Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2016) Volume 6, Issue 2
This study was designed to determine the preliminary phytochemical compounds, to study the potent bioactive compounds using GC-MS analysis and antioxidant potentials of Amaranthus hybridus and Amaranthus cruentus leaves after slicing, sun drying, and shade drying and cooking processing techniques. Ascorbic acid, total phenol content and antioxidant potential were determined by volumetric, Folin Ciocalteu reagent method and DPPH respectively. After preliminary screening for secondary metabolites evaluation, biochemical components where screened by GC-MS techniques. Results of phytochemical evaluation confirmed the presence of phytosterols, tannins, glycosides, saponins, flavonoids and terpernoids in both samples whereas alkaloids and anthraquinones were absent. Interestingly GC-MS analysis revealed the presence of fourteen compounds in A. cruentus and eighteen in A. hybridus. Phytol (39% and 40%) and 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (13% and 17%) were the most abundant components in the two extracts. The total phenol content of SDS A. hybridus was significantly higher (p < 0.05) than that of any treatment of A. cruentus. SHDU of both samples revealed higher antioxidant activities and vitamin C content while cooking reduced drastically the Vitamin C content in both samples. In view of the results of this study, it could be concluded that various processing methods trough make green leafy vegetables more palatable by reducing some anti-nutrients content and extend their shelf-life. However they influenced on the phenolic content and antioxidant behavior and caused significant decrease (P < 0.05) in ascorbic acid contents of the green leafy vegetables. Combination of these leafy vegetables with others foodstuffs may be recommended to satisfactorily meet the RDA.
Keywords: ICP-OES, GC-MS analysis, Amaranthus, Antioxidant activity
Traditional leafy vegetables form a part of the diets and incomes of rural and urban households in Cameroon by providing adequate amounts of protein, many vitamins, dietary fiber and other important nutrients which are usually in short supply in daily diets [1]. However their contribution of vitamins and other nutrients is limited due to the presence of anti-nutritional factors that render some of the nutrient unavailable for human beings. Epidemiological evidence has clearly shown that diets based on fruits and vegetables are associated with a lower risk of several degenerative diseases, such as cancers [2] and cardiovascular diseases [3]. This association is often attributed to different antioxidant components, such as some vitamins like vitamin C, polyphenols and other phytochemicals compounds. An antioxidant is a substance that has the ability to delay the oxidation of a substrate by inhibiting the initiation or propagation of oxidizing chain reactions caused by free radicals [4]. It plays important roles to prevent fats and oils to becoming rancid and protects human body from detrimental effects of free radicals. There have been concerns about the widely use of synthetic antioxidant around the world and they have been scrutinized because of their possible toxic effects and promoters of carcinogenesis [4]. Hence, strong restrictions have been placed on their application and there is a trend to substitute them with naturally occurring antioxidants [5]. Fruits and vegetables are major sources of dietary antioxidant [6] which help in cellular defenses and prevent cellular comportments against oxidative damage [7]. A. hybridus and A. cruentus locally called “Felon” and “red felon” in West region of Cameroon are widely distributed throughout the state of Cameroon and particularly in western region. Despite the fact they are rich sources of protein, many vitamins, and minerals and possess antioxidant potentials, they are underutilized when compared to the introduced varieties due to the flavor and unfamiliar taste impacted on the food [8,9].
They grow abundantly during the rainy season however; they are highly perishable and sometimes unpalatable when taken raw [10]. Hence they are subjected to various processing treatments which extend their shelf life and also improve the bioavailability of their constituent nutrients and palatability. Among those processing methods, sun drying is the cheapest and most commonly used in Cameroon compared to shade drying and cooking. The present study was undertaken to assess the effects of different conventional processing techniques (sun drying, shade drying and cooking sliced and non-sliced) common in Cameroon on phytochemical, antioxidant activity, total phenol and vitamin C content in A. hybridus and A. cruentus leaves.
