, unstressed, with all leaves appearing healthy [23]. Number of inflorescences, leaf color and chlorophyll content of the leaves were measured in August, when plants were at their peak flowering time and the rainy season. Leaf color was measured using a Chroma meter (CR-400, Konica Minolta Group, Japan) with L*, a*, and b* mode. The L* values ranged from black (L*=0) to white (L*=100). The a* values ranged from red (a*=100) to green (a*=−100). The b* values ranged from yellow (b*=100) to blue (b*=−100). Chroma C* was calculated as (a*2 + b*2)1/2 as a measure of color saturation or intensity. The hue angle, h, was calculated as tan-1 (b*/a*). When a* was less than 0 and b* was greater than 0, h was 180 + tan-1 (b*/a*). The value of h falls on a 360° color wheel, with 0°, 90°, 180°, and 270° representing red -purple, yellow, bluish-green and blue respectively. The third leaf from the top of each plant was measured. The chlorophyll content of the leaves was measured in nine leaves per container using a SPAD-502 meter (Minolta Camera Co., Ltd, Osaka, Japan).
Statistical Analysis
Data were subjected to analysis of variance (ANOVA) using the SAS 9.1 software package (SAS version 9.1, SAS Institute, Cary, NC). Significant mean separation was indicated by Duncan’s multiple range tests. Statistical significance was defined as p≤0.05.
The plant growth of Mentha suaveolens was significantly different in the three substrates (Table 1). There was an interactive effect between substrate and hydrogel rate on leaf number (interaction p<0.001) and visual rating (interaction p<0.001). The plant height (p<0.001), leaf length (p<0.001), leaf width (p<0.001), growth index (p< 0.001) and visual rating (p<0.001) of Mentha suaveolens were the worst in substrate C1P4 when they were measured in June with less rainfall. However, the lowest leaf number (p<0.001) was found in substrate C1P1. In substrate C1P4, all of the plant growth measures were highest with a concentration of 2.0 kg-m-3 of hydrogel. In substrate C1P1, the plant height and visual rating at the concentration of 2.0 kg-m-3 of hydrogel in substrate C1P1 was the highest. There was an interactive effect between substrate and hydrogel concentration on the total number of inflorescences of Mentha suaveolens, with a significantly greater number of inflorescences in substrate C1P4 than in the other substrates (Figure 1). The total number of inflorescences per plant in substrate C1P4 treated with 0.25, 0.5, and 1.0 kg-m-3 hydrogel was increased significantly, especially at the rate of 1.0 kgm-3, compared to the other hydrogel treatments or control in all substrates. In substrate C1P4, the number of inflorescences was 1.27-, 1.24- and 1.39- fold more than those in the control. As shown in Table 2, there was an interactive effect between substrate and hydrogel concentration on a* (interaction p=0.004), b* (interaction p<0.001), chroma (C*) (interaction p<0.001) and SPAD value (interaction p< 0.001) of Mentha suaveolens, with the lowest a* value but the highest values of z and chroma (C*) in C4P1. There were also significant differences between the three substrates in the values of lightness (L*) (p=0.002). However, there was no significant difference in hue angle (h). The addition of 2.0 kg-m-3 hydrogel increased the value of a* (more green) in C4P1. However, addition of hydrogel decreased the value of a* (less green) in C1P4. The values of b* and chroma (C*) were higher under the 2.0 kg-m-3 treatment than under other treatments or control in C1P4. In substrate C1P1, the values of b* and C* were decreased at a concentration of 1.0 and 2.0 kg-m-3 hydrogel. The effect of the hydrogel on plant growth of Mentha suaveolens was most obvious in substrate C1P4, which was associated with higher hydrogel content under drought stress, especially at a rate of 2.0 kgm-3. Moreover, the highest growth of Mentha suaveolens was