Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Research Article - (2021)Volume 9, Issue 10
The experimental setup to examine the response of plant against salinity should be controlled and consistent as the fluctuating environment conditions confound the exact plant response to saline stress. In vitro techniques, provides the prerequisite uniform environmental conditions to study the impact of salinity on plant growth and offer a means to focus exclusively on physiological and biochemical processes, which contribute to salinity tolerance.
The present study is an attempt to investigate the response of D. Sissoo against salinity stress at cellular level. The possible outcome of this work can be helpful in figuring out the exact optimum as well as lethal concentration(s) of salt stress which can be further used to develop salt tolerant cell lines of this species.
Effect of salinity; Callus culture; Dalbergia sissoo; Physiological; Biochemical processes; Plant growth
The genus Dalbergia belongs to the legume family Fabaceae), subfamily Papilionoideae.Dalbergia is a tropical genus containing 100 species of trees, shrubs and lianas. Dalbergia sissoo is a medium to large tree of about 25 meters high with grey yellow trunk, 2-3 meters in diameter. Leaves are leathery, pinnately compound, leaflets are alternate. They are broad, ovate, acuminate, glabrescent, petiolate with fine pointed tip. The sapwood is white to pale brown is dark brown in colour. This species reproduces mostly by seed and it can thus form dense thickets. The flowering period is March-May. It is found in riverine environments where sunlight and moisture are plentiful. Bangladesh, Bhutan, India, Malaysia, Pakistan are the exotic range and Cameroon, Ethiopia, Indonesia, Iraq, Israel, Kenya, Mauritius, Nigeria, Sudan, Tanzania, Thailand, Togo, US, Zimbabwe are native range.
D. sissoo has many reputed medicinal properties and have been used culturally for a variety of ailments including: skin diseases, blood diseases, syphilis, stomach problems, dysentry, nausea, eye and nose disorders, aphrodisiac, and expectorant, among others. Indian rosewood also has insecticidal and larvicidal properties, as well as resistance to some wood boring insects. Herbal preparation of D. sissoo and Datura stramoium with cow urine can be used as a potent antiseptic preparation for prevention and treatment of chronic bacterial infections. People use twigs of sissoo to clean their teeth, root is astringent. D. sissoo is best known economic timber species of the rosewood genus sold internationally, but it is also used as fuel wood and for shade and shelter. After teak, it is the most important cultivated timber tree of Bihar, which is the largest producer of D. sissoo timber in India. This is a highly valued hardwood timber species, providing an additional income source in agroforestry systems, mostly in India and Pakistan, but increasingly, elsewhere. According to Chaturvedi, ‘shisham (D. sissoo) is a friend of the farmer, as well as the forester; a tree which pays rich dividends’. Planting D. sissoo on farmlands in Pakistan is economically feasible and the use of D. sissoo as an agroforestry component with a wheat crop has been shown to be financially superior to wheat monocropping, generating a higher net present value than other alternatives over a period of 20 years. D. sissoo is a nitrogen-fixing species widely used in agroforestry, moreover it is also acknowledged for its environmental benefits such as increasing soil fertility, reducing soil erosion etc.
Salinity is a major abiotic stress. A saline soil is generally (approximately 40 mM NaCl) at 25°C and has an exchangeable sodium of 15%. The yield of most crop plants is reduced at this ECe, though many crops exhibit yield reduction at lower. It has been estimated that worldwide 20% of total cultivated and 33% of irrigated agricultural lands are afflicted by high salinity. It has been estimated that more than 50% of the arable land would be salinized by the year 2050. Main causes of salinity are weathering of rocks, capillary rise from shallow brackish groundwater, instruction of sea water along the cost, impeded drainage and other human causes are irrigation without proper drainage system, industrial influents over use of fertilizers etc. Salinity in agricultural fields is thus a severe constraint to crop growth and productivity in many regions and the situation has become a global concern. Excess salt in soil or in solutions interferes with several physiological and biochemical processes, resulting in problems such as ion imbalance, mineral deficiency, osmotic stress, ion toxicity and oxidative stress; these conditions ultimately interact with several cellular components, including DNA, proteins, lipids, and pigments in plants, impeding the growth and development of a vast majority of crops [1]. The protection of crops against salinity induced damage has become a global challenge. The development of salt-tolerant crops that can tolerate high levels of salinity in the soils would be a practical solution of such problem.
Plant tissue culture is a collection of techniques used to maintain or grow plant cells, tissues or organs under sterile conditions on a nutrient culture medium of known composition. Plant tissue culture is widely used to produce clones of a plant in a method known as micropropagation. Also, plant tissue culture techniques provide a promising and feasible approach to develop salt-tolerant crop plants. Haploid culture, double haploidy, somaclonal variation, and in vitro-induced mutagenesis have been used to create variability to improve salinity tolerance in crop plants. Cell and tissue culture techniques have been used to obtain salt-tolerant plants through in vitro culture approaches: selection of mutant cell lines from cultured cells and subsequent plant regeneration and in vitro screening of plant germplasm for salt tolerance.
