Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2016) Volume 6, Issue 3
This study seeks to optimize injection molding and sintering process parameters obtained using the Taguchi method. Mold flow analysis software was applied to simulate the injection molding process to improve mold design. Shrinkage rates of critical dimensions from both injection molding and sintering process analysis could be used to improve mold design. Density, Vickers hardness, and shrinkage rates of zirconia dental implants were carried out on the sintered dental implants, respectively, using Archimedes principle, Vickers hardness tester, and optical projectors. The simulation results show that the injection molding process with optimal parameters significantly reduced shrinkage rates as compared to results using the initial parameters. On the other hand, the measurement results show that zirconia dental implants sintered with optimal design parameters could achieve a density of 5.96 g/cm3, a hardness of Hv 1489 ± 131, and a shrinkage rate of 20.82 ± 0.70%. Analysis of variance (ANOVA) results revealed that the most influential factor was the sintering temperature, which was thus used as the key variable in the subsequent single factorial experiments. With sintering temperatures controlled between 1400°C to 1500°C, density increased from 5.96 g/cm3 to 6.00 g/cm3, hardness from 1489 ± 131 to 1489 ± 71, and the shrinkage rate from 20.82 ± 0.70% to 21.34 ± 0.20%.
<Keywords: Zirconia; Dental implant; Injection molding; Debinding; Sintering
Yttria-tetragonal zirconia polycrystal ceramics (Y-TZP) have attracted considerable interest for use in dental implants due to their high strength and toughness, chemical stability, biocompatibility, and good wear resistance [1,2]. Zirconia produced at different temperatures can result in crystallography forms in monoclinic, tetragonal, and cubic phases [3,4]. The optimal mechanical properties of these three forms are achieved using zirconia in its tetragonal form [5], which can be obtained by adding a stabilizer, such as MgO, CaO or Y2O3. Furthermore, tetragonal stabilized zirconia with the addition of 3 mol% Y2O3 (3Y-TZP) exhibits excellent mechanical properties, and has been wildly applied [6,7]. In this study, 3 mol% yttria-stabilized zirconia powder was used to produce dental implants. Zirconia powder is used to produce dental implants through two main processes: injection molding and sintering. Shrinkage during both processes can affect the dimensions of the final implant, raising challenges for mold design. This study seeks to identify optimal parameters to minimize shrinkage rates during injection molding and to ensure sufficient implant density and hardness during sintering. The shrinkage rates obtained from the sintering process are calculated to improve future mold designs.
Taguchi method
The use of traditional trial-and-error methods in the injection molding industry is time consuming and inefficient. The Taguchi experiment design method was adopted to select an optimal set of parameters through the smallest number of experiments due to a special design of orthogonal arrays [8], thus reducing experimental time and costs. This approach has been widely applied to determine process parameters in various studies [9-11]. The Taguchi method uses the signal-to-noise (S/N) ratio to measure the performance of process response as follows: S/N=(expected output)/(unexpected output)=(useful energy/wasted energy)=(effect of mean)/variable around mean) [8]. According to the objective of the experimental response variables, the characteristics of the S/N ratio are classified as nominal-the-best (NTB), smaller-the-better (STB), or larger-the-better (LTB) [8]. The combination of factors and levels that maximizes the S/N ratio is the optimal parameter setting. This study uses STB and LTB to respectively obtain the performance characteristics for injection molding and sintering. Analysis of variance (ANOVA) establishes how the significance of each factor is related to output [8]. It shows the relationship between factors and the contribution given to analysis the impact of factors in regards to the target quality, thereby facilitating the discovery of significant factors.
Injection molding simulation
Mold flow analysis software was used to evaluate the plastic flow within the mold to ensure molding quality. The Taguchi experiment design method was adopted to determine optimal parameters for the injection molding which formed an orthogonal array of L27(37). The control factors and levels of L27 are shown in Table 1. The quality objective of injection molding was set as minimizing the shrinkage rate; therefore, the S/N ratio of STB was used to apply the following equation:
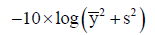 (1)
(1)
Where 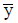 is sampling mean, s is sampling standard deviation.
is sampling mean, s is sampling standard deviation.
| A | B | C | D | E | F | G | |
|---|---|---|---|---|---|---|---|
| Control factors | Injection speed (%) | Injection pressure (%) | Packing time(sec) | Packing pressure(Mpa) | Material temperature(°C) | Mold temperature(°C) | Cooling Time(sec) |
| Level 1 | 20% | 70% | 0.5 | 55 | 140 | 50 | 5 |
| Level 2 | 50% | 80% | 1 | 75 | 150 | 60 | 10 |
| Level 3 | 90% | 90% | 1.5 | 95 | 160 | 65 | 15 |
Table 1: L27(37) experimental parameters.
Sample preparation
3 mol% yttria-stabilized zirconia powder was obtained from Tosoh (Japan) with 5.2 wt% Y2O3 and a total impurity content of less than 0.1 wt% excluding HfO2. Dental implants were produced by injection molding using the set of optimal parameters obtained using the Taguchi method. After injection molding process, dental implants proceeded to sinter.
Sintering
The Taguchi experiment design method was adopted to determine optimal parameters for the sintering which formed an orthogonal array of L9(34). The quality objective for sintering was set as maximizing density; therefore, the S/N ratio of LTB was applied using the following equation:
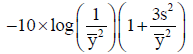 (2)
(2)
where 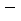 is sampling mean, s is sampling standard deviation.
is sampling mean, s is sampling standard deviation.
To determine the levels of holding temperature, samples were carried out by Thermogravimetric analysis/ Differential scanning calorimetry (TGA/DSC) in advance, with a heating and cooling rate of 10°C/min to a maximum temperature at 800°C for a 10 min holding time. To assess the effect of heating rates, the experiment was separated into two parts prior to experiment: (1) with a heating rate of 4°C/min to 1350°C and held for 2 hr (process A); (2) with water and thermal de-binding performed in advance, followed by sintering (process B). The implants were immersed in deionized water for 24 hours at room temperature during water debinding, with thermal de-binding temperatures of at 320°C and 420°C according to the results of TGA/DSC.
Structure and properties
Microstructural analysis was performed with a scanning electron microscope (SEM) to observe the binder before and after the sintering process. The hardness of ground and polished sintered dental implants was assessed using Vickers hardness testing machine. Density was calculated according to Archimedes principle. Shrinkage rates of critical dimensions were measured by optical projector, the one piece structure of Y-TZP dental implant and the critical dimensions are shown in Figure 1.
Analysis of injection molding
Figures 2a and 2b respectively shows the results of S/N effect plot and ANOVA for L27. According to S/N effect plot, the set A3B2C3D3E1F1G3 was found to produce the best results with an injection speed of 90%, injection pressure of 80%, packing time of 1.5 sec, packing pressure of 95 Mpa, material temperature of 140°C, mold temperature of 50°C, and cooling time of 15 sec (with injection speed and pressure respectively maintained at 178.254 mm/sec and 250 Mpa). Of all factors, cooling time had the greatest contribution of 29.79% which based on the analysis results of ANOVA. The results indicate that the most important factor was the cooling time which affected shrinkage rates during injection molding process, followed by the mold temperature, which was responsible for 25.70% of the resulting shrinkage As shown in Table 2, the optimal design improved shrinkage rates by 50% above the initial design. Average injection molding shrinkage rates of 0.50 ± 0.08% suggest that shrinkage may be an insignificant factor in future molds designs.
| Initial design | Optimal design | |
|---|---|---|
| Critical dimensions | Shrinkage rate (%) | |
| â? | - | - |
| â?¡ | 0.70 | 0.44 |
| â?¢ | 0.71 | 0.47 |
| â?£ | 0.77 | 0.58 |
Table 2: Shrinkage of critical dimensions of injection molding.
Analysis of sintering process
De-binding parameters analysis by TGA/DSC: Melting or vaporization of the subjects produced an endothermic or exothermic reaction. Endothermic peaks are shown in Figure 3 with temperature periods of 50 to 80°C, 260 to 380°C, and 400 to 500°C. The binder melting period took place at temperatures between 50 to 80°C, as evidenced by the lack of obvious weight changes at this temperature period. The first and second binder vaporization periods respectively took place at temperatures between 260 to 380°C and 400 to 500°C, as evidenced by the obvious weight changes at these temperatures. Within these temperature periods, the heating rates should be slower to ensure enough time to eliminate the binder [12,13].
Sintering processes: Process A of Figure 4 shows that implants sintered from room temperature to 1350°C without binder melting and vaporization resulted in surface cracking. Process B of Figure 4 shows that binder removal with water and thermal de-binding before sintering resulted in no surface cracks, suggesting that implants need to be subjected to water and thermal de-binding prior to sintering.
Optimum sintering parameters by Taguchi method: Figure 5 shows the sintering process and control factors of L9. In L9, four control factors including (A) sintering temperature, (B) holding time for sintering temperature, (C) holding temperature for the first binder vaporization period, and (D) holding temperature for the second binder vaporization period and levels are shown in Table 3. According to the analysis of TGA/DSC, the factorial levels of the holding temperature for the first binder vaporization period were set at 280, 320, and 360; for the second binder vaporization period at 420, 440, and 460. Zirconia assumes a tetragonal structure at temperatures above 1170°C; therefore, the factorial levels of the sintering temperatures were set at 1200°C, 1300°C and 1400°C. All implants were sintered according to Figure 5 which shows the heating rate for each steps of sintering process. Figures 6a and 6b respectively shows the results of S/N effect plot and ANOVA. According to S/N effect plot, the optimal design was A3B3C3D3, with a sintering temperature at 1400°C, a holding time for 3hr, a holding temperature at 360°C for the first binder vaporization, and a holding temperature at 460°C for the second binder vaporization. Of all factors, sintering temperature had the greatest contribution of 95.17% which based on the analysis results of ANOVA. The result showed that the sintering temperature has the greatest impact on density.
| Control factors | Level 1 | Level 2 | Level 3 |
|---|---|---|---|
| A. Sintering temperature | 1200 | 1300 | 1400 |
| B. Holding time | 1 | 2 | 3 |
| C. Holding temperature (1st binder vaporization period) | 280 | 320 | 360 |
| D. Holding Temperature (2nd binder vaporization period) | 420 | 440 | 460 |
Table 3: L9(34) experimental parameters.
Observation of microstructure
Figure 7 shows the microstructure before and after sintering at 1350°C. The figure indicates that the binder was apparently distributed inside the implants prior to de-binding and sintering. The vaporization of the binder increased porosity, but sintering eliminated the pores and increased implant density due to diffusion. Sintering at 1350°C produced an implant density of 5.70 g/cm3, while that of implants which did not undergo de-binding and sintering was 3.45 g/cm3.
Properties analysis
Taguchi method results show that the optimal sintering temperature is 1400°C, and the S/N ratio for factor A increased with temperature, as shown in Figure 6a. As a result, the following discussion focuses on the influence of sintering temperatures between 1200°C to 1500°C. Figure 8 shows the density measurement results. Implant density was lower in implants sintered at temperatures below 1400°C (5.23 g/cm3 at 1200°C and 5.55 g/cm3 at 1300°C) due to insufficient densification. At sintering temperatures above 1400°C, the implants’ relative density of implants was nearly 99% of the theoretical value. As shown in Figure 9, sintering temperatures between 1200°C to 1300°C resulted in lower hardness values due to insufficient density, while the density of implants sintered at higher temperatures increased to approximately Hv 1450. Hardness could not be further increased because of full densification, and would start decreasing within a specific temperature range [14-16]. As presented in this study, the implants hardness slightly decreased after 1400°C, and further sintering did not significantly improve hardness. Table 4 shows shrinkage rates of critical dimensions for various sintering temperatures. The shrinkage rate in critical dimension â? is negligible, as shown by the stable angle before and after sintering. Shrinkage rates increased with temperature, with sintering at 1200°C and 1300°C producing lower shrinkage rates of 12.86 ± 0.72% and 19.05 ± 0.51% due to lower density. However, at temperatures of 1400°C and above, the shrinkage value increased from 20.82 ± 0.70% to 21.34 ± 0.20%. These shrinkage rates should be taken into consideration in future injection mold designs.
| Average shrinkage rate (%) | ||||
|---|---|---|---|---|
| Critical dimensions Sintering temperatures | â? | â?¡ | â?¢ | â?£ |
| 1200°C | - | 13.05 | 13.58 | 11.94 |
| 1300°C | - | 19.37 | 19.28 | 18.56 |
| 1400°C | - | 21.48 | 20.82 | 20.16 |
| 1450°C | - | 21.02 | 21.23 | 23.02 |
| 1480°C | - | 21.16 | 21.57 | 21.34 |
| 1500°C | - | 21.14 | 21.56 | 21.32 |
Table 4: Average shrinkage of critical dimensions.
A optimal design parameter set was determined to include an injection speed of 90%, an injection pressure of 80%, a packing time of 1.5 sec, a packing pressure of 95 Mpa, a material temperature of 140°C, a mold temperature of 50°C, and a cooling time of 15 sec (with the injection speed and pressure respectively maintained at 178.254 mm/ sec and 250 Mpa). The simulation of the injection molding process with the optimal parameters improved shrinkage rates by 50% above the results obtained using the initial parameters. Results show that the obtained shrinkage rates could be a negligible factor in future mold design because the impact of the experimental data was so low (0.50 ± 0.08%). On the other hand, the optimal design parameters for sintering include a sintering temperature of 1400°C, a holding time of 3 hr, and temperatures of 360°C and 460°C, respectively for the first and second binder vaporizations. The measurement results show that zirconia dental implants sintered with optimal design parameters could achieve a density of 5.96 g/cm3, a hardness of Hv 1489 ± 131, and a shrinkage rate of 20.82 ± 0.7%. The density, hardness and shrinkage rate of 3Y-TZP zirconia dental implants increased with sintering temperatures in the range of 1400°C to 1500°C, with density increasing from 5.96 g/cm3 to 6.00 g/cm3, hardness from 1489 ± 131 to 1489 ± 71, and the shrinkage rate from 20.82 ± 0.70% to 21.34 ± 0.20%.