Research Article - (2018) Volume 4, Issue 3
Effect of Seasonal Variation on Anaerobic Treatment of Organic Municipal Solid Waste-II: Population Dynamics of Bacteria and Archaea Communities
*Corresponding Author: Ogbonna CB, Department of Microbiology, Faculty of Science, University of Port Harcourt, Port Harcourt, Nigeria, Tel: +234-7061103614 Email:
Abstract
This study was designed to investigate the effect of seasonal variation on population dynamics of bacteria and archaea communities during anaerobic treatment of organic municipal solid waste. The waste was subjected to anaerobic treatment inside one-stage 250 L-capacity batch-type mesophilic poly-tank reactors with used volume of 230 L, substrate concentration of 5.53% total solids, rumen juice as the source of microbial inoculum and a retention time of 84 days. The first Anaerobic Digestion (ADH) treatment was conducted during the dry season (between February and April, 2016) while the second Anaerobic Digestion (ADC) treatment, a repeat of the first process, was conducted during the rainy season (between July and October, 2016). To monitor performance of the anaerobic treatment process, populations of selected bacteria and archaea groups and biodegradation of the feed where estimated with time using standard methods. Inside the ADH system, the population of Acetoclastic Methanogens (AMA), Hydrogenotrophic Methanogens (HMA), Strict Anaerobic Bacteria (SAB) and Facultative Anaerobic Bacteria (FAB) ranged from not-detected to 8.76 × 106 CFU/ml, not-detected to 7.93 × 106 CFU/ml, 1.45 × 106 CFU/ml to 4.18 × 107 CFU/ml and 1.2 × 106 MPN/ml to 7.5 × 107 MPN/ml respectively with time. Inside the ADC system, the population of AMA, HMA, SAB and FAB ranged from not-detected to 1.46 × 106 CFU/ml, not-detected to 1.05 × 106 CFU/ml, 9.2 × 105 CFU/ml to 1.85 × 107 CFU/ml and 2.4 × 106 MPN/ml to 1.2 × 108 MPN/ml respectively with time. Biodegradation of the feed inside the ADH system and the ADC system, increased to 97.21% and 75.86% respectively after 84 days. Microbial populations inside the ADH-system increased significantly more than the microbial populations inside the ADC-systems with time, leading to a significantly (p<0.05) better performance of the ADH-system than the ADC-system with respect to biodegradation of the feed. Therefore, seasonal variation appears to have influenced the population dynamics of microbial communities during anaerobic degradation of the waste.
Keywords: Municipal solid waste; Anaerobic treatment; Seasonal variation; Microbial community
Introduction
Anaerobic conversion of biomatter to biogas usually includes four stages namely hydrolysis, acidogensis, acetogenesis and methanogenesis, in which various microbial groups play distinct roles [1]. Successful biogas production is based on a stable and adaptable microbial community structure which depends on the type of substrate used and several physico-chemical conditions in the bioreactor. Monitoring those and the dynamics of microbiota is important for planning and optimizing the biogas process, avoiding critical points and reaching the maximum methane yield [2]. The study of microbial communities represents another area of research in the field of anaerobic digestion [3-5]. It is well known that, a deeper knowledge into microbial community dynamics, would provide information in order to, for example, predict system performance under a given set of conditions, or design engineered systems to foster the development of specific communities and to optimize the process for gas production [6].
Engineered systems offer a controlled environment in which complex microbial communities can be studied using cultural and modern culture-independent techniques that can provide an unbiased view of community composition [5]. The research for understanding microbial ecology to improve the efficiency and robustness of AD systems is still on-going [4,6]. Culture-independent molecular techniques have been used to characterize AD-associated microbial communities under a range of process configurations, conditions, feedstocks and using different inocula [6-14]. These studies have provided substantial insight into the biogas producing microbial communities as it relates to the process performance. The aim of the current study was to investigate the effect of seasonal variation on population dynamics of bacteria and archaea community structure during anaerobic treatment of organic municipal solid waste.
Materials and Methods
Anaerobic digestion set-up
The anaerobic digestion process was set-up as described by Stanley et al. [15]. One-stage 250 L-capacity anaerobic digesters (AD) were configured for batch-type mesophilic reactors with useful volumes of around 230 L, rumen juice as the source of microbial inoculum and a retention time of 84 days. The first ADH process was conducted in duplicate during the dry season (between February and April, 2016) while the ADC process was conducted in duplicate during the rainy season (between July and October, 2016).
Preparation, characterisation and anaerobic digestion of the substrate
Preparation and pre-treatment of the rumen juice has been described by Stanley et al. [15]. Collection and pre-treatment of Organic Fraction of Municipal Solid Waste (OFMSW) as well as preparation of the substrate have been described by Stanley et al. [15]. The feed for anaerobic digestion was formulated to arrive at the desired substrate concentration (%) shown in Table 1 as described in Stanley et al. [15]. After preparation, samples of the substrate were collected to determine some of its physical, chemical and microbiological properties using standard methods and the result is presented in [15].
| System | Substrate | PW:FW | DS (Kg) | WC (Kg) | *WS (Kg) | *RJ (kg) | *WA (kg) | Total (kg) | %TS |
|---|---|---|---|---|---|---|---|---|---|
| ADH | OFMSW | 1:4 | 12.69 | 3.21 | 15.90 | 6.36 | 207.74 | 230.00 | ~5.53 |
| ADC | OFMSW | 1:4 | 12.74 | 3.16 | 15.90 | 6.36 | 207.74 | 230.00 | ~5.53 |
Table 1: Composition of the Feed for Anaerobic digestion.
Collection of samples and determination of physico-chemical parameters
To monitor the anaerobic treatment process of the waste, slurry samples from the AD were collected at weekly and bi-weekly intervals (during a period of 84 days) to determine important physicochemical and microbiological parameters which could influence process performance with respect to biodegradation of the feed [1]. Daily online bio-digester Process Temperature (PTM) was measured using digital thermometers with probes (SCT-lilliput, Scichem Tech) which extended into the AD. Weekly process pH was determined using a digital hand-held pH meter (SCT-lilliput, Scichem Tech) as described by Ogbonna et al. [15]. Total organic carbon (TOC), biochemical oxygen demand (BOD) and chemical oxygen demand (COD) were determined using Standard Methods [16]. Substrate Degradability (SD) and Degree of Digestion (DD) of the feed were determined using values of BOD and COD and TOC respectively as described by Schnurer and Jarvis [1]. Total sulphate (SO42-) was determined using the Nephelometeric protocol described in Standard methods [16]. Total nitrate (NO3-) was determined using the spectrophotometric protocol described in Standard methods [16].
Enumeration of bacteria and archaea populations
Microbial populations were evaluated by counting select groups based on metabolic capacity and oxygen sensitivity respectively. The metabolic groups selected included the populations of Sulphate Reducing Bacteria (SRB), Nitrate Reducing Bacteria (NRB), Cellulolytic Bacteria (CEB), Proteolytic Bacteria (PRB), Butyrate Oxidizing Bacteria (BOB), Propionate Oxidizing Bacteria (POB), AMA and HMA respectively. They were enumerated anaerobically using the agar roll-tube technique described by Holdeman et al. [17,18]. The oxygen-sensitive groups selected included the populations of FAB and SAB. The population of FAB were enumerated using the MPN (n=3) method described in Lozano et al. [19]. The MPN result was interpreted with appropriate MPN tables from Oblinger and Koburger and reported in MPN/ml of digester sample [20]. The populations of SAB were enumerated using the agar roll-tube method described by Holdeman et al. respectively [17,18]. The media were prepared as described for the cultivation of methanogenic and non-methanogenic anaerobes as well FAB [18].
During incubation (at 30° C between one and two weeks), the cultures inside the tubes were monitored for microbial growth. The growth of AMA and HMA were monitored under UV-(black) light for the presence of blue-auto fluorescent colonies due to the exhibition of factor 420 blue-fluorescence specific to methanogens [1,21]. The growth of SRB was monitored for black deposits on or around colonies due to the reduction of SO42- to sulphide [22]. The growth of NRB was monitored for red coloration/deposits on or around colonies due to the reduction of NO3- to nitrite after the addition of few drops of Griess reagent [23]. The growth of CEB and PRB was monitored for the development of colonies with zone of clearance around them. However, the growth of POB, BOB and SAB was respectively monitored for the development of colonies [18]. Colonies were counted and recorded as observed.
Medium for acetoclastic methanogenic archaea (AMA): The growth medium for AMA which was prepared using a modified basal medium of Wolfe, was composed of the following in 1 L-capacity Erlenmeyer flask with butyl rubber cork: 1 L of sterile distilled water, 1.0 g of NH4Cl, 0.6 g of NaCl, 5.0 g of NaHCO3, 0.3 g of KH2PO4, 0.3 g of K2HPO4, 0.16 g of MgCl2.6H2O, 0.01 g of CaCl2.2H2O, 10 ml of oligoelement and vitamin solutions [18], 1.0 g of yeast extract, 1.0 ml of resazurin solution (1% w/v), 15 g of agar powder and the amounts of substrate used per L of medium was 6.0 g of sodium acetate, final pH was 7.2.
Medium for hydrogenotrophic methanogenic archaea (HMA): The growth medium for HMA which was prepared using a modified basal medium of Wolfe, was composed of the following in 1 L-capacity Erlenmeyer flask with butyl rubber cork: 1 L of sterile distilled water, 1.0 g of NH4Cl, 0.6 g of NaCl, 5.0 g of NaHCO3, 0.3 g of KH2PO4, 0.3 g of K2HPO4, 0.16 g of MgCl2.6H2O, 0.01 g of CaCl2.2H2O, 10 ml of oligoelement and vitamin solutions [18], 1.0 g of yeast extract, 1.0 ml of resazurin solution (1% w/v), 15 g of agar powder and the amounts of substrate used per L of medium was 6.0 g of sodium acetate, final pH was 7.2.
Medium for propionate oxidizing bacteria (POB): The growth medium for POB which was prepared using a modified basal medium of Wolfe, was composed of the following in 1 L-capacity Erlenmeyer flask with butyl rubber cork: 1 L of sterile distilled water, 1.0 g of NH4Cl, 0.6 g of NaCl, 5.0 g of NaHCO3, 0.3 g of KH2PO4, 0.3 g of K2HPO4, 0.16 g of MgCl2.6H2O, 0.01 g of CaCl2.2H2O, 10 ml of oligoelement and vitamin solutions [18], 1.0 g of yeast extract, 1.0 ml of resazurin solution (1% w/v), 15 g of agar powder and the amounts of substrate used per L of medium was 5.0 g of sodium propionate, final pH was 7.2.
Medium for butyrate oxidizing bacteria (BOB): The growth medium for BOB which was prepared using a modified basal medium of Wolfe, was composed of the following in 1 L-capacity Erlenmeyer flask with butyl rubber cork: 1 L of sterile distilled water, 1.0 g of NH4Cl, 0.6 g of NaCl, 5.0 g of NaHCO3, 0.3 g of KH2PO4, 0.3 g of K2HPO4, 0.16 g of MgCl2.6H2O, 0.01 g of CaCl2.2H2O, 10 ml of oligoelement and vitamin solutions [18], 1.0 g of yeast extract, 1.0 ml of resazurin solution (1% w/v), 15 g of agar powder and the amounts of substrate used per L of medium was 4.0 g of sodium butyrate, final pH was 7.2.
Medium for sulphate reducing bacteria (SRB): The growth medium for SRB which has been modified from Moissl-Eichinger was composed of the following in 1 L-capacity Erlenmeyer flask with butyl rubber cork: 1 L of sterile distilled water, 1.0 g of NH4Cl, 1.0 g of Na2SO4, 5.0 g of NaHCO3, 0.3 g of KH2PO4, 0.3 g of K2HPO4, 0.2 g of MgSO4 7H2O, 0.01 g of CaCl2.2H2O, 0.02 g of FeSO4 7H2O, 10 ml of oligoelement and vitamin solutions [18], 1.0 g of yeast extract, 1.0 ml of resazurin solution (1% w/v), 15g of agar powder, 0.2 g of sodium thioglycolate and the amounts of substrates used per L of medium were 4ml of sodium lactate and 0.4 g of ascorbic acid, final pH was 7.2 [22].
Medium for nitrate reducing bacteria (NRB): The growth medium for NRB which has been modified from Labat and Garcia was composed of the following in 1 L-capacity Erlenmeyer flask with butyl rubber cork: 1 L of sterile distilled water, 2.0 g of NaNO3, 5.0 g of NaHCO3, 0.3 g of KH2PO4, 0.3 g of K2HPO4, 0.16 g of MgCl2 2H2O, 0.01 g of CaCl2.2H2O, 10 ml of oligoelement and vitamin solutions [18], 1.0 g of yeast extract, 1.0 ml of resazurin solution (1% w/v), 15 g of agar powder, 0.2 g of sodium thioglycolate and the amounts of substrates used per L of medium were 2 ml of sodium lactate and 4 g of sodium acetate, final pH was 7.2 [23].
Medium for cellulolytic bacteria (CEB): The growth medium for CEB which was prepared using a modified basal medium of Wolfe, was composed of the following in 1 L-capacity Erlenmeyer flask with butyl rubber cork: 1 L of sterile distilled water, 1.0 g of NH4Cl, 2.0 g of NaCl, 5.0 g of NaHCO3, 0.3 g of KH2PO4, 0.3 g of K2HPO4, 0.16 g of MgCl2.6H2O, 0.01 g of CaCl2.2H2O, 12.5 ml of oligoelement and vitamin solutions [18], 2.0 g of yeast extract, 1.0 ml of resazurin solution (1% w/v), 5.0 g of cellulose powder (CMC), 0.5 g of sodium thioglycolate and 15 g of agar, final pH was 7.2.
Medium for proteolytic bacteria (PRB): The growth medium for PRB which was prepared using a modified basal medium of Wolfe, was composed of the following in 1 L-capacity Erlenmeyer flask with butyl rubber cork: 1 L of sterile distilled water, 1.0 g of NH4Cl, 2.0 g of NaCl, 5.0 g of NaHCO3, 0.3 g of KH2PO4, 0.3 g of K2HPO4, 0.16 g of MgCl2.6H2O, 0.01 g of CaCl2.2H2O, 12.5 ml of oligoelement and vitamin solutions [18], 2.0 g of yeast extract, 1.0 ml of resazurin solution (1% w/v), 3.0 g of fish powder, 3 g of meat powder, 0.5 g of sodium thioglycolate and 15 g of agar, final pH was 7.2
Medium for strict anaerobic bacteria (SAB): The growth medium for SAB which was prepared using a modified basal medium of Wolfe, was composed of the following in 1L-capacity Erlenmeyer flasks with butyl rubber corks: 1 L of sterile distilled water, 1.0 g of NH4Cl, 2.0 g of NaCl, 5.0 g of NaHCO3, 0.3 g of KH2PO4, 0.3 g of K2HPO4, 0.16 g of MgCl2.6H2O, 0.01 g of CaCl2.2H2O, 12.5 ml of oligoelement and vitamin solutions [18], 2.0 g of yeast extract, 1.0 ml of resazurin solution (1% w/v), 5.5 g of D-glucose, 3.0 g of sodium acetate, 0.5 g of sodium thioglycolate and 15 g of agar, final pH was 7.2.