Environmental stresses affect many aspects of tree physiology and metabolism, and can negatively impact tree growth, development and distribution. Stress responses and tolerance mechanisms involve the prevention or alleviation of cellular damage, the re- establishment of homeostasis and growth resumption. Because forest trees are sessile and continue to develop over many growing seasons, mechanisms have evolved that allow trees to respond to changes in environmental conditions. Adaptation to environmental stresses is controlled by molecular networks, and significant progress using transcriptomics, proteomics and metabolomics has facilitated discoveries of abiotic stress-associated genes and proteins involved in these cascades. Primary stresses, such as drought, salinity and cold, are often interconnected and cause cellular damage and secondary stresses, such as osmotic and oxidative. The initial stress signals (e.g., osmotic and ionic effects or changes in temperature or membrane fluidity) trigger downstream signalling process (es) and transcriptional controls, which activate stress-responsive mechanisms to re-establish cellular homeostasis and to protect and repair damaged proteins and membranes. Inadequate responses at one or more steps in the signalling and stress-gene-activation process might ultimately result in irreversible changes in cellular homeostasis and in the destruction of functional and structural proteins and membranes, leading to cell death.
Genetic transformation is now a widely used procedure for introducing genes from distant genepools into many plant species for the development of stress tolerant plants and considerable efforts have been made to produce stress-tolerant plants using this technique [2]. Physiologically, many processes are affected by salinity, but reduced cell growth, leaf area, biomass and yield are the most important ones. Reduction in growth is a common phenomenon of salt stressed plants, which has also been observed in cultured cells, tissues or organs on medium supplemented with NaCl. In fact, slower growth is an adaptive feature for plant survival under stress and the extent of salt tolerance often appears to be inversely related to growth rate [3]. Leaf photosynthetic capacity depends on physiological characteristics such as chlorophyll content, Rubisco activity and photosystem efficiency. Reduction in the chlorophyll content of the plant is accompanied by a lower efficiency of PS II and senescence. According to photosynthesis rates are usually lower in plants exposed to salinity. Leaf injury is another screening parameter for salinity tolerance and can be measured by membrane damage, premature loss of chlorophyll or damage to the photosynthetic apparatus [4].
During salinity induced oxidative stress, several cytotoxic Reactive Oxygen Species (ROS) are continuously generated in the mitochondria, peroxisomes and cytoplasm, which can destroy the normal metabolism through oxidative damage of lipids, proteins and nucleic acids [5]. ROS mainly comprises of superoxide radicals (O2− ), hydrogen peroxide (H2O2), hydroxyl radicals (OH ) and singlet oxygen. In course of evolution, plant cells have developed complex antioxidant defense system both enzymatic (SOD, APX, GPX, GR, CAT, etc.) and non-enzymatic (ascorbate, glutathione, tocopherol, carotenoids, flavonoids, etc.) to protect themselves against saltstress [5-7]. In many studies differences have been found inlevels of expression and activity of antioxidative enzymes; thesedifferences are sometimes associated with more tolerant genotypes,and sometimes with the more sensitive genotypes [8]. Superoxide Dismutase (SOD) is ametallo enzyme which catalyzes the dismutation of superoxide into oxygen and H2O2 [4].H2O2 reacts with various cellular targets inducing damage to proteins and DNA and also cause lipid peroxidation. This H2O2 is removed by the activity of peroxidase and catalase [7]. Ascorbate Peroxidase (APX) is the most important peroxidase, catalyzing the reduction of H2O2 to water using the reducing power of ascorbate [6]. Catalases (CAT), located mostly in peroxisomes and glyoxisomes, are tetramerichomo proteins that are involved in catalyzing the reduction of H2O2 to water. Glutathione Reductase (GR) plays a crucial role in ascorbate–glutathione cycle by maintaining the GSH (reduced glutathione)/GSSG (oxidized glutathione) ratio favourable to ascorbate reduction. These ROS scavenging pathways are found in almost all the cellular compartments-the water–water cycle is found in chloroplasts; the ascorbate-glutathione cycle is found in chloroplast, cytosol, mitochondria, apoplast and peroxisomes; the Glutathione Peroxidase (GPX) cycle and CAT are found in peroxisomes.
Under salt-stress, plants restrict the uptake of salt and adjust their osmotic pressure by the synthesis of compatible organic solutes. Compatible solutes are low molecular weight, highly soluble compounds that are usually nontoxic at high cellular concentrations. These solutes include proline, sucrose, polyols, trehalose and Quaternary Ammonium Compounds (QACs) such as glycine betaine, alaninebetaine, prolinebetaine, choline O-sulfate, hydroxyprolinebetaine and pipecolatebetaine [9]. Proline, is one of moststudied compatible solute playing a predominant role in protectingplants from osmotic stress. Under salt stress, several functions are proposed for the accumulation of proline in tissues which include osmotic adjustment, carbon and nitrogen reserve for growth after stress resistance, detoxification of excess ammonia, stabilization of membranes, protecting photosynthetic activity and mitochondrial functions, and scavenging of free radicals [10]. However, the significance of proline accumulation in osmotic adjustment is still controversial and varies according to the species [10].
Mohammad Asif et al., conducted a study in 2011; phytochemical investigation and evaluation of anti-nociceptive activity of ethanolic extract of Dalbergia sissoo (Roxb.) bark [11]. They concluded that (300, 500 and 1000 mg/kg) doses of extract exhibited significant and dose dependent anti-nociceptive activity which may be due to presence of flavanoids.
He conducted a study in 2011, Evaluation of anti-helminthic activity of Dalbergia sissoo Roxb. The study indicated the potential usefulness of Dalbergia sissoo Roxb, against helminthic infections [12].
They both have studied the influence of of NaCl salinity on growth, dry-matter production and leaf photosynthesis of seedlings of Eucalyptus camaldulensis Dehnh and Dalbergia sissoo Roxb. Imposing 4 levels (40, 80, 120 and 160 mM) of NaCl in pot culture [13]. Salinity upto 160mM did not affect the plant survival, but did affect plant growth dry matter production depending upon the species and salt concentration. NaCl reduced leaf number and dry-weight of all the plant components but increased stem dry weight, especially in E. camaldulensis. Salinization also stimulated total dry-matter productionat all the salinity levels in E. camaldulensis but only at 40 Mm in D. sissoo.
He has investigated the germination, seedling vigour, lipid peroxidation and proline metabolism in Catharanthus roseus seedlings under salt stress. Seeds were grown with different concentrations (15, 30, 45 and 60 mM) of sodium chloride (NaCl). They found that germination was delayed at lower salinity levels and inhibited athigher salinity regimes [14].
He has evaluated the physiological responses of plants to the salinity stress [15]. They quantified the impact of salinity on different traits, such as relative growth rate, water relations, transpiration, and transpiration use efficiency, ionic relations, photosynthesis, senescence, yield components.
They have studied the response of plant cells to salt stress on embryo derived calli of rice (Oryza sativa L.) in order to identify cellular phenotypes associated with the stress [16]. Callus was grown on agar-solidified media containing 0%, 1% and 2% (w/v) NaC1 for 24days. They found that the callusgrowth decreased markedly with increasing NaCl concentrationin the medium.
They have compared the saline stress and alkaline stress on the germination, growth, photosynthesis, ionic balance and activity of anti-oxidant enzymes of Lathyrus quinquenerviusto elucidate the physiological adaptive mechanism of plants to alkaline stress (high pH) [17].
Physiologically, many processes are affected by salinity, but reduced cell growth, leaf area, biomass and yield are the most important ones. Reduction in growth is a common phenomenon of salt stressed plants, which has also been observed in cultured cells, tissues or organs on medium supplemented with NaCl. In fact, slower growth is an adaptive feature for plant survival under stress and the extent of salt tolerance often appears to be inversely related to growth rate [3]. Photosynthesis rates are usually lower in plants exposed to salinity [4]. Leaf injury is another screening parameter for salinity tolerance and can be measured by membrane damage, premature loss of chlorophyll or damage to the photosynthetic apparatus. However, these methods can only discriminate between genotypes tolerating low or moderate salinities [8]. Antioxidant defense system is positively associated with salt tolerance in plants, therefore, in some studies, in vitro selected salt-tolerant plantlets have been characterized by estimation of antioxidative enzymes mainly SOD, APX, CAT and GR [7]. Lipids play an important role as the structural constituent of most of the cellular membranes [4]. Therefore, the level of Malondialdehyde (MDA), produced during peroxidation of membrane lipids, is often used as an indicator of oxidative damage. Selected tolerant callus lines of Solanum tuberosum subjected to NaCl showed an increase in lipid peroxidation [3]. Most of works so far done in relation to in vitro selection are based on ion-homeostasis and compatible solutes mainly proline pool. When salinity results from an excess of NaCl, the most common type of salt stress, the concentrations of sodium (Na+) in the plant increase and concentrations of potassium (K+) is reduced. This is called salt-specific or ion-excess effect of salinity [18]. For overcoming salt stress, plants have evolved protective mechanisms that allow them to acclimatize. These mechanisms include osmotic adjustment that is usually accomplished by accumulation of compatible solutes such as proline, glycine betaine and polyols.
It is estimated that 20% of the irrigated land in the world is affected by salinity [2]. Excess salt in soil or in solutions interferes with several physiological and biochemical processes, resulting in problems such as ion imbalance, mineral deficiency osmotic stress, ion toxicity and oxidative stress; these conditions ultimately interact with several cellular components, including DNA, proteins, lipids, and pigments in plants, impeding the growth and development of a vast majority of crops [1].
The development of salt-tolerant crops that can tolerate high levels of salinity in the soils would be a practical solution of such problem [2]. Different strategies are in progress for the development of NaCl-tolerant plants. In vitro selection procedure and Agrobacterium-mediated transformation offer a meaningful tool for development of such tolerant lines [7]. Since first report in Nicotiana sylvestris, many attempts have been made to produce salt tolerant plants using in vitro techniques [19, 20]. This is being done using a number of systems (callus, suspension cultures, somatic embryos, shoot cultures, etc.) which are screened for variation in their ability to tolerate relatively high levels of salt (NaCl) in media [21]. In majority of salinity studies, the salt used is NaCl. Several researchers, however, have compared the response of other C1- and SO2- salts including KCl,Na2SO4 and MgSO4 during in vitro screening.
During salinity induced oxidative stress, several cytotoxic Reactive Oxygen Species (ROS) are continuously generated in the mitochondria, peroxisomes and cytoplasm, which can destroy the normal metabolism through oxidative damage of lipids, proteins and nucleic [5]. ROS mainly comprises of superoxide radicals (O2- ), hydrogenperoxide (H2O2), hydroxyl radicals (OH•) and singlet oxygen (1O2). In course of evolution, plant cells have developed complex antioxidant defense system both enzymatic (SOD, APX, GPX, GR, CAT, etc.) and non-enzymatic (ascorbate, glutathione, -tocopherol, carotenoids, flavonoids, etc.) to protect themselves against saltstress [6,7]. In many studies differences have been found in levels of expression and activity of antioxidative enzymes; these differences are sometimes associated with more tolerant genotypes, and sometimes with the more sensitive genotypes [8]. Superoxide Dismutase (SOD) is ametalloenzyme which catalyzes the dismutation of superoxide into oxygen and H2O2 [4].H2O2 reacts with various cellular targets inducing damage to proteins and DNA and also cause lipid peroxidation. This H2O2is removed by the activity of peroxidase and catalase [7]. Ascorbate Peroxidase (APX) is the most important peroxidase, catalysing the reduction of H2O2 to wate rusing the reducing power of ascorbate [6].
Salinity causes ion-specific stresses resulting from altered K+/Na+ ratios leads to build up in Na+ and Cl- concentrations that are detrimental to plants. The alteration of ion ratios in plants is due to the influx of Na through pathways that function in the acquisition of K+. Maintenance of a high cytosolic K+/Na+ ratio is a key requirement for plant growth under high concentration of salt [2]. Ion exclusion mechanism can provide a degree of tolerance to relatively low concentrations of NaCl but will not work at high concentrations of salt, resulting in the inhibition of key metabolic processes and concomitant growth inhibition [2]. Enhancement in proton pumping activity would furnish plasma membrane Na+/H+ antiporter with a driving force to expel Na+ out of the cytoplasm into the apoplast and thus reducing cytosolic Na+ load [5]. The NHX-type antiporters i.e. Na+/H+ located in tonoplast have been reported to increase salt-tolerance in many plant species by driving Na+ accumulation in vacuole [22].
Under salt stress, several functions are proposed for the accumulation of proline in tissues which include osmotic adjustment, carbon and nitrogen reserve for growth after stress resistance, detoxification of excess ammonia, stabilization of membranes, protecting photosynthetic activity and mitochondrial functions, and scavenging of free radicals [23]. However, the significance of proline accumulate on in osmotic adjustment is still controversial and varies according to the species [10].
During past years, in vitro selection for cells exhibiting increased tolerance to water or drought stress has been reported [24]. Polyethylene Glycol (PEG), sucrose, mannitol or sorbitol was used by several workers as osmotic stress agents for in vitro selection. But in most cases, PEG has been used to stimulate water stress in plants.
In vitro selection is an attractive alternative approach for development of stress tolerant lines [25]. In vitro selection through enhanced expression of Pathogenesis-Related (PR) proteins, antifungal peptides or biosynthesis of phytoalexins is an important tool for desirable plant selection [18, 25]. This technology is easy and cost effective compared to the transgenic approach for the improved disease tolerance. The possibility of in vitro selection for disease resistance was first reported by Carlson (1973) in tobacco for Pseudomonas syringae. Since then, lines resistant to fungal, bacterial and viral pathogens have been isolated in many species [25-29].
Some successful reports of in vitro selection for disease resistance in woody species involve peach, lemon, mango and grapes. Hammerschlag [27] regenerated disease resistant plant of peach by screening embryogenic callus obtained from zygotic embryos against culture filtrate produced by a pathogenic bacterium Xanthomonas campestris cv. pruni. The nucellar embryogenic cultures of two polyembryonic cultivars of mango selected against the culture filtrate of Colletotrichum gloeosporioides exhibited resistance to the fungus in vitro [28]. On similar lines, screened proembryogenic mass of grapes against culture filtrate produced by Elsinoe ampelina, the causal agent of anthracnose disease and reported that regenerated plants showed enhanced resistance to the pathogen. Such studies have also been shown to be useful assays in testing for resistance in wheat [29, 25, 30] etc. Selected tobacco mosaic virus resistant tobacco in vitro using callus lines infected with tobacco mosaic viruses itself [25, 31].
Chitinases and 1,3-glucannases are the most important PR proteins expressed by diseased plants. Recent reports have shown that activation of chitinase gene has been widely used for improving disease tolerance in plants [32, 33]. Induced disease resistance by the expression of chitinase enzymes through fungal culture filtrate was observed in Citrus, mango [28], etc.
In recent years, considerable progress has been made regarding the development and isolation of stress mainly disease and salt tolerant cell/callus lines using in vitro technique. In vitro selection will save the time required for developing disease resistant and abiotic stress tolerant lines of important plant species. In vitro selected variants should be finally field-tested to confirm the genetic stability of the selected trait. The problem of loss of regeneration ability during in vitro selection can be overcome by the use of explants with high morphogenic potential which may ensure successful regeneration. Our knowledge about the molecular mechanisms operational during stress tolerance under in vitro conditions in plants is also limited. In vitro selection in corporate with molecular and functional genomic scan provide a new opportunity to improve stress tolerance in plants relevant to food production and environmental sustainability.
The present work was carried out in the Tissue Culture Lab of Division of Genetics & Tree Propagation at Forest Research Institute, Dehradun.
Laboratory requirements
• Chemical balance.
• Autoclave.
• pH meter
• Volumetric flasks & beakers for media preparation.
• Culture vessels (conical flasks and Petri dishes).
• Oven for drying glassware.
• Microwave oven for media preparation.
• Laminar flow wood for aseptic transfer.
• Culture room with constant temperature.
• Culture racks with fluorescent light controlled by a timer.
• Temperature controlling facilities.
• Refrigerator for keeping stock solutions and chemicals.However a standard tissue culture laboratory should provide facilities for:-
• Washing and storage of glassware, plastic ware.
• Preparation, sterilization and storage of nutrients media.
• Aseptic manipulation of plant material.
• Observation of cultures.
• Maintenance of culture under controlled conditions.
• Acclimatization of in vitro developed plants.
One for glassware washing, storage and media preparation and a culture room to store cultures and a third inoculation room to transfer ex-plant into media. A tissue culture laboratory requires large quantities of good quality water and provision for waste water disposal.
Reagents prepared
Stock solution (Major) 1000 ml: 7.4 g of MgSO4.7H2O, 3.4 g of KH2PO4 and 8.8 g of CaCl .H O were dissolved in 1000 ml distilled water.
Stock solution (Minor) 100 ml: 0.0166 g of KI, 0.124 g of H3BO4, 0.446 g of MnSO4.4H2O, 0.172 g of ZnSO4.7H2O, 00005 g of CuSO .5H O, 0.005 g of CoCl .6H O and 0.005 g of Na2MO4 .2H2O were dissolved in 100 ml distilled water.
Stock solution (Vitamins) 100 ml: 2 g of Myo-Inositol, 0.01 g of Nicotinic Acid, 0.01 g of Pyridoxine HCl, 0.01 g of Thymine HCl and 0.04 g of Glycine were dissolved in 100 ml distilled water.
Stock solution (Iron) 100 ml: 0.746 g of Na EDTA and 0.556 g of FeSO4.7H2O were dissolved separately in 40 ml of water by heating and constant stirring. The two solutions were then mixed and pH was adjusted to 5.5. The solution was made upto100 ml with distilled water.
Stock solution (BAP) 25 ml: 25 mg of BAP was dissolved in 25 ml distilled water with the help of a few drops of NaOH.
Glassware and plastic ware washing
Glassware used was of Borosil brand. Detergents especially designed for washing laboratory glassware and plastic ware were used. After soaking in detergent solution for a suitable period (preferably overnight) the apparatus was thoroughly rinsed first in tap water and then in distilled water. If the glassware used had dried agar sticking to the sides of the tubes or jars, it was better to melt it by autoclaving at low temperature. To recycle glassware that had contaminated tissues or media, it was extremely important to autoclave them without opening the closure so that all the microbial contaminants were destroyed. The washed apparatus was placed in wire baskets or trays to allow maximum drainage and dried in an oven at about 75 degrees Celsius, for an hour, and stored in a dust- proof cupboard (Figure 1).

Figure 1: Washed apparatus placed in wire baskets in the washing room.
Sterilisation
The dried glassware was allowed to cool for some time before removing them from the oven. The required amount of jam bottles were taken out of the oven in trays. Their already- washed sun-dried caps were thoroughly wiped with cotton, moistened with 95% alcohol (ethanol).The caps were then used for sealing the jam bottles. The forceps and scalpels were dipped in 95% ethanol, burned and cooled. After cooling they were first wrapped in aluminium foil, and then in paper before autoclaving. Similarly, petridishes were subjected to the same sterilisation procedures as the forceps and scalpels, and wrapped in aluminium foil and paper. Cotton tubes, for holding the forceps and scalpel during the experiment, were also prepared and wrapped on top with aluminium foil and paper. The jam bottles, along with the forceps, scalpel, petridishes and cotton tubes were subjected to autoclaving for 15 minutes at a temperature of 121 degrees Celsius and 15 psi (Figure 2).