observed with the 1.0 kg-m-3 and 2.0 kg-m- 3 treatments, perhaps because the capacity for water retention of the soil and the efficiency of water use increased with the addition of hydrogel under drought conditions [24-26]. This finding is also consistent with those of other studies which showed the effect of hydrogel on plant growth and hydrogel used to improve the transplant success of seedlings [12,13,27-30]. Akhter et al. reported that the addition of hydrogel could slow soil moisture loss and delay the wilting time of seedlings by four to five days [31]. The C4P1 and C1P1 substrates contained more coir components that could hold more water to maintain the structural integrity of plants and supply nutrients under drought conditions [32,33]. Cho et al. have found that gerbera has greater growth in coir-based substrates with more organic matter content than in rockwool, especially in root growth [34]. Xia et al. have studied the effects of relative moisture content of organic substrates on the growth of tomatoes and have shown that the physiological characteristics and fruit yield of tomatoes were improved significantly with increasing moisture content [35]. It has also been suggested that a substrate with high coir content can increase plant growth of Mentha suaveolens under drought conditions. However, the total number of inflorescences per plant of the Mentha suaveolens grown in C1P4 was significantly greater than that of plants grown in C4P1 and C1P1 under rainy conditions. Moreover, C4P1 with high coir content resulted in brighter, more yellow and more yellow-green leaves than did the other two substrates. The data on SPAD value in this study agreed with that of the color measurement. The light purple flowers of Mentha suaveolens start blooming in early July and continue through late September and the peak blooming period occurs from late July to the middle of the August. The period of peak flower blooming corresponded to the rainy season, when there is heavy rainfall. Leaf greenness can be affected by many factors, such as growth stage and water stress to the middle of August [36,37]. In our study, there was heavy rainfall during the peak flowering time, which may result in waterlogging and there by affect inflorescence number and leaf color. Rowe et al. reported that high levels of organic substrate may result in shrinkage because of decomposition. Increased coir could result in lush growth that may suffer damage under frequent rainfall, resulting in frequent wetting cycles. In our study, the higher coir content of substrate C4P1 increased the content of water available for plant growth in June, when there was little rainfall, resulting in greater growth and visual rating than for substrate C1P4. However, the intensity of rainfall in August reduced plant growth and ornamental quality. These results confirmed that the growth of Mentha suaveolens was significantly affected by the composition of substrate in the dry season [38]. On this, the growth of Mentha suaveolens was the best in C4P1 and the worst in C1P4 in June under drought conditions. However, data on the total number of inflorescences and the ornamental quality of Mentha suaveolens plant suggests that the 0.25, 0.5, and 1.0 kg-m-3 treatments of C1P4 and control, and the 2.0 kg-m-3 treatment of C1P1, were more beneficial than were other treatments and more inflorescence and better ornamental quality in C1P4 and C1P1 than in C4P1 in August during the rainy season. This result might indicate that reducing water loss by adding the coconut coir dust seems to improve the growth of Mentha suaveolens plant under drought condition, but produces less flowering and ornamental quality under rainy condition. These experimental conditions suggest that effective strategies to improve growth and promote flowering yield might partially be amended in substrate C1P4 with 1.0 kg-m-3 hydrogel for the rainy season or the dry season in green roofs.