Medium for facultative anaerobic bacteria (FAB): The growth medium for FAB was composed of the following in 1 L-capacity Erlenmeyer flask with butyl rubber cork: 1 L of sterile distilled water, 1.0 g of NH4Cl, 2.0 g of NaCl, 5.0 g of NaHCO3, 0.3 g of KH2PO4, 0.3 g of K2HPO4, 0.16 g of MgCl2.6H2O, 0.01 g of CaCl2.2H2O, 12.5 ml of oligoelement and vitamin solutions [18], 2.0 g of yeast extract, 1.0 ml of resazurin solution (1% w/v), 5.5 g of D-glucose, 3.0 g of sodium acetate, 0.5 g of sodium thioglycolate, 6 g of agar, final pH was 7.2.
Metagenomic analysis of archaea community structure
DNA extraction and PCR amplification: To determine the dynamics of microbial community structure during anaerobic digestion of the waste inside the ADH and ADC systems, slurry samples were collected at day 1, 14, 35, 56 and 84 respectively and stored at -20°C before further analysis. Total DNA was extracted and purified (in Lahor Research Institute, Nigeria) using the Zymo Research (ZR) fungal/bacterial DNA extraction 96-well format according to the manufacturer’s instruction. The quality of DNA was then verified by agarose gel electrophoresis [12]. Extracted DNA was stored at -20°C until further use. Bacteria and archaea 16S ribosomal RNA genes were amplified by polymerase chain reaction (94o C for 3 min, followed by 35 cycles at 94°C for 30 s, 54°C for 30 s, and 72°C for 1 min and a final extension at 72°C for 7 min) using primers 8 F-FAM (5’-AGA GTT TGA TCM TGG CTC AG-3’)/1492R (5’-GGT TAC CTT GTT ACG ACT T-3’) and Arc 109 F-FAM (5’-ACK GCT CAG TAA CAC GT-3’)/Arc915R (5’-GTG CTC CCC CGC CAA TTC CT-3’) respectively [12]. The 5’-ends of primers 8F and Arc109 F were labelled with 6-carboxyfluoresceinphosphoramidite (FAM). PCR reactions were performed in a 25 µL mixture containing 12.5 µL of quick load one taq one step PCR master mix (2x), 1.25 µL of forward primer (20 µM), 1.25 µL of reverse primer (20 µM), 5.0 µL of nuclease free water and 5.0 µL of template DNA.
Sequencing/analysis of sequences: After the quality of the PCR products were verified by agarose gel electrophoresis, the PCR products were cleaned using the ExoSAP protocol as instructed by the manufacturer. Next, the cleaned PCR products were subjected to labelling (or sequencing) reactions using the ABI V3.1 Big dye kit according to manufacturer’s instructions. Next, the labelled products were cleaned with the Zymo Seq clean-up kit as instructed by the manufacturer (ZR). Next, the ultra-pure (or cleaned) DNA samples were loaded (or injected) on ABI 3500 XL analyzers with a 50 cm array using POP7. Following this, sequence data generated were analyzed with the Geneious package (version 9.0.5) and phylogenetic trees were then constructed using neighbour joining.
Result and Discussion
Population dynamics of digester bioindicator microbes
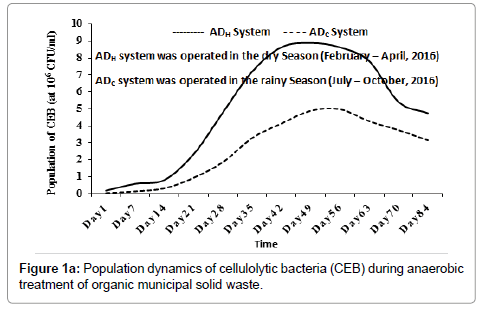
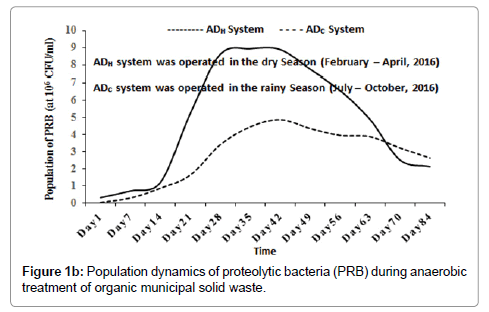
Inside the ADH operated during the dry season (between February and April, 2016), the average population of CEB increased from 1.82 × 105 CFU/ml (at day 1), peaked at 8.89 × 106 CFU/ml (around day 49) and then decreased progressively to 4.74 × 106 CFU/ml (around day 84). Inside the ADC operated during the rainy season (between July and October, 2016), the population of CEB increased from 4.10 × 104 CFU/ml (at day 1), peaked at 4.98 × 106 CFU/ml (around day 56) and then decreased progressively to 3.15 × 106 CFU/ml (around day 84) as shown in Figure 1a. The population of PRB inside the ADH system increased from 3.33 × 105 CFU/ml (at day 1), peaked at 8.95 × 106 CFU/ml (around day 35) and then decreased progressively to 2.13 × 106 CFU/ml (around day 84). Inside the ADC system, the population of PRB increased from 5.0 × 104 CFU/ml (at day 1), peaked at 4.87 × 106 CFU/ml (around day 42) and then decreased progressively to 2.46 × 106 CFU/ml (around day 84) as shown in Figure 1b. In this study, the population dynamics of CEB and PRB were used as bioindicators of hydrolysis and acidogenesis inside the bio-digesters (ADH and ADC) [1]. Their population dynamics suggested that hydrolysis and acidogenesis may have increased peaked and then decreased inside the bio-digesters with time.
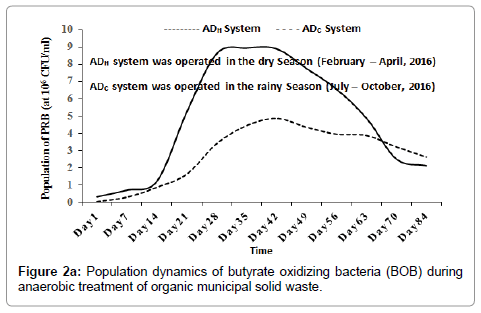
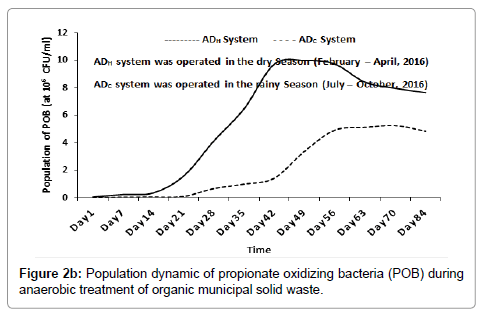
Inside the ADH system, the population of BOB increased from 3.70 × 104 CFU/ml (at day 1), peaked at 6.99 × 106 CFU/ml (around day 49) and then decreased progressively to 4.82 × 106 CFU/ml (around day 84). In the ADC system, the population of BOB increased from 8.0 × 103 CFU/ml (at day 1), peaked at 3.24 × 106 CFU/ml (around day 63) and then decreased progressively to 2.87 × 106 CFU/ml (around day 84) as shown in Figure 2a. Inside the ADH system, the population of POB increased from 5.10 × 105 CFU/ml (at day 1), peaked at 9.99 × 106 CFU/ml (around day 49) and then decreased progressively to 7.64 × 106 CFU/ml (around day 84). Inside the ADC system, the population of POB increased from 4.0 × 103 CFU/ml (at day 1), peaked at 5.25 × 106 CFU/ml (around day 70) and then decreased slightly to 4.84 × 106 CFU/ml (around day 84) as shown in Figure 2b. The population dynamics of BOB and POB were used as bioindicators of anaerobic oxidation (or acetogenesis) inside the ADH and ADC systems [1,19,23]. As the result indicated, acetogenesis may have increased, peaked and then decreased with time during the anaerobic treatment process inside the ADH system and the ADC system respectively.
Inside the ADH system, the population of AMA peaked at 8.76 × 106 CFU/ml (around day 56) and then decreased slightly to 8.63 × 106 CFU/ml (around day 84). Inside the ADC system, the population of AMA peaked at 1.46 × 106 CFU/ml (around day 63) and then decreased slightly to 1.21 × 106 CFU/ml (around day 84) as shown in Figure 3a. Likewise, the population of hydrogenotrophic methanogens (HMA) inside the ADH system peaked at 7.93 × 106 CFU/ml (around day 56) and then decreased slightly to 7.42 × 106 CFU/ml (at around day 84). The population of hydrogenotrophic methanogens (HMA) inside the ADC system increased, peaked at 1.05 × 106 CFU/ml (around day 49) and then decreased progressively to 7.20 × 105 CFU/ml (at around day 84) as shown in Figure 3b. The population dynamics of AMA and HMA were used as bioindicators of methanogenesis in both ADH and ADC systems. As the result indicates, methanogenesis seemed to have increased, peaked and the decreased with time inside the ADH system and the ADC system respectively [1].
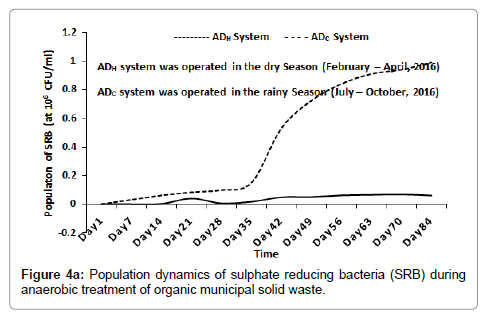
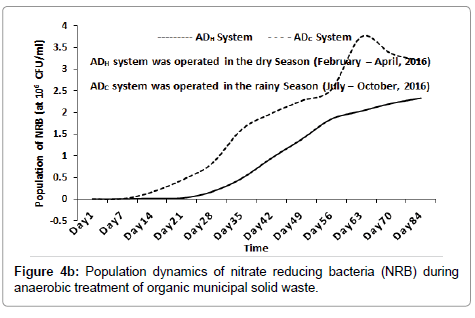
Inside the ADH system, the population of SRB increased, peaked at 6.9 × 104 CFU/ml (around day 70) and then decreased slightly to 6.1 × 104 CFU/ml (at around day 84). Inside the ADC system, the population of SRB increased and peaked at 9.9 × 105 CFU/ml (around day 84) as shown in Figure 4a. Inside the ADH system, the population of NRB increased progressively to 2.33 × 106 CFU/ml (around day 84). Inside the ADC system the population of NRB increased, peaked at 3.75 × 106 CFU/ml (around day 63) and then decreased slightly to 3.19 × 106 CFU/ml (around day 84) as shown in Figure 4b. The population dynamics of SRB and NRB were used as bioindicators of process instability inside both ADH and ADC systems with time. [1,24,25]. As the result shows, the population of SRB and NRB inside both AD (ADH and ADC) increased with time during the anaerobic treatment process. This may have been as a result of their consumption of SO42- and NO3- contained in the feed [24,26].
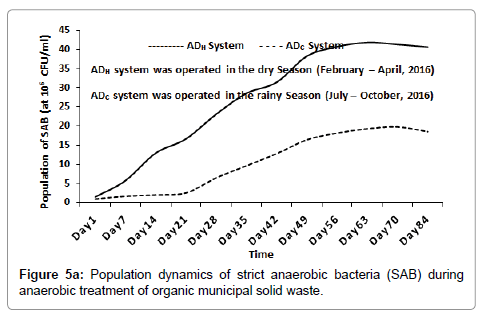
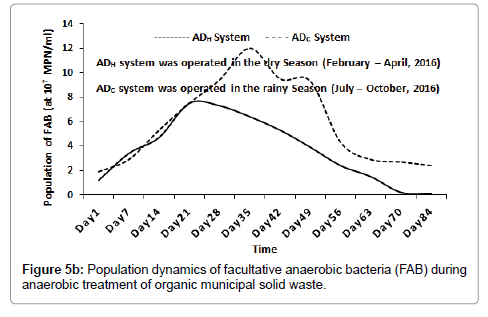
In terms of oxygen sensitivity (or O2 requirement), the population of SAB inside the ADH system increased from 1.45 × 106 CFU/ml (at day 1), peaked at 4.18 × 107 CFU/ml (around day 63) and then decreased slightly to 4.06 × 107 CFU/ml (around day 84). Inside the ADC system, the population of SAB increased from 9.2 × 105 CFU/ml (at day 1) and peaked at 1.85 × 107 CFU/ml (around day 84) as shown in Figure 5a. The population of FAB inside the ADH system increased from 1.2 × 107 MPN/ml (at day 1), peaked at 7.5 × 107 MPN/ml (around day 21) and then decreased progressively to 1.2 × 106 MPN/ml (around day 84). Likewise, inside the ADC system, the population of FAB increased from 1.9 × 107 MPN/ml (at day 1), peaked at 1.2 × 108 MPN/ml (around day 35) and decreased progressively to 2.4 × 106 MPN/ml (around day 84) as shown in Figure 5b.
Bacterial and archaeal population dynamics inside the AD appear to resemble the sigmoid pattern of growth usually observed with microbial batch cultures [27]. This was expected since the anaerobic treatment processes were conducted using the batch technology [1]. As the result suggested, hydrolysis, acetogenesis and methanogenesis appear to have progressed much better inside the ADH operated during the dry season when compared to the ADC operated during the rainy season of 2016 as shown in Figures 1-3 respectively. This is because the population of bacteria and archaea groups which may be responsible for these stages of anaerobic digestion of the waste were higher inside the ADH system than those recorded inside the ADC system with time [1,28]. In terms of oxygen requirement, the population of SAB was significantly higher inside the ADH system compared to the ADC system with time (Figure 5a). However, the population of FAB inside the ADC system was significantly higher at some point than the population of FAB inside the ADH system (Figure 5b). In fact, the period when the population of FAB peaked inside both systems appeared to have correlated with the same period when the process pH became significantly acidic. According to literature, acidogenesis which is the second phase of anaerobic digestion process, is usually influence by the activities of mostly FAB groups who are the fermenters [1,28]. Therefore, it may be possible to attribute the lower (or acidic) pH recorded inside the ADC system over time to its significantly higher number of FAB. This increased acidic nature inside the ADC system may have contributed to its reduced performance with respect to biodegradation of the feed compared to that observed inside the ADH system with time. This is because at lower (or acidic) pH, the methanogens are most affected negatively, consequently leading to a reduced biomass conversion efficiency especially if acidity increases above their carrying capacity [1,28].
Archaea community dynamics
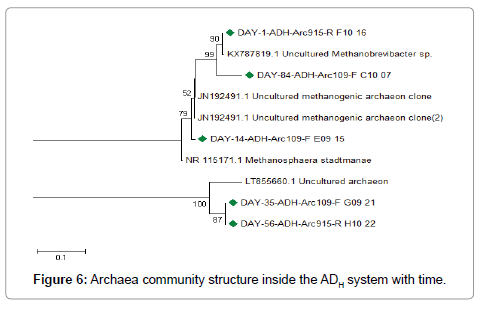
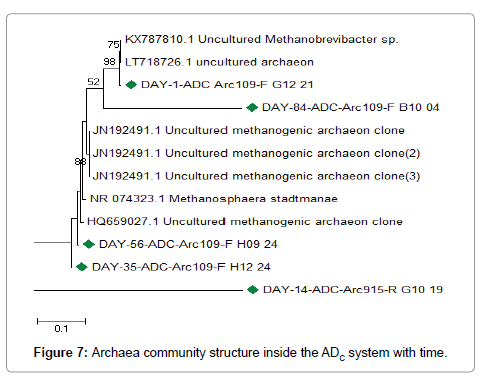
At day 1, the archaea community inside the ADH system operated during the dry season of 2016 appears to have been dominated by Methanobrevibacter species followed by other uncultured archaeon clones. Inside the ADC system operated during the rainy season of 2016, the archaea community appears to have been dominated by uncultured archaeon. However, one uncultured Methanobrevibacter species appears to have been present at the time. Around day 14, archaea related uncultured methanogenic archaeon clones, Methanosphaera stadmanae, uncultured archaeon clones were represented inside the ADH system. Inside the ADC system, archaea related to Methanosphaera stadmanae, uncultured archaeon clones and uncultured methanogenicarchaeon clones were represented. Around day 35, the archaea community inside the ADH system was represented by archaea related to Methanosphaera stadmanae, uncultured methanogenic archaeon clones and uncultured archaeon clones. Inside the ADC system, archaea related to uncultured archaeon clones, uncultured methanogenic archaeon clones and Methanosphaera stadmanae were represented. Around day 56, archaea related to uncultured archaeon clones, uncultured methanogenic archaeon clones and Methanosphaera stadmanae were also represented inside the ADH system. Inside the ADC system, the archaea community appears to have been dominated by uncultured archaeon clones and uncultured methanogenic archaeon clones respectively. Around day 84, the dominant archaea inside the ADH system were related to uncultured archaeon clones. Inside the ADC system, archaea related to uncultured archaeon clones appeared to have dominated the archaea community, however, others related to uncultured methanogenic archaeon clones and Methanosphaera stadmanae were present.