Figure 2: Already-washed sun-dried caps that were thoroughly sterilized using autoclave.
Media preparation
(For 1litre media) Less than 1litre (about 500 ml) of autoclaved distilled water was taken in a dispenser, with clear volume markings. 30 g of Pure Sucrose was weighed in the weighing machine and dissolved in the dispenser filled with autoclaved distilled water. Sucrose acts as the carbon and energy source. While autoclaving the medium, it is converted into glucose and fructose. 3.55 g of M.S. (Murashige & Skoog) Supplement was also added. MS supplement contains desired salt compositions to induce organogenesis and regeneration of plants in cultured tissue. This was followed by addition of the stock solutions that had been prepared earlier- 50ml of MgSO4 +KH2PO4, 50 ml of CaCl2, 5 ml of Minor solution, 5 ml of Vitamins and, 5 ml of Iron EDTA. These solutions contain various macronutrients (N, P, K, Ca, Mg and S), micronutrients (Fe, Mn, Zinc, B, Cu and Mo), inorganic nutrients (KNO3 from KH2PO4) and vitamins for plant growth and survival. After adding the stock solutions the consequent solution in the dispenser becomes moderately acidic (about pH 4.3). pH has to be maintained at 5.6-5.8 for preparation of media. Therefore this was corrected by adding drops of Sodium Hydroxide solution, through a micropipette; till its pH was shown to be 5.7 in the pH meter. 6.7 g of Agar (HI media brand, 0.7%) was then added. Agar is a polysaccharide obtained from seaweed. It does not react with the media constituents and are not digested by plant enzymes. They remain stable at all feasible incubation temperatures. The solution in the dispenser was then made up to 2litre by adding additional autoclaved distilled water. This was then hard-boiled in a microwave. The solution was taken out at regular intervals and stirred to facilitate homogenous dissolving of the agar. After boiling it was taken out and the necessary hormones were added-1 mg/l BAP (Benzyl Amino Purine)+0.5 mg/l 2, 4-D (2,4-Dicholorophenoxyacetic acid)for sub-culturing of Shisham calli. BAP is a Cytokinin which induces cell division, modification of apical dominance and shoot differentiation. The media (liquid) was poured into the jam bottles in equal amounts and sealed with their caps. The jam bottles were labeled for easy identification and to prevent mixing up with other glassware. The jam bottles, containing the media, were then autoclaved for 40 minutes at 121 degrees Celsius and 15 psi. After autoclaving, the jam bottles were taken out and kept aside for the media to solidify.
Plant material
Calli of Dalbergia sissoo (Shisham).
Working in the laminar air-flow cabinet: The working surface of the laminar air-flow cabinet was wiped with 95% alcohol. All the equipment required in the cabinet- spirit lamp, spirit, alcohol, cotton, conical flask (for supporting the cotton tube), jam bottles (containing media), forceps, scalpel and petridishes-were kept in it and subjected to sterilisation by U.V. light for 20 minutes. The U.V. light was switched off and the parent callus to be sub-cultured was then brought to the laminar air-flow cabinet prior to commencement of work in it. A surgical mask was put on and hands were sterilised before work began. The surface of the laminar was again wiped with alcohol and the wrapped forceps, scalpel and petridishes were opened, dipped in alcohol and burned. The forceps and scalpel (fixed with a new blade) were burnt red-hot over the spirit lamp. A cotton tube was taken out and filled with alcohol and the forcep and scalpel were dipped in it after burning. The forcep and scalpel were burned at regular intervals while sub-culturing. After cooling of the burnt equipment sub- culturing began. The parent callus was taken out of the jam bottle in which it was growing and placed in a petridish. It was cut into small pieces with the help of the forceps and scalpel. Dead parts, which were coloured black or brown, and residual media, were removed. Each cut piece was then placed in a jam bottle each and the bottles were immediately closed. After induction of all the pieces of calli the jam bottles were placed in the culture room where there is sufficient light and favourable temperature which promotes growth. Shisham callus was sub-cultured on 31-01-2018.
Sub-culturing the calli with different concentrations of NaCl in the media: The same protocol for preparing the glassware and other equipment and their sterilization, as done for sub- culturing calli, was followed. 20 jam bottles and additional 9 conical flasks were prepared. Each of the conical flasks was for different types treatments. Nine treatments of different NaCl concentration were planned (0 mM, 25 mM, 50 mM, 75 mM, 100 mM, 125 mM, 150 mM, 175 mM, 200 mM). But a slightly different protocol was followed for preparation of media to accommodate different concentration of NaCl. After addition of major and minor stock solution 3.55 MS supplement and 10 g sucrose were added. pH was corrected to 5.7 with the help of NaOH solution, instead of 6 g Agar, was weighed and added. When media appeared transparent then media divided into each flask. Salt was added to each conical flask (representing different treatments).
Treatment 1:- 0 mM (control) 0 g
Treatment 2:- 25 mM (0.1461 g/100 ml)
Treatment 3:- 50 mM (0.292 g/100 ml)
Treatment 4:- 75 mM (0.438 g/100 ml)
Treatment 5:- 100 mM (0.584 g/100 ml)
Treatment 6:- 125 mM (0.730 g/100 ml)
Treatment 7:- 150 mM (0.876 g/100 ml)
Treatment 8:- 175 mM (1.022 g/100 ml)
Treatment 9:- 200 mM (1.168 g/100 ml)
Each flask containing media were boiled in the microwave. The media of each flask was then poured in jam bottle, 3 replicate of each treatment has been taken. The bottles were then labelled TnRn, where T is Treatment, R is Replicate, and n is treatment no. or replicate no. for example; T1R3 represented Treatment-1 Replicate-3. They were then autoclaved for 15 minutes at 121 degree Celsius and 15 psi. The media were then removed and kept aside to facilitate solidification.
Culturing and weighing of the salt-stressed calli: All the equipment, glassware and media were placed in the laminar after the surface of the laminar was sterilised with alcohol. Additionally, a small digital weighing machine was also gently wiped with cotton, moistened with alcohol, and kept with the rest of the equipment in the laminar. They were treated to U.V. light for 20 minutes. The calli that were sub-cultured earlier was brought from the culture room for further sub-culturing in the media of different salt concentrations. Same procedure, as for sub-culturing previous calli, was followed except that time initial weight of each piece of cut callus was weighed on the weighing machine and noted. Each callus was then put into different jam bottles representing different treatments and replicates. Each callus was roughly cut into a dimension of 1 cm × 1 cm to attain almost uniform weight. Then jam bottle placed into culture room for incubation (Figure 3).

Figure 3: Culture room for incubation of bottles.
The final weight of each replicate was taken after 10days and noted. The laminar was well-cleaned and its surface wiped with alcohol after each experiment/usage. Sub-culturing of calli in media of different salt concentrations was done on 10-03-2018 and their final readings were taken on 1-04-2018 and same treated calli were then dried in oven for 24 hours and and then dry weight was taken. Initial and final weights of the calli were taken to determine relative weight, which symbolises growth. Calli were blotted with cotton before taking final weights so as to remove any residual liquid media on it (Figures 4-6).

Figure 4: Weighing of the salt stressed calli.

Figure 5: Callus of D. sissoo..

Figure 6: Salt treated calli.
Effects of light intensity on inflorescence formation
The treatment of D. sissoo is treated with three replicates to measure the Fresh Weight Initial (IW), Fresh Final Weight (FW), Relative Growth Rate (RGR), Dry Weight (DW) and Relative Water Content (RWC) on the effect of salinity on growth of callus culture in Dalbergia sissoo (Table 1).
| Treatments with 3 replicates | Fresh Initial Weight (IW) | Fresh Final Weight (FW) | Relative Growth Rate (RGR) | Dry Weight (DW) | Relative Water Content (RWC) |
|---|---|---|---|---|---|
| T0R1 | 0.43 | 0.53 | 0.23 | 0.0564 | 8.3 |
| T0R2 | 0.45 | 0.5 | 0.11 | 0.0573 | 7.72 |
| T0R3 | 0.46 | 0.44 | -0.04 | 0.05 | 7.8 |
| T1R1 | 0.48 | 0.62 | 0.29 | 0.0414 | 13.97 |
| T2R2 | 0.55 | 0.52 | -0.05 | 0.045 | 10.55 |
| T3R3 | 0.45 | 0.44 | -0.02 | 0.043 | 9.23 |
| T2R1 | 0.55 | 0.51 | -0.07 | 0.0548 | 8.3 |
| T2R2 | 0.51 | 0.61 | 0.19 | 0.052 | 10.73 |
| T2R3 | 0.47 | 0.58 | 0.23 | 0.057 | 9.17 |
| T3R1 | 0.5 | 0.59 | 0.18 | 0.0586 | 9.06 |
| T3R2 | 0.52 | 0.57 | 0.09 | 0.053 | 9.75 |
| T3R3 | 0.56 | 0.62 | 0.1 | 0.0565 | 9.97 |
| T4R1 | 0.54 | 0.64 | 0.18 | 0.0723 | 7.85 |
| T4R2 | 0.6 | 0.5 | -0.16 | 0.07 | 6.14 |
| T4R3 | 0.45 | 0.47 | 0.04 | 0.0875 | 5.96 |
| T5R1 | 0.58 | 0.5 | -0.13 | 0.0572 | 7.74 |
| T5R2 | 0.44 | 0.62 | 0.4 | 0.05 | 11.4 |
| T5R3 | 0.45 | 0.51 | 0.13 | 0.055 | 8.27 |
| T6R1 | 0.57 | 0.65 | 0.14 | 0.075 | 7.66 |
| T6R2 | 0.57 | 0.67 | 0.17 | 0.07 | 8.57 |
| T6R3 | 0.54 | 0.56 | 0.03 | 0.0675 | 7.29 |
| T7R1 | 0.55 | 0.54 | -0.01 | 0.0548 | 8.85 |
| T7R2 | 0.5 | 0.55 | 0.1 | 0.052 | 9.57 |
| T7R3 | 0.58 | 0.64 | 0.1 | 0.05 | 11.8 |
| T8R1 | 0.53 | 0.53 | 0 | 0.565 | 8.38 |
| T8R2 | 0.54 | 0.6 | 0.18 | 0.052 | 10.53 |
| T8R3 | 0.52 | 0.52 | 0 | 0.055 | 9.4 |
Table 1: Reading of treated calli of D. Sissoo.