| Substrates | Hydrogel rate (kg·m-3) | Plant height (cm) | Leaf number (per plant) | Leaf length (mm) | Leaf width (mm) | Growth index | Visual rating |
|---|---|---|---|---|---|---|---|
| z C4P1 |
Control | 21.88bcdy | 144.7ab | 31.70cd | 21.84def | 18.25cd | 3.6abc |
| 0.25 | 22.67bcd | 152.5ab | 30.56def | 21.47def | 18.54bcd | 3.9ab | |
| 0.5 | 22.58bcd | 159.3a | 31.56cd | 23.00bcde | 18.21cd | 3.9ab | |
| 1.0 | 22.87b | 152.5ab | 32.88abcd | 22.95bcde | 18.64bcd | 4.0a | |
| 2.0 | 24.13ab | 130.0bcd | 31.99cd | 23.06bcde | 19.46abc | 3.8ab | |
| C1P1 | Control | 23.12b | 113.7def | 34.83abc | 25.58ab | 20.27ab | 3.2c |
| 0.25 | 22.97b | 104.5ef | 32.20bcd | 23.11bcde | 19.05bc | 3.3bc | |
| 0.5 | 22.17bcd | 108.5def | 32.87abcd | 24.13bcd | 19.04bc | 3.8ab | |
| 1.0 | 23.53b | 96.0f | 36.36a | 27.18a | 19.58abc | 3.5abc | |
| 2.0 | 26.08a | 132.0bcd | 35.25abc | 24.92abc | 20.90a | 4.0a | |
| C1P4 | Control | 19.55e | 121.5cde | 27.62ef | 21.77def | 16.22e | 2.0d |
| 0.25 | 20.48ed | 113.2def | 27.27f | 19.48f | 17.18de | 2.4d | |
| 0.5 | 20.70cde | 120.2cde | 27.74ef | 19.62f | 17.12de | 3.1c | |
| 1.0 | 22.00bcd | 138.2abc | 31.19cde | 20.64ef | 18.52bcd | 3.3bc | |
| 2.0 | 22.10bcd | 145.5ab | 36.08ab | 22.78cde | 18.18cd | 3.9ab | |
| Significance | Substrate | *** | *** | *** | *** | *** | *** |
| Hydrogel | *** | NS | *** | ** | ** | *** | |
| Interaction | NS | *** | NS | NS | NS | *** |
z C4P1: Coir 80%, Perlite 20%; C1P1: Coir 50%, Perlite 50%; C1P4: Coir 20%, Perlite 80% (% by vol.) supplemented with five concentrations of hydrogel, 0 (Control), 0.25, 0.5, 1.0, and 2.0 kg·m-3 (hydrogel: medium (w/v), dry weight basis)
y Means in the same row followed by the same letter are not significantly different at p ≤ 0.05 level by Duncan’s multiple range test.,**, *: p<0.001, p<0.01, p<0.05, respectively. NS: not significant
Table 1: Plant height, leaf number, leaf length, leaf width growth index and visual rating of Mentha suaveolens (apple mint) grown in three different green roof substrates supplemented with five concentrations of hydrogel in June (dry season).
| Substrates | Hydrogelrate (kg·m-3) | L* | a* | b* | C* | H | SPAD value |
|---|---|---|---|---|---|---|---|
| z C4P1 |
Control | 43.39aby | −18.32cde | 31.20ab | 36.18ab | 120.44ab | 25.46d |
| 0.25 | 44.29a | −18.19cde | 30.12abc | 35.18abc | 121.14ab | 27.84d | |
| 0.5 | 44.40a | −18.46de | 32.70a | 37.54a | 119.44b | 25.63d | |
| 1.0 | 43.67ab | −18.76e | 31.89ab | 37.00a | 120.47ab | 27.80d | |
| 2.0 | 44.41a | −17.47abcd | 30.10abc | 34.80abcd | 120.14ab | 27.04d | |
| C1P1 | Control | 43.73ab | −18.40de | 32.33a | 37.21a | 119.68ab | 34.49ab |
| 0.25 | 43.19ab | −17.88bcde | 30.05abc | 34.96abc | 120.77ab | 33.77b | |
| 0.5 | 43.78ab | −18.58de | 32.51a | 37.45a | 119.80ab | 34.20ab | |
| 1.0 | 41.68b | −16.92ab | 27.33cd | 32.15de | 121.74ab | 33.06bc | |
| 2.0 | 41.94b | −17.14abc | 27.96cd | 32.80cde | 121.51ab | 34.33ab | |
| C1P4 | Control | 42.32ab | −16.50a | 26.89d | 31.55e | 121.55ab | 31.97bc |
| 0.25 | 42.56ab | −17.50abcd | 29.32bcd | 34.16bcde | 120.89ab | 32.59bc | |
| 0.5 | 42.02b | −17.58abcde | 28.16cd | 33.20cde | 121.99ab | 36.59a | |
| 1.0 | 42.41ab | −17.49abcd | 27.75cd | 32.80cde | 122.23a | 33.97b | |
| 2.0 | 43.81ab | −18.40de | 31.62ab | 36.58ab | 120.19ab | 31.13c | |
| Significance | Substrate | ** | ** | *** | *** | NS | *** |
| Hydrogel | NS | NS | NS | NS | NS | NS | |
| Interaction | NS | ** | *** | *** | NS | *** |
z C4P1: coir 80%, perlite 20%; C1P1: coir 50%, perlite 50%; C1P4: coir 20%, perlite 80% (% by vol.) supplemented with five concentrations of hydrogel, 0 (control), 0.25, 0.5, 1.0, and 2.0 kg·m-3 (hydrogel: medium (w/v), dry weight basis)
y Means in the same row followed by the same letter are not significantly different at p ≤ 0.05 level by Duncan’s multiple range test.