There was a shift in archaea community structure during anaerobic treatment of OFMSW inside the ADH system and the ADC system with time as indicated in Figures 6 and 7 respectively. Generally, the archaea community inside both ADH and ADC systems appears to have been dominated by uncultured archaeon and uncultured methanogenic archaeon clones as seen in Figures 6 and 7 respectively. Some of the methanogenic archaea that were also observed inside both ADH and ADC belonged to Methanobrevibacter and Methanosphaera. Methanogenic archaea species belonging to these generae have been implicated in biogas (i.e., methane) production inside anaerobic digestion systems treating various forms of organic wastes in previous studies [1,14,29-35]. Species belonging to these two methanogenic archaea generae have also been implicated in hydrogenotrophic methanogenesis while utilizing either acetate, formate or alcohol, etc. as substrate [1,29,34,35]. This indicates that hydrogenotrophic methanogenesis may have been active inside the bio-digesters (i.e., ADH and ADC systems) with time. It also implies that the dominant methanogenic pathway may have been hydrogenotrophic methanogenesis rather than acetoclastic methanogenesis during anaerobic treatment of the organic solid waste inside both ADH and ADC systems [1]. GenBank accession number(s) for the nucleotide sequence(s) isolated during anaerobic treatment of OFMSW inside the bio-digesters (ADH and ADC) are presented in Table 2.
| Nucleotide Sequence | Accession Number |
|---|---|
| SUB3878683 DAY_1_ADC_Arc109-F_G12_21 | MH177267 |
| SUB3878683 DAY-1-ADH-Arc915-R_F10_16 | MH177268 |
| SUB3878683 DAY-14-ADC-Arc915-R_G10_19 | MH177269 |
| SUB3878683 DAY-14-ADH-Arc109-F_E09_15 | MH177270 |
| SUB3878683 DAY-35-ADC-Arc109-F_H12_24 | MH177271 |
| SUB3878683 DAY-35-ADH-Arc109-F_G09_21 | MH177272 |
| SUB3878683 DAY-56-ADC-Arc109-F_H09_24 | MH177273 |
| SUB3878683 DAY-56-ADH-Arc915-R_H10_22 | MH177274 |
| SUB3878683 DAY-84-ADH-Arc109-F_C10_07 | MH177275 |
Table 2: Gen Bank accession number(s) for the nucleotide sequence(s) isolated during anaerobic digestion of organic municipal solid waste inside the anaerobic digesters (ADH and ADC).
Biodegradation of the feed
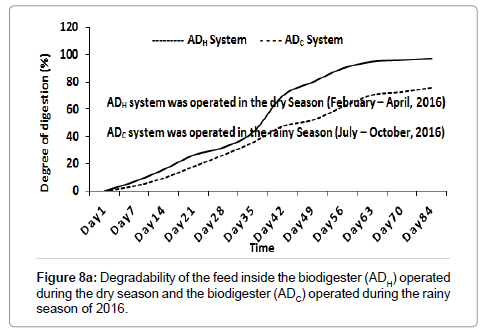
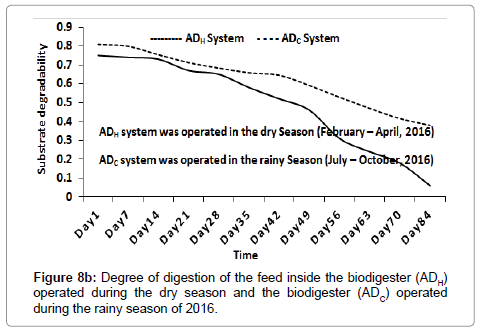
Degradability (i.e., BOD to COD ratio) of the feed inside the ADH operated during the dry season (2016) and the ADC operated during the rainy season (2016) decreased from 0.75 and 0.81 to 0.06 and 0.38 respectively after 84 days (Figure 8a). The DD of the feed (which is a function of TOC) inside the ADH system and the ADC system increased to 97.21% and 75.86% respectively after 84 days (Figure 8b). The DD of the feed inside the ADH operated during the dry season (2016) was significantly (P<0.05) higher than the DD of the feed inside the ADC operated during the rainy season (2016). This may be attributed to the fact that the populations of bacteria and archaea inside the ADH operated during the dry season (2016) may have adapted better to their environment compared to the much lower bacterial and archaea populations observed inside the ADC operated during the rainy season of 2016 [1].