Plant sample collection and treatment: The leaves of Amaranthus sp. were obtained from cultivated farmlands located at Foto, Dschang city of West region of Cameroon from April 2013 to September 2013. The collected samples were thoroughly mixed, their stalks and dust removed and divided in two groups: one group was chopped before sun drying, shade drying and cooking while the other group was sun and shade dried without any chopping. These selected green leafy vegetables were carefully plucked and each group was divided into three portions. The first portion was sun-dried for about 5 hrs daily by turning 4-5 times daily for 2-3 days at 24-26°C with a relative humidity between 50-70% till they were properly dried while the second portion was spread on cotton sheets and kept in well ventilated room for about six days till they become crisp and brittle to touch. The last part was cooked by weighing one kilogram and washing before blanching in hot water at 100°C for 10mins. Cooking samples were cooled with water, the excess of water removed and sun-dried for 3 days. Each dried sample was pulverized into fine flour and packaged in name labelled polythene bags and stored in a cool dry place at 25 ± 2°C until used for various analysis.
Qualitative screening of phytochemicals in aqueous extracts of Amaranths leaves: Phytochemical screening of the leaves for anthraquinones, alkaloids, glycosides, phytosterols, tannins, saponins, flavonoids and terpernoids was carried out according to methods of Mace [11]; Finar [12]; Evans [13] and Kokate [14].
Gas chromatography-mass spectroscopy (GC-MS) analysis: The GC-MS analysis was carried out using a Clarus500 Perkin-Elmer (Auto system XL) Gas Chromatograph equipped and coupled to a mass detector Turbo mass gold-Perkin Elmer Turbomass 5.2 spectrometer with an Elite-5MS (5% Diphenyl / 95% Dimethyl poly siloxane), 30 m × 0.25 μm DF of capillary column. The instrument was set to an initial temperature of 110°C, and maintained at this temperature for 2 min. At the end of this period the oven temperature was rose up to 280°C, at the rate of an increase of 5°C/min, and maintained for 9 min. Injection port temperature was ensured as 200°C and Helium flow rate as one ml/min. The ionization voltage was 70 eV. The samples were injected in split mode as 10:1. Mass spectral scan range was set at 45-450 (m/z). Using computer searches on a NIST Version 2.0 were used MS data library and comparing the spectrum obtained through GC-MS compounds present in the plants sample was identified.
Total phenolic contents: The total phenolic contents (TPC) of leaves extract of A. hybridus and A. cruentus was determined according to the method described by Malik and Singh [15]. Aliquots of the extracts were taken in a 10 ml glass tube and made up to a volume of 3 ml with distilled water. Then 0.5 ml folin ciocalteau reagent and 2 ml Na2CO3 (20%) were added sequentially in each tube. The test solutions were warmed for 1 minute, cooled and absorbance was measured at 650 nm against the reagent used as a blank. A standard calibration plot was generated at 650 nm using known concentrations of Gallic acid. The concentrations of phenols in the test samples were calculated from the calibration plot and expressed as grams of Gallic acid equivalents (GAE) per 100 grams dry-weigh basis (g GAE / 100 g dwb).
Antioxidant activity (DPPH free radical scavenging activity) of methanolic extract: The antioxidant activity of Amaranthus extracts were measured from the bleaching of the purple-colored in terms of hydrogen donating or radical scavenging ability using the stable 2,2- Diphenyl-1-picrylhydrazyl (DPPH) free radical activity by method described by Nooman with slight modifications. Zero point five ml of (0.238 mg/ml) DPPH in methanol was fluxed with equal volume of plants extracts at various concentrations, fluxed well and kept in dark for half an hour. The change in color from deep violet to light yellow was then measured at 515 nm in ultra violet spectrophotometer. The test samples were measured in three replicates and Ascorbic acid was used as reference standard.
The scavenging activity of each extract was calculated using the following equation:
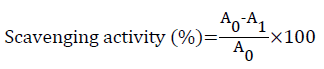
With A0 being the absorbance of the control reaction (containing all reagents except for the extract) and A1 the absorbance of the extract.
Determination of ascorbic acid (Vitamin C): Ascorbic acid was determined titremetrically according to the method described by Adeboye [16].
Statistical analysis: Statistical analyses were performed using SPSS package program version 20.0. Data were analysed by one way analysis of variance (ANOVA), followed by Duncan’s multiple range post-host test. Results are expressed as mean ± standard deviation of triplicate samples. Differences were considered significant at P < 0.05.
Results
Qualitative screening of phytochemicals: Phytochemical evaluation of aqueous extract of samples determines the biologically active non nutritive compounds that contribute to the flavor, color and others characteristics of plants. It revealed the presence and the degree in which they are found of a wide array of phytochemicals including phytosterols, tannins, glycosides, saponins, flavonoids and terpernoids. Terpernoids, tannins, phytosterols, and glycosides were most common and present in almost both vegetables whereas alkaloids, anthraquinones were absent (Table 1). Most of the phytochemical compounds were not detected in SDS, CS, and SDU A. hybridus while in A. cruentus , it was in SHDS. Alkaloids and anthraquinones were not detected in both samples.
| Treatments | Alkaloids | Anthraquinones | Flavonoids | Glycosides | Phytosterols | Saponins | Tannins | Terpernoids |
|---|---|---|---|---|---|---|---|---|
| Amaranthus hybridus | ||||||||
| SDS | - | - | ± | - | + | - | + | - |
| SHDU | - | - | ± | - | + + | - | + | + |
| SHDS | - | - | + | - | + | - | + | + |
| CS | - | - | - | + | - | + | - | + |
| SDU | - | - | - | + | - | + | - | + |
| Amaranthus cruentus | ||||||||
| SDS | - | - | - | + | + + | - | + | + |
| SHDU | - | - | - | ++ | + | + | + | + |
| SHDS | - | - | - | + | - | + | - | + |
| CS | - | - | - | + | ++ | - | + | + |
| SDU | - | - | ++ | + | + | - | + | + |
Table 1: Qualitative screening of phytochemicals of aqueous extracts of amaranths leaves, ++: appreciable amount; +: present; -: absent; ±: doubtful; SDS: Sun Drying and Slicing; SHDS = Shade Drying and Slicing; SDU = Sun drying and unslicing; SHDU = Shade Drying and Unslicing; CS = Cooking and Slicing.
GC-MS analysis: Gas chromatography-Mass spectroscopy is a precious tool for reliable detection of bioactive constituents. GC-MS was carried out in ethanol leaves extract of sun dried A. hybridus (Figure 1), cooked A. hybridus (Figure 2), sun dried A. cruentus (Figure 3) and cooked A. cruentus (Figure 4). Their retention time (RT), compound name, molecular formula and percentage composition (%) are tabulated in Table 2 for sun dried A. hybridus and sun dried A. cruentus and Table 3 for cooked A. hybridus and cooked A. cruentus . The peaks in the chromatogram were integrated and were compared with the database of spectrum of known components stored in the GC-MS library which allowed the identification of nineteen compounds for both cooked samples and twenty-two compounds for both dried sample belonging to the various groups.
| No. | Name of the compound | Molecular Formula | A. hybridus | A. cruentus | ||
|---|---|---|---|---|---|---|
| RT | Peak Area % | RT | Peak Area % | |||
| 1 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 11 | 13.51 | 11.1 | 17.49 |
| 2 | E-2-Tetradecen-1-ol | C14H28O | 11.3 | 7.73 | 11.3 | 8.4 |
| 3 | 2-Tridecen-1-ol, (E)- | C13H26O | 11.5 | 6.26 | 11.5 | 5.69 |
| 4 | Phytol | C20H40O | 14.1 | 39.1 | 14.2 | 40.68 |
| 5 | 1-Eicosanol | C20H42O | 19.2 | 2.75 | 19.2 | 3.22 |
| 6 | Z,Z-2,5-Pentadecadien-1-ol | C15H28O | 20.6 | 1.72 | 20.6 | 4.49 |
| 7 | 3-Hexadecyloxycarbonyl-5-(2-hydroxyethyl)-4-methylimidazolium ion | C24H45N2O3 | 22 | 1.42 | 22 | 3.73 |
| 8 | Squalene | C30H50 | 23.5 | 2.42 | 23.5 | 2.67 |
| 9 | 6,9,12-Octadecatrienoic acid, phenylmethyl ester, (Z,Z,Z)- | C25H36O2 | 26.4 | 4.38 | 26.3 | 3.5 |
| 10 | 1b,5,5,6a-Tetramethyl-octahydro-1-oxa-cyclopropa[a]inden-6-one | C13H20O2 | 27.9 | 3.53 | 27.9 | 4.27 |
| 11 | 5à-Androstan-16-one, cyclic ethylene mercaptole | C21H34S2 | 30.7 | 2.02 | ||
| 12 | 1b,5,5,6a-Tetramethyl-octahydro-1-oxa-cyclopropa[a]inden-6-one | C13H20O2 | 32.4 | 2.14 | ||
| 13 | Z,Z,Z-1,4,6,9-Nonadecatetraene | C19H32 | 27.1 | 4.73 | ||
| 14 | Benzenemethanol, 2-(2-aminopropoxy)-3-methyl- | C11H17NO2 | 8.81 | 2.59 | ||
| 15 | 9-Oxabicyclo[3.3.1]nonan-2-one, 6-hydroxy- | C8H12O3 | 12.3 | 1.14 | ||
| 16 | 1,4-Dioxaspiro[4.5]decane, 8-(methylthio)- | C9H16O2S | 17.5 | 2.23 | ||
| 17 | Benzeneethanamine, 2,5-difluoro-á,3,4-trihydroxy-N-methyl- | C9H11F2NO3 | 12.9 | 0.92 | ||
| 18 | Imidazole, 2-amino-5-[(2-carboxy)vinyl]- | C6H7N3O2 | 14.1 | 1.41 | ||
| 19 | Pentanal | C5H10O | 8.18 | 1.64 | ||
| 20 | Benzeneethanamine, 2-fluoro-á,3-dihydroxy-N-methyl- | C9H12FNO2 | 12.3 | 1.01 | ||
| 21 | 2-Aminononadecane | C19H41N | 17.8 | 1.36 | ||
| 22 | Cyclopenta[c]furo[3',2':4,5]furo[2,3-h][1]benzopyran-11(1H)-one, 2,3,6a,9a-tetrahydro-1,3-dihydroxy-4-methoxy- | C17H14O7 | 31.2 | 1.85 | ||
Table 2: Components identified in the ethanolic extract of sun dried Amaranthus hybridus and Amaranthus cruentus, RT = Retention Time.
Eighteen compounds were identified in sun dried A. hybridus and fourteen in sun dried A. cruentus (Table 2). Ten compounds were present in both samples and among them, phytol and 3,7,11,15- Tetramethyl-2-hexadecen-1-ol appeared with the highest peak area follow by E-2-Tetradecen-1-ol and 2-Tridecen-1-ol, (E)- respectively. Some others compounds like Z,Z,Z-1,4,6,9-Nonadecatetraene, 5à- Androstan-16-one, cyclic ethylene mercaptole and 1b,5,5,6a- Tetramethyl-octahydro-1-oxa-cyclopropa[a]inden-6-one were only present in A. hybridus and absent in A. cruentus while compounds like Pentanal, Benzeneethanamine, 2-fluoro-á,3-dihydroxy-N-methyl- and 2-Aminononadecane where represented in A. cruentus but absent in A. hybridus.
The results of the identified compounds of the leaves of cooked A. hybridus and A. cruentus showed 17 and 15 peaks indicating the presence of seventeen and fifteen fourteen phytocomponents with the retention time ranging from 11.08 to 33.01 and 11.08 to 31.40 in A. hybridus and A. cruentus respectively. Thirteen compounds were as well as present in cooked A. hybridus and cooked A. cruentus . Phytol and 3,7,11,15-Tetramethyl-2-hexadecen-1-ol were the mains compounds in both extract with the highest peak area. Components like Benzeneethanamine, 2-fluoro-á,3-dihydroxy-N-methyl- and Cyclopenta[c]furo[3',2':4,5]furo[2,3-h][1]benzopyran-11(1H)-one, 2,3,6a,9a-tetrahydro-1,3-dihydroxy-4-methoxy- were present in A. cruentus but not in A. hybridus while others known as Spiro[androst-5-ene-17,1'-cyclobutan]-2'-one, 3-hydroxy-, (3á,17á)-, Propanoic acid, 2-methyl-, 2-ethyl-1-propyl-1,3-propanediyl ester, Z,Z,Z-1,4,6,9-Nonadecatetraene and 8,9,9,10,10,11-Hexafluoro-4,4- dimethyl-3,5-dioxatetracyclo [5.4.1.0(2,6).0(8,11)]dodecane were present in A. hybridus but absent in A. cruentus .
Total phenol content (TPC)
Phenolic compounds are very important plant constituents because they exhibit antioxidant activity by inactivating free radicals or preventing decomposition of hydrogen peroxides into free radicals [17]. Many reports support the use of antioxidant supplements in reducing the level of oxidative stress and slowing or preventing the development of complications associated with diseases [18]. Figure 5 shows the contents of total phenols that were measured by Folin- Ciocalteau reagent in terms of Gallic acid equivalent.
The average quantity of phenolic compounds present in A. hybridus was found to range from 0.819 ± 0.0016 g GAE / 100 g dwb to 2.759 ± 0.0025 g GAE / 100 g dwb. In all treatments done on A. hybridus , SDS extract exhibited the highest (p < 0.05) (2.759 ± 0.0025 g GAE / 100 g dwb) value of antioxidant followed by 2.380 ± 0.0163 g GAE / 100 g dwb for SHDU. The lowest values were found in CS (0.909 ± 0.0021 g GAE / 100 g dwb). On the other hand, SHDU treatment for A. cruentus contained the highest content of phenolic 2.100 ± 0.0099 g GAE / 100 g dwb. The total phenolic compounds were very similar (p = 0.05) for SHDS and CS, except for SDS which were slightly lower (p < 0.05). The result shows that the treatment influences the extractability of phenolic compounds.
Antioxidant activity
DPPH·is a stable nitrogen-centred free radical the colour of which changes from violet to yellow upon reduction by either the process of metal chelators, singlet oxygen quenchers and atoms or electrondonation [19,20] and to capture free radicals. Substances which are able to perform this reaction can be considered as antioxidants and therefore radical scavengers [21]. Results of the activity of free radical scavenging of A. hybridus and A. cruentus are presented in Figure 6.
Figure 6: Free Radical Scavenging activity of methanol extracts from leaf samples A. hybridus and A. cruentus SDS: Slicing and Sun Drying; SHDS: Slicing and Shade Drying; SDU: Unslicing and Sun Drying; SHDU: Unslicing and Shade Drying; CS: Slicing and Cook. Bars carrying the same letters are not significantly different (P > 0.05).
The total antioxidant activity of A. hybridus was generally higher (p < 0.05) than that of A. cruentus . The highest DPPH scavenging activity was observed in SHDU (79.205 ± 1.721%) followed by SDS (68.452 ± 2.513%). Moderate was found in SHDS (61.360 ± 3.477%), while the low value was found in CS (60.540 ± 3.258 A. hybridus . However low to moderate DPPH scavenging activity was found in A. cruentus and the highest (p < 0.05) scavenging potential was observed in SDS (59.575 ± 1.296%), while low values were present in SHDS (39.073 ± 0.236%), and the least value in SHDU (18.089 ± 1.425%).Vitamin C.
The vitamin C contents of two green leafy vegetables as affected by different processing methods are indicated in Table 3. Vitamin C content in shade dried vegetables was significantly higher than that of sun dried and cooked samples (P < 0.05). However, uncutting led to significantly high loss in vitamin C content compared to cutting samples. For processing condition in general, A. hybridus had the higher destruction of vitamin C while the least loss was observed in A. cruentus .
| No. | Name of the compound | Molecular Formula | A. hybridus | A. cruentus | ||
|---|---|---|---|---|---|---|
| RT | Peak Area % | RT | Peak Area % | |||
| 1 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 11.1 | 17.07 | 11.1 | 10.05 |
| 2 | E-2-Tetradecen-1-ol | C14H28O | 11.3 | 4.85 | 11.3 | 2.51 |
| 3 | 2-Tridecen-1-ol, (E)- | C13H26O | 11.5 | 8.79 | 11.5 | 4.33 |
| 4 | Phytol | C20H40O | 14.2 | 50.03 | 14.2 | 70.34 |
| 5 | trans-2-Undecen-1-ol | C11H22O | 14.6 | 2.65 | 14.6 | 2.45 |
| 6 | 2-Aminononadecane | C19H41N | 17.8 | 0.46 | 17.8 | 0.5 |
| 7 | 1-Eicosanol | C20H42O | 19.2 | 0.97 | 19.2 | 0.89 |
| 8 | Z,Z-2,5-Pentadecadien-1-ol | C15H28O | 20.6 | 0.65 | 20.6 | 0.57 |
| 9 | 3-Hexadecyloxycarbonyl-5-(2-hydroxyethyl)-4-methylimidazolium ion | C24H45N2O3 | 22 | 0.59 | 21.9 | 0.54 |
| 10 | Squalene | C30H50 | 23.5 | 3.25 | 23.5 | 2.26 |
| 11 | 6,9,12-Octadecatrienoic acid, phenylmethyl ester, (Z,Z,Z)- | C25H36O2 | 26.4 | 1.38 | 26.3 | 0.23 |
| 12 | 1b,5,5,6a-Tetramethyl-octahydro-1-oxa-cyclopropa[a]inden-6-one | C13H20O2 | 27.8 | 3.55 | 27.7 | 1.87 |
| 13 | 5à-Androstan-16-one, cyclicethylenemercaptole | C21H34S2 | 30.6 | 1.31 | 30.6 | 1.41 |
| 14 | Spiro[androst-5-ene-17,1'-cyclobutan]-2'-one, 3-hydroxy-, (3á,17á)- | C22H32O2 | 32.3 | 1.29 | ||
| 15 | 8,9,9,10,10,11-Hexafluoro-4,4-dimethyl-3,5-dioxatetracyclo[5.4.1.0(2,6).0(8,11)]dodecane | C12H12F6O2 | 33 | 0.5 | ||
| 16 | Propanoic acid, 2-methyl-, 2-ethyl-1-propyl-1,3-propanediyl ester | C16H30O4 | 12.4 | 1.65 | ||
| 17 | Z,Z,Z-1,4,6,9-Nonadecatetraene | C19H32 | 27.1 | 1 | ||
| 18 | Benzeneethanamine, 2-fluoro-á,3-dihydroxy-N-methyl- | C9H12FNO2 | 12.4 | 1.36 | ||
| 19 | Cyclopenta[c]furo[3',2':4,5]furo[2,3-h][1]benzopyran-11(1H)-one, 2,3,6a,9a-tetrahydro-1,3-dihydroxy-4-methoxy- | C17H14O7 | 31.4 | 0.69 | ||
Table 3: Components identified in the ethanolic extract of cooked Amaranthus hybridus and Amaranthus cruentus , RT = Retention Time.
Qualitative screening of phytochemicals
Phytochemical analysis conducted on the plant extracts revealed the presence of constituents which are known to exhibit physiological activities. Analysis of the vegetables extracts revealed the presence of phytochemicals such as tannins, flavonoids, saponins, phytosterols, glycosides and terpenoids. Tannins are water soluble phenolic compounds with the ability to precipitate proteins from aqueous solution. They because decreased feed consumption in animals, bind dietary protein and digestive enzymes to form complexes that are not readily digestive [22]. They are also responsible to the damage to the intestinal tract, toxicity of tannins absorbed from gut, and interference with the absorption of iron and a possible carcinogenic. The extracts were also revealed to contain saponins which are known to produce inhibitory effect on inflammation [23]. Saponins have been shown to possess beneficial (cholesterol-lowering) by binding cholesterol, making it unavailable for absorption and deleterious (permeabilisation of the intestine) properties. Saponins have the property of precipitating and coagulating red blood cells. Some of the characteristics of saponins include formation of foams in aqueous solutions, hemolytic activity and bitterness [24]. Glycosides are known to lower the blood pressure [25].
GC-MS analysis
In this study, the heights of the peak point out the relative concentration of the presented components and apart from few components, the two species of Amaranthus got almost the same composition. Phytol was found to have the highest concentration in both species. Phytol is one part of chlorophyll and important in plant biosynthesis. It is suggested to be a diterpene compound and it may act as antimicrobial, anti-inflammatory and anticancer diuretic [26,27] Phytol is a reactive oxygen species-promoting substance. In an extensive study, experiments showed that treatment of rats with phytol increased oxidative burst in vivo and thereby corrected the effect of the genetic polymorphism in arthritis prone Ncf1DA [28]. Phytol and its isomer 3,7,11,15-Tetramethyl-2-hexadecen-1-ol are the precursor for the manufacture of synthetic forms of vitamin E and Vitamin K1 [29]. Plants use phytol and its metabolites as chemical deterrents against predation. It also acts as effective adjuvants and also increases the titers of all major Immunoglobulin G (Ig G) subclass and is also capable of inducing specific effect to T cells responses [30]. Several investigations revealed in vitro antioxidant activity of diterpenes (phytol) [31] meanwhile the polyunsaturated fatty acid 6,9,12-Octadecatrienoic acid, phenyl methyl ester, (Z,Z,Z)- a conjugated linolenic acid are known an antioxidant that can protect membranes from harm. When humans as well as rodents are fed free phytol, a high proportion is absorbed and converted in vivo to phytonic acid [32] which is a natural retinoid. It shows antidiabetic activity in type II diabetic patients, linolenic acid and their conjugates also can normalize impaired glucose tolerance in Zucker diabetic fatty acids [33]. On the other hand, fatty acids are important to every cells in the body for normal growth, especially of the blood vessels and nerves and to keep the skin and other tissues youthful and supple through their quality [34]. These are nutrients which are invaluable for the production and movement of energy throughout the body, regulation of transportation of oxygen and are vital in maintaining the integrity of cell structure as well as unique ability to lower cholesterol levels of the blood [35]. Squalene is a triterpene found to occur in leaf extract. It is a main unsaturated lipid that resents advantages for skin as an emollient, antioxidant for hydration and for its antitumor activities [36]. It is an intermediate metabolite in cholesterol synthesis possessing Immunostimulating, hypolipidemic, cholesterol reducing, anti-carcinogenic and antiinflammatory activity [37]. The primary therapeutic use of Squalene currently is as an adjunctive therapy in a variety of cancers [37] ant it has an antioxidant property [38]. Alcohols are known to possess bactericidal rather than bacteriostatic activity against vegetative cells [39]. Aldehydes, notably N-valeraldehyde are known to possess powerful antimicrobial activity [39]. It has been proposed that an aldehyde group conjugated to a carbon double bond is a highly electronegative arrangement which may explain their activity [40] suggesting that an increase in electronegativity increases antibacterial activity [41]. Such electronegative components eg, proteins and nucleic acids can therefore inhibit the growth of microorganisms [39].
Total phenol content (TPC)
Phenolic compounds inhibit auto-oxidation of unsaturated lipids, thus preventing the formation of oxidized low density lipoprotein (LDL), which induce cardiovascular disease. The higher increase was found in SDS (2.759 ± 0.0025 g GAE / 100 g dwb) and SHDU (2.380 ± 0.0163) A. hybridus . This increase may be due to the fact that, wounding (e.g., cutting) increases phenolic metabolism in vegetables tissue with the production and accumulation of soluble phenolic compounds (mainly hydrocycinnomic acid, systemin) that subsequent react to produces wound-induced tissue browning. Oboh et al., [40] reported that sun drying of green leafy vegetables caused a significant increase in total phenol content. This investigation explains that the drying process may result in high or low levels of total phenol content depending on the type of phenolic compounds present in the plant material and their location in the cells [41]. The trend of results were generally higher than what Barku et al., [5] found in Amaranthus spinosus (48.01 ± 2.0 mg of GAE / g of extract and those underlined by Ganiyu Oboh [42], in A. cruentus (0.3-0.6 g / 100 g). They also showed that the treatments influence the extractability of the phenolic compounds present. That difference in the total phenol content of both processed cannot be categorically stated. However, it may be attributed to the vegetables themselves (bioactive structures), the drying and cooking method, the bioavailability of phenolic [43], temperature, the localization of the structures in the vegetables, cutting, chopping, stability of the structure to heat, the synergic activity of the structures and the reaction systems assayed [44]. Standards used to express the TPCs; the colour measurement of Folin-ciocalteau which was nonspecific on and perhaps the presence of other components that can react with Folin-Ciocalteau reagent such as ascorbic acid and vitamin E.
Antioxidant activity: DPPH
The results summarized in Figure 7 indicated that the two species of amaranth were found to interact with DPPH radicals and thereby stabilize their hyperactivity. Between both samples, unsliced shade and sun dried were found to be most effective as DPPH radical scavenger. This increase agree with Liu et al. [45] to the fact that, drying increase the antioxidant activity suggesting the possibility of new compounds formation with antioxidant activity. Oboh et al. [46] reported that sun drying of green leafy vegetables caused a significant increase of TPC, reducing property and free radical scavenging activity; however others antioxidants like carotenoids and vitamin C may be lost. They explained that this increase may be due to the formation of Maillard reaction products during drying process. In addition, the benefits of high level in SHDU can be attributed to the fact that shade drying minimizes the degradation of heat sensitive compounds such as antioxidant phenols because the dehydration of the plant material is performed at low temperature [47]. The increased of antioxidant capacity in dried samples were not in agreement with Mueller-Harvey, [48] who stated that heating treatment not only deactivates enzymes but also degrades phytochemicals and some phenolic compounds decompose rapidly in direct sunlight or if dried at elevated temperature. It has also been evidenced by Tomaino et al., [49] that drying process would generally result in a depletion of naturally occurring antioxidants because, intense and / or prolonged thermal treatment may be responsible for a significant loss of natural antioxidants, as most of these compounds are relatively unstable as against some compounds which are heat stable [50]. The moderate antioxidant activity in SHDS and CS could be due to cutting and slicing as they induced a rapid enzymatic oxidation of natural antioxidants [51] then caused a depletion of that activity. These researchers clearly explain that the antioxidant activity obtained after drying process may be higher or lower based on the type of phenolic compounds present and their location in the cell. Cooking resulted in high level of activities of antioxidants mainly due to the fact that boiling water could completely activate the degradative enzymes presents in plant materials. This significance is in concomitance with the literature of Oboh [46], who attributed that tannin break down to simple phenol by high temperature, which increasing of the number of compounds with free hydroxyl groups. It could also be due to the production of new none nutrients antioxidants or the formation of novel compounds such as Maillard reaction products with antioxidant activity [52]. Generally, variation in antioxidant activity of the vegetables may be influenced by phenomena such as environmental factors (sunlight, temperature, raining, etc.), collection period, variety, and chemical composition, maternity at harvest, growing condition, and soil state [53].
Figure 7: The Vitamin C (mg / 100g) of green leafy vegetables as affected by various processing methods SDS: Slicing and sun drying; SHDS: Slicing and shade drying; SDU: Unslicing and sun drying; SHDU: Unslicing and shade drying; CS: Slicing and cook. Bars carrying the same letters are not significantly different (P > 0.05).
Vitamin C
The loss of vitamin C in green leafy vegetables is a function of the processing method employed in its preparation. The level of vitamin C in boiled samples of both vegetables was significantly lower than those of dried samples. The loss is attributed to the thermosensitive, labile and hydrosoluble nature of that vitamin which is easily leached into the boiling medium [54]. Sun drying compared to shade drying appears to be a poor method of processing owing to the higher percentage losses of vitamin C in sun dried samples. Besides, wilting is another factor that could be responsible for the vitamin losses during sun drying [55]. The losses observed when vegetables were sundried are in agreement with work of Oshodi [56] who confirmed that vitamin C in vegetables is temperature dependant. The low level of vitamin C content in slicing samples compared to uncutting samples is due to the fact that, cutting is one the way by which oxidases contained within the cells are set free to oxidize and thereby destroy the vitamin C content. Shade drying of amaranth would be able to meet RDA of ascorbic acid for adolescent and adults (60 mg/day) as their ascorbic content were observed to be higher than the value [57,58].
The leaves of Amaranthus have very high phenolic and antioxidant activity. However, the various studied processing methods influenced the extractability of phenolic and antioxidant compounds. Cooking revealed the lowest vitamin C content, Shade drying and unslicing shown lower antioxidant activity while sun drying and slicing underlined the highest value in both samples. Each compound identified in leaf extract of Amaranthus has its own biological importance and further study of this plant’s phytochemical by in vitro methods can prove its nutritional status in future. However, isolation of individual phytochemical constituents and subjecting it to biological activity will definitively give fruitful results. Recognizing the need for identification of such green leafy vegetable, which are believed to be nutritious, may help in achieving nutritional (micronutrient) security. Antinutrients present in the both amaranth could affect the bioavailability of essential nutrients if the vegetables are not properly processed. Furthermore, a process to eliminate undesirable characteristics of the leaves is necessary if their nutritional potential is to be fully realized and utilized.
This research was funded by the ‘CV Raman International Fellowship for African Researchers’ by the Department of Science and Technology (DST) and Ministry of External Affairs (MEA), Government of India through the Federation of Indian Chambers of Commerce and Industry (FICCI).