***, **, *: p<0.001, p<0.01, p<0.05, respectively. NS: Not Significant
Table 2: Ornamental quality and chlorophyll contents of Mentha suaveolens (Apple mint) leaf grown in three different green roof substrates supplemented with five concentrations of hydrogel in August (rainy season).
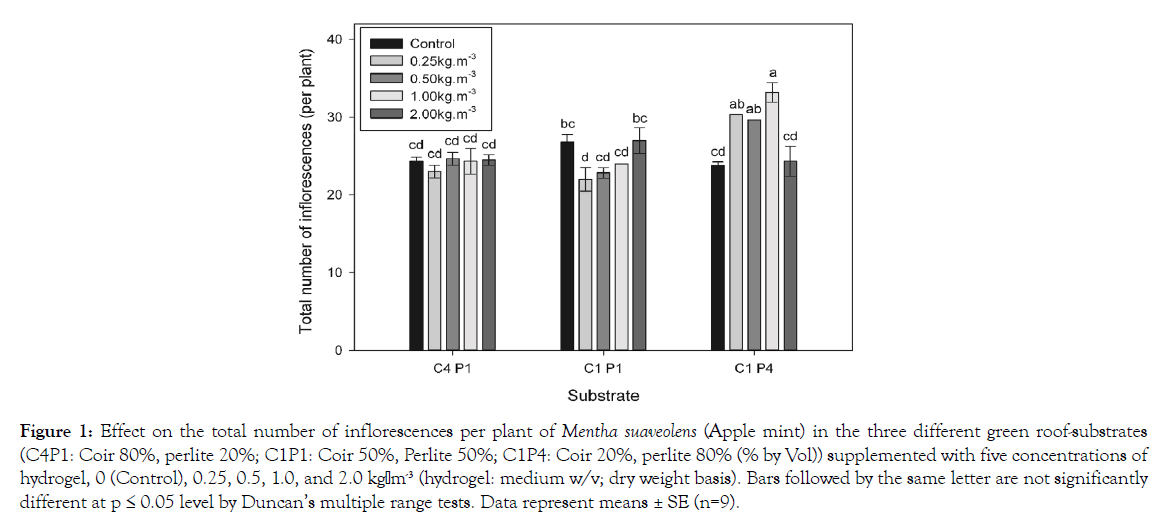
Figure 1: Effect on the total number of inflorescences per plant of Mentha suaveolens (Apple mint) in the three different green roof-substrates (C4P1: Coir 80%, perlite 20%; C1P1: Coir 50%, Perlite 50%; C1P4: Coir 20%, perlite 80% (% by Vol)) supplemented with five concentrations of hydrogel, 0 (Control), 0.25, 0.5, 1.0, and 2.0 kg·m-3 (hydrogel: medium w/v; dry weight basis). Bars followed by the same letter are not significantly different at p ≤ 0.05 level by Duncan’s multiple range tests. Data represent means ± SE (n=9).
Mentha suaveolens grew better in C4P1 or C1P1 than in C1P4 in June under drought conditions. However, C4P1 with high coir content decreased the inflorescence number and ornamental quality during peak flowering time; the heavier rainfall may result in waterlogging. In contrast, the effect of hydrogel on the number of inflorescences and on the ornamental quality of Mentha suaveolens was most obvious in substrate C1P4, which was associated with higher hydrogel content under rainy conditions. Therefore, we suggest that substrate C1P4 with 1.0 kg-m-3 hydrogel was optimal for Mentha suaveolens grown on green roofs, because the plants showed stable growth in these conditions, regardless of growth concerns in times of drought and ornamental quality concerns in the rainy season.
Citation: Hui XU, Yeum KJ, Yoon Y, Jin-Hee JU (2019) Effect of Hydrogels in Three Substrates on Growth and Ornamental Quality of Apple Mint (Mentha suaveolens) in Unirrigated Green Roofs. J Horttic 6:260
Received: 14-Sep-2019 Accepted: 03-Oct-2019 Published: 10-Oct-2019
Copyright: © 2019 Hui XU, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited