Journal of Pollution Effects & Control
Open Access
ISSN: 2375-4397
ISSN: 2375-4397
Research Article - (2014) Volume 2, Issue 2
Drainage water reuse in Egypt represents a major potential water resource for expanding the cultivated area. El-Noubaria canal irrigates 2.8 million feddans. Alexandria Drinking Water Treatments intakes; mainly El-Noubaria WTP and K.40 WTP take their water from El-Noubaria canal and El-Alamein WTP obtains raw water from El-Nasr canal branched at K. 55 from El-Noubaria canal, which suffers from pollution by agriculture drainage water. A main quantity of such drainage water is coming from Omumm Drain. The present study focuses on the impact of the drainage water of El- Omumm Drain by mixing of its water directly into El-Noubaria canal, which serves agriculture uses and drinking water. Electrical conductivity (EC), was increased in Noubaria canal after receiving point at Nasr canal intake (site III) by 83.9, 109, 118, 81.8%, at Noubaria WTP intake (site IV) by 43.8, 5, 69.5, 43.4% and at K40 WTP (site V) by 66.7, 49.7, 78.6, 67.8% during winter, spring, summer and autumn, respectively due to the attribute of Omumm Drain effluent.. a-BHC, b-BHC, heptachlor, diendrin and endrin, were increased after mixed fresh water with Omumm Drain effluent at Nasr canal intake (km 55), and the average observed values were 2.65, 1.1, 2.4 and 1.3 μg/l for a-BHC, b-BHC, heptachlor and diendrin while endrin not detected during summer and autumn. The ANOVA test showed effects of sites on EC were significant The ANOVA test showed the effect of sites on COD were significant and the seasonal effects on BOD were significant.
Keywords: Noubaria canal; Omumm drain; Water quality assessment
In the 1970s, a policy was formulated for reusing drainage water for irrigation. Because irrigation water was scarce, reuse of drainage water became an essential element in water management starting in the late 1980s after a series of studies by the Drainage Research Institute and others [1]. This reuse was made possible by the extensive network of open drains and subsurface drains that was being developed. Of all the countries in arid regions, Egypt has gone farthest in reusing drainage water. In Upper Egypt, drainage water is reused by gravity flow. In Lower Egypt; reuse is by lifting water to the irrigation canals. In implementing the reuse policy, 25 centralized mixing stations were built on the main drains in the Nile Delta, managed by the MWRI Mechanical and Electrical Department (MED) [2]. Upper limits were set on the salinity of the water that can be used for mixing. The official reuse of drainage water was 2.9 BcM/yr in 1984–85, it increased to 4.4 BcM/yr in 1996–97 [1]. The Water Policy Reform Program of 1999 projected further increases in reuse to 8.4 BcM/yr by 2017. This water will be required to meet demand for new horizontal expansion projects, including those now coming on stream, as well the increased water demand from the domestic and industrial sectors. The national policy seeks a 20 percent increase the volume of water available by 1997–2017. Of this, a fifth would have to come from increased reuse. In the context of water quality management, agriculture must be seen as a widespread non-point source of pollution. Pollutants include leached salts, nutrients like nitrogen and pesticides [2].
These non-point sources will be collected in agricultural drains to form point sources of pollution for the River Nile, lakes and irrigation canals in case of mixing water for reuse. Although there are different mechanisms for retaining the pollutants by passing the polluted water through soil, the non-point sources of pollution may influence the groundwater quality.
In the downstream direction, the water quality gradually deteriorates due to the poorly treated wastewater discharges from both domestic and industrial activities and uncontrolled mixing with water from polluted drains. Therefore, they contain high levels of various pollutants, such as fecal bacteria, heavy metals and pesticides. Some drains should be considered as open sewage system that badly due to the production of hydrosulfide [3].
With reuse, all kinds of pollutants are diffused throughout the entire water network. Reuse of drainage water is no longer limited to irrigation and drainage only but involves wastewater disposal, drinking water supply, and aquatic ecology as well. A number of mixing stations were closed, mainly because the drinking water supply from the irrigation canals was jeopardized by the inflow of low-quality drainage water. Of the 25 mixing stations in the delta, five stopped operations and two were interrupted for extended periods. The closed mixing stations represent a capacity of 1.56 BcM/yr, about a third of total capacity. The largest mixing station no longer in operation is on the Omumm Drain in the West Delta, where water supplies are polluted not so much by agricultural production water as by untreated municipal and industrial wastewater from the Abu Hommos and Shereshtra area.
Omumm drain water is mixed with Noubaria canal at k. 46.5 to meet the increasing demand of irrigation water for new reclaimed lands. This mixing of such polluted water causes some problems to the drinking water treatment plants along the Noubaria canal. The pollution of mixed that irrigation water from Omumm drain result in adverse effect on physical, chemical and biological characteristics of Noubaria canal. Omumm drainage system receives untreated and semi-treated wastewater from Abou-Hommos city, Hosh Isa city and Abou-El Matameer city.
The enrichment and distribution of metals in water column depends on combination of environmental, physico-chemical and biological factors [4].
This study has been performed to follow the effect of El-Omumm Drain effluents on seasonal variation of pH: dissolved oxygen; heavy metals, pesticides, biological characteristics and major inorganic compounds in surface water of El-Omumm Drain; at the point of discharge, and also before and after the discharge point. Comparison with five selected locations along the Noubaria canal was carried out in order to determine the effect of waste disposal discharge into the Noubaria canal water and obtain information, which may help in giving information about variability in Noubaria canal quality.
Description of the area of investigation
The area of investigation is 46.5 Km to the North of Kanater Bolin and. five different sampling sites are chosen along the Omumm drain. Figure 1a shows the selected sites. It must be noted that, site (II) which represents, Omumm Drain, is the site which receives domestic and agriculture wastes from south Behara governorate then pour its content into the Noubaria canal at site III. Figure 1b shows the position of each site. Accordingly the sites under investigation are divided into three classes the first include station II which is at Omumm drain itself. The second include sites I, which are located before, and III, IV, V after Omumm Drain respectively.
Sixty water samples were collected from the five sites. Fifteen samples were taken per season, starting from June 2012 to May 2013. From subsurface of each sites two samples were taken using water collectors. The experimental procedures of the studied parameters were carried out according to standard methods (SMWW, 2012) as follows:
Physical parameters
The different parameters were determined according to the standard methods [5].
pH: The pH was measured using an analytical pH meter (CG- 840 SCOTT, Germany); the instrument was calibrated using standard pH buffers (4, 7, and 10) [5].
Dissolved Oxygen (DO): DO was determined by using the modified Winkler method according to standard methods (SMWW, 2012)[5] and expressed as (mg/l).
Electrical conductivity: The electrical conductance of water was measured by using EC-meter model (Janway 3000) after calibration by 0.1 KCl (1413 μmos/cm) (SMWW, 2012) [5].
Temperature: The temperature was measured using an analytical thermometer embedded in (Electrical Conductivity - Jenway 4320) [5].
Turbidity: Turbidity was measured by a nephelometric method (NTU) using turbidimeter (Hach model 2100N), Hach Co., USA. Formazin turbidity standard were prepared weekly and used as a reference according to Standard Methods for Water and Wastewater Treatment [5].
Inorganic parameters
Total hardness: Calcium and magnesium (total hardness) were determined using EDTA titimetric method according to APHA 1999. the ethylene diamine tetra acetic acid (EDTA) form a chelated soluble complex when added to a solution of certain metal cations. The calcium and magnesium were determined by using Eriochrome black T as indicator at pH 10. The calcium ions are determined alone by using Mureoxide as an indicator at high pH (12-13) by adding NaOH, the magnesium is estimated as the difference between the total hardness and calcium as CaCO3. The concentrations of calcium and magnesium are expressed as mg/l [5].
Sulphate: It is determined by turbidimetric method according to standard methods. This method depends on the precipitation of sulphate ion in acidic medium using of barium chloride forming barium sulphate crystals. Barium sulphate suspension was carried out using HACH turbidimeter model 2100A (USA). Sulphate concentration was determined by comparison with standard values [5].
Chloride: Chloride ions was determined according to standard methods with titration of 50 ml sample against standard silver nitrate solution (Mohr’s method) and expressed as mg/l [5].
Ammonia: Ammonia was determined by “Nesslerization method” according to standard methods. This method depends on the reaction of water samples containing ammonia as ammonia salt with Nessler`s reagent in the presence of Rochelle salt. The resultant yellow colour due to complexation between ammonia and Nessler`s reagent is proportional to the concentration of the present ammonia. This colour was measured spectrophotometrically at wave length 420 nm by using (UV/VIS) spectrophotometer Lambda 6 Perkin Elmer instrument [5] (Table 1).
| Source of Variation | SS | df | MS | F | P-value | F crit |
| season | 0.406561 | 3 | 0.13552 | 1.054605 | 0.4043 | 3.490295 |
| sites | 6.087091 | 4 | 1.521773 | 11.84228 | 0.000395 | 3.259167 |
| Error | 1.54204 | 12 | 0.128503 | |||
| Total | 8.035692 | 19 |
Table 1: ANOVA test for ammonia.
Nitrate: Nitrate was determined according to standard methods; 50 ml of sample were filtered and then acidified to less than pH 2, where measurements were done at 220 nm using a spectrophotometer Cecil 2040, (Perkin E. Co, Germany).The instrument was calibrated weekly by using nitrate standard solution at 0.5, 1.0, 2.0, 4.0, 6.0, 8.0, 10.0, 12.0, 14.0 mg/l to produce calibration curve [5].
Nitrite: Nitrite was determined according to standard methods; through formation of a reddish purple azo dye produced at pH 2.0 to 2.5 by coupling diazotized sulfanilamide with N-(1-naphthyl)- ethylenediamine dihydrochloride (NED dihydrochloride). 50 ml of sample were filtered and then acidified to less than pH 2, where measurements were done at 543 nm using a spectrophotometer Cecil 2040, (Perkin E. Co, Germany).The instrument was calibrated weekly by using nitrite standard solution at 0.5, 1.0, 2.0, 4.0 mg/l to produce calibration curve [5].
Chemical Oxygen Demand (COD): This method is called Open Reflux Method. Chemical oxygen demand is used as a measure of the oxygen equivalent to the organic matter content of the sample. A sample is refluxed in strongly acid solution with a known excess of potassium dichromate. After digestion, the remaining unreduced K2Cr2O7 is titrated with ferrous ammonium sulphate to determine the amount of K2Cr2O7 consumed and the oxidizable organic matter is calculated in terms of oxygen equivalent (SMWW, 2012) [5].
Biological Oxygen Demand (BOD): Determination of BOD was carried out using a 5-day BOD test according to standard methods. Basically the BOD was measured dissolved oxygen consumed by aerobic microorganisms as they metabolise the complex unstable molecules of pollutions e.g. proteins, carbohydrates, lipids….etc. into CO2, H2O, sulphate….etc. as simple stable inorganic compound [5].
Heavy metals: Four metals included Al, Fe, Ba and B were measured in water samples using (coupled plasma 400 emission spectrometer Perkin Elemer Emission Spectrometer) according. The Preservation and digestion were carried out as follows:
• Preservation: Samples were preserved immediately after collection by acidifying with concentrated HNO3 to pH<2 by adding 5 ml nitric acid to l liter water samples. After acidification store in refrigerator.
• Digestion: Heavy metals were extracted in water samples using digestion by nitric acid method as follows below.
Mix well 500 ml of sample in a beaker at hot plate, add 10 ml nitric acid bring slow boil, evaporate on a hot plate till volume become 200– 100 ml before precipitation occurs, continue heating add another 10 ml of nitric acid until the volume reached to 100–80 ml until digestion is complete. The samples do not dry during digestion, mix well and complete to 100 ml by distilled water, and take portion of their solution for required determination. Four metals included Al, Fe, Ba and B were determined in the digested solution using inductively coupled plasma emission spectrometry [5].
Organic parameters
TOC: TOC was determined according to standard methods; 50 ml of sample were filtered through 0.45 μm membrane filter and then acidified to less than pH 2, where measurements were done at 254 nm using a spectrophotometer Cecil 2040, (Perkin E. Co, Germany).The instrument was calibrated weekly by using dipotassium phthalate at 0.5, 1.0, 2.0, 4.0, 6.0, 8.0, 10.0, 12.0, 14.0, 16.0, 18.0, 20.0 mg/l to produce calibration curve [5].
Chlorinated pesticides: The liquid-liquid extraction (LLE) gas chromatographic (GC) method is used to monitor both the PCBs and the organochlorine pesticides simultaneously. This method has excellent sensitivity. One liter of sample is extracted with methylene chloride. The extract is dried and exchanged to hexane during concentration. If other determinations having essentially the same extraction and concentration steps are to be performed, a single sample extraction is sufficient. The extract is separated by gas chromatography and the compounds are measured with an electron capture detector according to EPA 508.1 method [6].
Microbiological examination
Determination of bacterial count: The enumeration of total bacterial count (TBC) in water sample was done using the spreading plate technique over Difco agar medium (plate count Agar ref. 247940, USA). The medium was prepared by adding 23.5 g to one litter distilled water. The medium was dissolved by boiling for 1 min, and then 20 ml of dissolved medium were transferred into 20 ml clean test tube. The tubes were then autoclaved for 20 minutes at 121°C, and then each tube was poured while at 45°C into a sterile Petri-dish. Water sample was serially diluted to a suitable concentration to get 30 – 300 single separated colonies. One hundred microlitter of the suitable dilution were transferred into the middle of the medium surface under aseptic conditions, and then the sample was distributed on the surface using sterile spreader. Plates were left for 10 min, then inverted and incubated at 30ºC for 24 hours. The resulting colonies were counted using CC 30, Ch, and Hern scientific colony counter [5].
Biological Examination
Determination of algal count: Algae were counted in 10 ml sample of raw water. The sample was centrifuged for 10 min at 4000 rpm. The supernatant was discarded, and then the pellet was dissolved in 1 ml of saline water, which was transferred to Sedgwick - rafter counting chamber that was covered by a slide cover. Algae were counted using Carl Zeiss Axiolab Optical Microscope fitted with 3CCD colour video camera attached to TV monitor. The count was done from the TV monitor [5].
Sixty samples were collected from five sites from June 2008 to July 2009. The first site at before mixing point by 1000 m (location I) at Abd- Bridge, the second point at Noubaria canal receive the discharge water from Omumm drain (location II) at k.m 46.5, the third point after receiving drainage water of Omumm drain to Noubaria canal by 8 k.m (location III) at Nasr canal intake, forth point at Noubaria WTP intake at K.m 81.3 along the Noubaria canal (location IV) and the end point at K.40 WTP intake at K.m 96.0 along the Noubaria canal (location V).
Water temperature showed noticeable seasonal trends with a lowest value (15.3°C) recorded in autumn and a highest (30.8ºC) in summer (Figure 1). The slight variations between different sites were mainly due to different sampling times. Temperature is a key parameter which influences physical, chemical and biological transformation in the aquatic environment [7]. It is evident that in Figure 2, electrical conductivity (EC), was increased in Noubaria canal after receiving point at Nasr canal intake (site III) by 83.9, 109, 118, 81.8%, at Noubaria intake (site IV) by 43.8, 5, 69.5, 43.4% and at K40 WTP (site V) by 66.7, 49.7, 78.6, 67.8% during winter, spring, summer and autumn, respectively due to the attribute of Omumm Drain effluent.. The ANOVA test showed effects of sites on EC were significant (Table 2).
| Source of Variation | SS | df | MS | F | P-value | F crit |
| season | 342816.6 | 3 | 114272.2 | 5.740621 | 0.011313 | 3.490295 |
| sites | 21327686 | 4 | 5331921 | 267.8564 | 1.28E-11 | 3.259167 |
| Error | 238870.7 | 12 | 19905.89 | |||
| Total | 21909373 | 19 |
Table 2: ANOVA test for electrical conductivity.
It is evident that in Figure 3, dissolved oxygen (DO), was decreased in Noubaria canal after receiving point at Nasr canal intake (site III) by 3.7, 7.0, 4.7, 2.0% during winter, spring, summer and autumn, respectively due to the attribute of Omumm Drain effluent and due to its consumption by oxidation of nitrogenous compounds, then the DO values return to increase at sites IV and V due to navigation activity and the dilution effect of the polluted water from Omumm Drain. DO was correlated negatively with nitrate, nitrite, sulphate and ammonium during the most seasons (Table 3). The dissolved oxygen content can be an indicator of organic loading nutrient input and biological activity. Cole and Saad [8] reported that, the decrease in DO during the hot seasons in surface water is due to the elevation of water temperature which decreases the solubility rate of atmospheric oxygen. The ANOVA test showed effects of sites on DO parameters were significant (Table 4).
| Temp | pH | EC | DO | TOC | COD | BOD | TH | SO4 | Cl | NH3 | NO3 | NO2 | Al | Fe | Ba | B | a-BHC | b-BHC | Heptachlor | TPC | FC | TAC | BGA | |
| Temp | 1.0 | |||||||||||||||||||||||
| pH | -0.4 | 1.0 | ||||||||||||||||||||||
| EC | -0.2 | -0.6 | 1.0 | |||||||||||||||||||||
| DO | 0.4 | 0.4 | -0.8 | 1.0 | ||||||||||||||||||||
| TOC | 0.0 | -0.3 | 0.2 | -0.1 | 1.0 | |||||||||||||||||||
| COD | -0.5 | -0.3 | 0.9 | -0.8 | 0.2 | 1.0 | ||||||||||||||||||
| BOD | -0.5 | -0.2 | 0.2 | -0.3 | 0.3 | 0.5 | 1.0 | |||||||||||||||||
| TH | -0.1 | -0.6 | 0.1 | -0.8 | 0.2 | 0.9 | 0.2 | 1.0 | ||||||||||||||||
| SO4 | -0.2 | -0.6 | 1.0 | -0.8 | 0.2 | 0.9 | 0.2 | 1.0 | 1.0 | |||||||||||||||
| Cl | -0.2 | -0.5 | 0.9 | -0.8 | 0.1 | 0.9 | 0.1 | 0.9 | 0.9 | 1.0 | ||||||||||||||
| NH3 | 0 | -0.6 | 0.8 | -0.8 | 0.2 | 0.7 | 0.3 | 0.9 | 0.8 | 0.7 | 1.0 | |||||||||||||
| NO3 | 0.4 | -0.7 | 0.6 | -0.4 | 0.0 | 0.2 | -0.3 | 0.6 | 0.6 | 0.6 | 0.6 | 1.0 | ||||||||||||
| NO2 | -0.4 | -0.3 | 0.7 | -0.5 | 0.2 | 0.6 | 0.0 | 0.6 | 0.7 | 0.6 | 0.3 | 0.4 | 1.0 | |||||||||||
| Al | -0.4 | -0.4 | 0.9 | -0.9 | 0.1 | 0.9 | 0.3 | 0.9 | 0.9 | 0.9 | 0.7 | 0.4 | 0.6 | 1.0 | ||||||||||
| Fe | -0.3 | -0.3 | 0.8 | -0.6 | 0.2 | 0.5 | 0.0 | 0.7 | 0.8 | 0.6 | 0.6 | 0.5 | 0.8 | 0.7 | 1.0 | |||||||||
| Ba | -0.1 | -0.5 | 0.8 | -0.8 | 0.2 | 0.7 | 0.2 | 0.9 | 0.8 | 0.8 | 0.8 | 0.5 | 0.4 | 0.9 | 0.7 | 1.0 | ||||||||
| B | 0.0 | 0.4 | 0.8 | -0.6 | 0.0 | 0.6 | -0.2 | 0.8 | 0.8 | 0.9 | 0.6 | 0.7 | 0.5 | 0.7 | 0.6 | 0.6 | 1.0 | |||||||
| a-BHC | -0.5 | -0.1 | 0.6 | -0.6 | 0.2 | 0.7 | 0.4 | 0.5 | 0.6 | 0.5 | 0.6 | 0.3 | 0.6 | 0.6 | 0.5 | 0.5 | 0.4 | 1.0 | ||||||
| b-BHC | -0.6 | 0.0 | 0.7 | -0.6 | 0.3 | 0.6 | 0.1 | 0.7 | 0.5 | 0.7 | 0.4 | 0.3 | 0.8 | 0.6 | 0.7 | 0.4 | 0.5 | 0.7 | 1.0 | |||||
| Heptachlor | -0.5 | 0.3 | 0.3 | -0.4 | -0.1 | 0.5 | -0.1 | 0.2 | 0.3 | 0.4 | 0.1 | 0.0 | 0.4 | 0.4 | 0.2 | 0.1 | 0.4 | 0.6 | 0.7 | 1.0 | ||||
| TPC | -0.1 | -0.6 | 0.9 | -0.8 | 0.1 | 0.8 | 0.2 | 1.0 | 0.9 | 0.9 | 0.8 | 0.6 | 0.5 | 0.9 | 0.6 | 0.9 | 0.8 | 0.5 | 0.5 | 0.2 | 1.0 | |||
| FC | 0.1 | -0.6 | 0.8 | -0.8 | 0.1 | 0.7 | 0.2 | 0.9 | 0.8 | 0.8 | 0.9 | 0.6 | 0.2 | 0.8 | 0.5 | 0.9 | 0.7 | 0.4 | 0.2 | 0.1 | 0.9 | 1.0 | ||
| TAC | 0 | 0.0 | -0.1 | 0.1 | -0.4 | -0.1 | -0.1 | -0.1 | -0.1 | -0.1 | -0.2 | 0.0 | -0.1 | 0.0 | -0.1 | -0.1 | -0.1 | -0.3 | -0.3 | -0.2 | -0.1 | -0.1 | 1.0 | |
| BGA | 0 | -0.4 | 0.7 | -0.8 | 0.2 | 0.5 | 0.0 | 0.7 | 0.7 | 0.7 | 0.6 | 0.5 | 0.3 | 0.7 | 0.4 | 0.7 | 0.6 | 0.5 | 0.5 | 0.4 | 0.7 | 0.7 | -0.4 | 1.0 |
Table 3: Correlation coefficient matrix between physical and chemical parameters during the present study.
| Source of Variation | SS | df | MS | F | P-value | F crit |
| season | 2.28214 | 3 | 0.760713 | 9.535138 | 0.001688 | 3.490295 |
| sites | 13.38088 | 4 | 3.34522 | 41.93056 | 5.85E- 07 | 3.259167 |
| Error | 0.97536 | 12 | 0.07978 | |||
| Total | 16.62038 | 19 |
Table 4: ANOVA test for DO.
pH values were in the alkaline side (7.66-8.34). The higher pH values during winter might be due to the blooming of algae, which were accompanied by photosynthetic activity and consumption of CO2 with expect pH elevation [9] pH showed a negative correlation with most studied parameters, Table 3. It is evident that in Figure 4, turbidity in Omumm Drain effluents were lower than the Noubaria water turbidity during the period of the present study.
It is evident that in Figures 5-7, TOC, COD and BOD were increased in Noubaria canal after receiving point at Nasr canal intake (site III) by average increasing percentage (47.5, 20.7 and 32.6%), the maximum increasing percentage were 58.8, 26.6 and 44.4% and the minimum increasing percentage were 30.6, 10 and 13.8%, respectively. the maximum and minimum values of TOC and BOD were observed during autumn and summer, respectively. While the maximum and minimum values of COD were observed during winter and autumn, respectively due to the attribute of Omumm Drain effluent. The ANOVA test showed effects of sites on COD were significant (Table 5) and the seasonal effects on BOD were significant (Table 6). Most waters contain organic matter that can be measured as total organic carbon (TOC). The high value of TOC observed in cold period; this due to the decrease in the current water stream which facilitates the growth of algae and other microorganisms which the main source of TOC. During the study period, the most BOD and COD values in the study area of El-Noubaria canal after the point of receiving the effluent of Omumm Drain were exceeds the limit of 6 mg/l set by the law 48/1982 as maximum permissible limit for drinking water. As regard to spatial variation. Ali, 1998, reported that, the concentration of most physical and chemical parameters were increased northward more than southern region of river Nile, mainly due to agricultural and industrial, sewage wastes pour in the northern region [10-12].
| Source of Variation | SS | df | MS | F | P-value | F crit |
| season | 2299.97 | 3 | 766.6567 | 8.485252 | 0.0027 | 3.490295 |
| sites | 7123.732 | 4 | 1780.933 | 19.71113 | 3.3E-05 | 3.259167 |
| Error | 1084.22 | 12 | 90.35167 | |||
| Total | 10507.92 | 19 |
Table 5: ANOVA test for COD.
| Source of Variation | SS | df | MS | F | P-value | F crit |
| season | 683.948 | 3 | 227.9827 | 9.161081 | 0.001987 | 3.490295 |
| sites | 59.028 | 4 | 14.757 | 0.592984 | 0.674374 | 3.259167 |
| Error | 298.632 | 12 | 24.886 | |||
| Total | 1041.608 | 19 |
Table 6: ANOVA test for BOD.
| Source of Variation | SS | df | MS | F | P-value | F crit |
| Season | 0.00092 | 3 | 0.000307 | 6.994297 | 0.005639 | 3.490295 |
| Sites | 0.008693 | 4 | 0.002173 | 49.58099 | 2.3E-07 | 3.259167 |
| Error | 0.000526 | 12 | 4.38E-05 | |||
| Total | 0.010139 | 19 |
Table 7: ANOVA test for Al.
It is evident that in Figures 8-10 the hardness total, sulphate and chloride were increased after receiving point (Nasr canal intake km 55) due to Omumm Drain effluent, the maximum increasing percentage of hardness total during winter while sulphate and chloride during autumn. Calcium and magnesium are the principle ions responsible for water hardness. The hardness in water is derived largely from contact with the soil and rock formations [10,11]. Rain water as it falls upon the earth is incapable of dissolving the tremendous amounts of solids found in many natural waters. The ability to dissolve is gained in the soil where carbon dioxide is released by bacterial action. The soil water becomes highly charged with carbon dioxide which exists in equilibrium with carbonic acid. Under low pH conditions that develop, basic materials, particularly limestone formations are dissolved [13]. In raw waters, calcium is the main source of hardness and found in large quantities in ground water versus surface water. Malik [14] reported that, the chloride ion is one of the major inorganic anion in wastewater because of the presence of sodium chloride as a common article of diet, as well as, chloride ion may be increase by industrial processes. The high values of hardness total, sulphate and chloride were observed in cold period; this due to the decrease in the current water stream and nearly the amount of Omumm Drain discharge on Noubaria canal. Hardness total, sulphate and chloride were showed a positive correlation with most studied parameters, Table 3.
It is evident that in Figures 11-13, ammonia, nitrate and nitrite were increased in Noubaria canal after receiving point at Nasr canal intake (site III) by average increasing percentage (29.3, 37.5 and 172.4%), the maximum increasing values were observed during summer, winter and spring respectively. Ammonia is rarely present in natural waters and originates mainly from fertilizers runoff. Bacteria usually convert ammonia in raw water to nitrite then to nitrate [15,16]. Train, 1979 [17] reported that, the toxicity of ammonia is increased with high alkalinity and pH values and in some cases ammonia may reach high toxic levels, High concentration of dissolved ammonia in cold session may be attributed to flushing agricultural land with irrigation water in order to plant winter crops. Considerable amounts of nitrogen fertilizers are leached from agricultural land into Noubaria canal. The high value of ammonia observed in cold period; this due to the decrease in the current water stream. Ammonia, nitrate and nitrite were showed a positive correlation with most studied parameters, Table 3.
Al, Fe, Ba and B are common pollutants which are widely distributed in aquatic environment. According to United Nation Environmental Program, the threshold toxicity values of these metals are 0.3, 5.00, 0.005, 0.05 mg/l, respectively [18]. Iron is a component of iron-porphyrins haem and ferroxins and it is essential for growth and well being of living organisms including man. However it likely to show toxic effects when organisms are exposed to levels higher than normally required. Heavy metals may enter the river water from the geology of catchments soil. Probable sources included: sewage plants industrial, agriculture, domestic effluents and urban run-off [4,19]. De- Groot and Allersma [20] reported that, metals can enter rivers from different sources: rocks and soils, dead and decomposed plants and animal matter, from man’s activity including the discharge of various treated and untreated liquid wastes to the water. Data in represent graphically in Figures 12-15 indicated that, the metals were increased after fresh Noubaria water mixed with the Omumm Drain effluent during the most season, and the increasing percentage were ranged 28- 100%, 0-78%, 10-210% and 32-455% for Al, Fe, Ba and B, respectively. ANOVA test showed spatial effect was significant on Al as shown in Table 7.
It is evident that in Figures 16-20 the a-BHC, b-BHC, heptachlor, diendrin and endrin, were increased after mixed fresh water with Omumm Drain effluent at Nasr canal intake (km 55), and the average observed values were 2.65, 1.1, 2.4 and 1.3 μg/l for a-BHC, b-BHC, heptachlor and diendrin while endrin not detected during summer and autumn. Pesticides are used for agricultural as well as public health purposes. Pesticide residues may seep from the soil into drains, irrigation water and finally into the main canal such as Noubaria canal, the leaching mainly depends on the type of pesticides, soil characteristics, hydrogeological conditions, climatic factors, agrotechnical factors and human factors [21].
It is evident that in Figures 21-23, the average increasing percentage after receiving polluted water from Omumm Drain were 110, 45.6 and 55% for total plate count (TPC), total coliform TC) and fecal coliform (FC), respectively. The high values were observed during winter due to the decrease in the current fresh water stream and nearly the amount of Omumm Drain discharge on Noubaria canal. As a result of poor wastewater treatment, high concentrations of coliform bacteria are found in the Noubaria canal, most of observed FC was exceed the Egypt standard [2].
It is evident that in Figures 24 and 25, the average increasing percentage after receiving polluted water from Omumm Drain were 15.4 and 14.4 for total algal count (TAC) and blue green algae (BGA), respectively. The high values were observed during autumn due to the decrease in the current fresh water stream and nearly the amount of Omumm Drain discharge on Noubaria canal.
Various water quality indexes have been developed in the past 40 years and the utility of a water quality index (WQI) were summarized as a follow: i) volumes of water quality data are summarized in a single index value in an objective, rapid, and reproducible manner; ii) the numerical scale of an index facilitates evaluation of “within class” variations, thereby allowing identification of changes in water quality at a site that would not precipitate a change within the classification system; iii) the index values may be related to a “potential water use” classification scheme to help determine the ecological potential of the water body; iv) the index and associated water body classification scheme may be used in operational management to identify surface waters requiring priority action; and v) the index facilitates communication with the layperson, while maintaining the initial precision of measurement [22].
It is evident that in Figure 26, the average value after receiving polluted water from Omumm Drain was 442 (poorest) for water quality index (WQI) and the increasing percentage ranged from 7.0 to 47.0% as a result of mixed polluted water from Omumm Drain to Noubaria canal at Nasr canal intake (km 55.0) [23]. Water quality index is the result of the sum of the products of water quality ratings (qi) and weighting each individual parameter (wi) divided by each Egypt standard value (Si), according to the following equation:
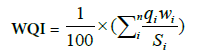
WQI, > 140% represents the poorest and <50% the highest water quality. In this study, class rating was adopted: 51–80%, Good; 81– 110%, Fair; 111–140%, Poor.
1. In order to conserve water quality in Noubaria canal; Ministry of Water Resource and Irrigation should be limit the mix of irrigation drainage water from Omumm drain with Noubaria canal and should be look for alternatives for using this water for agriculture only.
2. Boustan canal locate north of Omumm effluent point and feed from Noubaria canal from the other side and can be take the effluent of Omumm drain which useful in agriculture uses.
3. Water treatment plants intakes should be protected according to Ministerial law No. 301/1995.
4. Limit the discharge of wastewater on irrigation drain and fresh water canal by extend the sanitary services to all rural area.