• Relative Growth Rate is calculated by the formula:
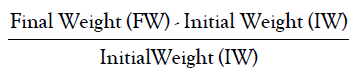
• Relative Water Content is calculated by the formula:
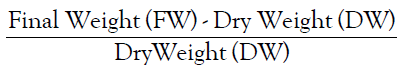
Analysis of Variance (ANOVA)
Relative water content (Table 2).
| Sum of squares | df | Mean square | F | Sig. | |
|---|---|---|---|---|---|
| Between Groups | 44.749 | 8 | 5.594 | 3 | 0.025 |
| Within Groups | 33.562 | 18 | 1.865 | ||
| Total | 78.311 | 26 |
Table 2: Reading (ANOVA) of Relative Water Content
Relative growth rate (Table 3).
| Sum of squares | Df | Mean square | F | Sig. | |
|---|---|---|---|---|---|
| Between groups | 0.033 | 8 | 0.004 | 0.186 | 0.99 |
| Within groups | 0.405 | 18 | 0.022 | ||
| Total | 0.438 | 26 |
Table 3: Reading (ANOVA) of relative growth rate.
Analysis of Variance (ANOVA) showed that NaCl treatments had significant effect on Relative Water Content (RWC). With increase in salt concentration the RWC decreased. Moreover, the growth of calli reduced in higher salt concentrations, however, the reduction in growth rate was not found statistically significant. 175 and 200 Mm concentration of NaCl resulted in browning of calli although no morphological change was noticed in of rest of salt concentrations as calli remained green in colour (Figure 7).
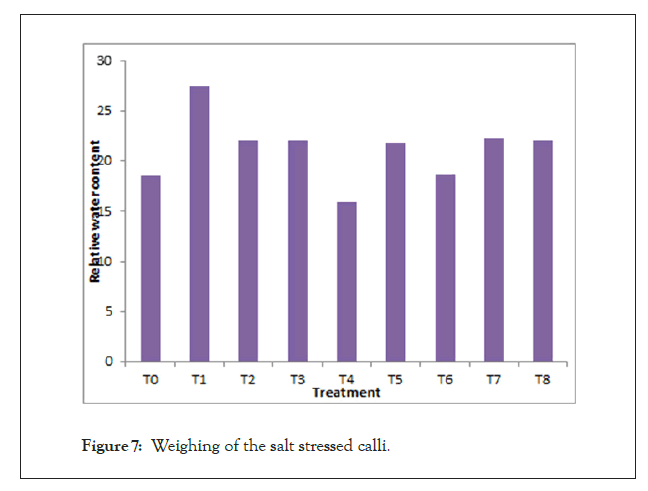
Figure 7: Weighing of the salt stressed calli.
Graph represents the maximum and minimum value of relative water content in different salt concentration treatment in calli of Dalbergia sissoo.
In the present study, the results showed that the growth of cultured calli was drastically reduced at higher concentrations of NaCl. The reduction in growth can be attributed to the nutritional imbalance due to an interference of salt ions, such as Na+ and Cl- with essential nutrients involved in both uptake and translocation processes. Moreover, the toxicity of Na+ can be the cause of browning and necrosis in Figure 8 of callus at 175 and 200 Mm NaCl concentrations. Salt stress in the form of different concentrations of NaCl had a significant impact on the RelativeWater Content (RWC) of callus cultures (Figure 8).
Figure 8: Callus of D. sissoo.
The present work was carried out with an aim to assess the impact of salinity which is one of the major environmental stress, on Dalbergia sissoo at the cellular level.
After analyzing the acquired data with the statistical software it was found that there was no statistical difference among the means of the various treatments. Further experiments may be conducted using media incorporated with higher salt concentrations to determine the salt-tolerance limits of shisham and its utilization in salt-affected areas. Moreover, biochemical analysis such as proline estimation, bioassays of antioxidant enzymes etc. can be carried out which can give a better perspective of the behavior of Dalbergia sissoo in salt stress.
Citation: Chamoli A (2021) Effect of Salinity on Growth of Callus Culture in Dalbergia sissoo. J Plant Biochem Physiol. 9:271.
Received: 28-Oct-2021 Accepted: 11-Nov-2021 Published: 18-Nov-2021 , DOI: 10.35248/2329-9029.21.9.271
Copyright: © 2021 Chamoli A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.