Dynamics of COD/SO42- and COD/NO3- ratios
Inside the ADH operated during the dry season (2016), COD/SO42- ratio ranged from 362.20 to 98.18 after 84 days (Figure 9a). The COD/SO42- ratio inside the ADC operated during the rainy season (2016) ranged from 12,845.40 to 101.47 after 84 days (Figure 9a). COD/SO42- ratio less than 3.0 is said to increase the competitive advantage of SRB in biogas producing anaerobic environment over the methanogens with respect to acetate and hydrogen consumption thereby limiting methane production [1,36]. However, in this study, COD/SO42- ratios inside both AD (ADH and ADC) were significantly greater than 3.0. Therefore, the SRBs may not have contributed significantly to process instability inside both ADH and ADC at any point in time. Furthermore, COD/NO3- ratio inside ADH operated during the dry season (2016) ranged from 74.15 to 271.35 after 84 days of the digestion process (Figure 9b). The COD/NO3- ratio inside the ADC operated during the rainy season (2016) ranged from 73.49 to 4,670.91 after 84 days of the digestion process (Figure 9b). Generally, lower COD/NO3- ratios promote process instability because the condition favors the population of NRB which usually out-compete the population of methanogens (under this condition) with respect to acetate consumption thereby limiting methane production [37,38]. However, in this study, COD/NO3- ratio inside both ADH and ADC systems was significantly higher than the threshold (between 2.0 and 5.0) required for an anaerobic treatment process to be considered unstable [37]. Therefore, the NRBs may not have contributed significantly to process instability inside both ADH and ADC at any point in time.
Temperature and pH dynamics
During the dry season when the first ADH of the waste was conducted, ambient temperature ranged from 30.3°C to 33.6°C with time. However, during the rainy season when the same ADC process was repeated, the ambient temperature ranged from 26.3°C to 30.5°C with time. Consequently, PTMH inside the ADH operated during the dry season and the ADC operated during the rainy season ranged from 29.7°C to 39.3°C and 26.8°C to 30.8°C respectively with time. This shows that the temperature recorded during the dry season (between February and April, 2016) was relatively higher than temperature observed during the rainy season (between July and October, 2016). Several authors have shown that the anaerobic digestion process of organic matter performs much better at higher temperatures to some degree [12,28,39,40]. This could be one of the reasons why the populations of microbes inside the ADH system (operated during the dry season, 2016) performed better than the populations of microbes inside the ADC system (operated during the rainy season, 2016) with respect to biodegradation of the feed. The process pH (which ranged from 6.67 to 5.32 with time) inside the ADH operated during the dry season (2016) was less acidic than the process pH (which ranged from 6.40 to 4.60 with time) inside the ADC operated during the rainy season (2016). This suggests that the ADH operated during the dry season (2016) may have been more favorable to the microbial populations taking part in the digestion process compared to the ADC operated during the rainy season (2016) with time [28]. It could be another reason why the ADH system (operated during the dry season, 2016) performed better than the ADC system (operated during the rainy season, 2016) with respect to biodegradation.
Conclusion
The aim of the study was to investigate the influence of seasonal variation on the dynamics of bacterial and archaeal populations/community structure during anaerobic treatment of OFMSW. From the study, it was observed that there was a temporal shift in the populations of bacteria and archaea community during anaerobic treatment of organic municipal solid waste. However, the microbial populations inside the ADH system appeared to have increased significantly more than the microbial populations inside the ADC systems with time. The presence of methanogenic species which belong to known genera such as Methanobrevibacter and Methanosphaera suggests that hydrogenotrophic methanogenesis may have been active inside the bio-digesters. It was also observed that the ADH process conducted between February and April (2016), during the dry season performed significantly (P<0.05) better than the ADC process conducted between July and October (2016), during the rainy season with respect to biodegradation of the feed. This is probably due to its higher process temperature, less acidic process pH and higher microbial populations with time. Seasonal variation appeared to have influenced the population dynamics of bacteria and archaea communities during anaerobic treatment of organic municipal solid waste.
References
- Schnurer A, Jarvis A (2010) Microbiological handbook for biogas plants. Swedish Gas Centre Report 207 Pp: 13-138.
- Cater M, Fanedl L, Logar RM (2013) Microbial Community Analyses in Biogas Reactors by Molecular Methods. Acta Chim Slov 60: 243-255.
- Wang J, Shen J, Wu Y, Tu C, Soininen J, et al. (2013) Phylogenetic beta diversity in bacterial assemblages across ecosystems: deterministic versus stochastic processes. ISME J 7: 1310-1321.
- Pholchan MK, Baptista Jde C, Davenport RJ, Sloan WT, Curtis TP (2013) Microbial community assembly, theory and rare functions. Front Microbiol4: 1-9.
- Zhou J, Liu W, Deng Y, Jiang YH, Xue K, et al. (2013) Stochastic assembly leads to alternative communities with distinct functions in a bioreactor microbial community. BioMed 4: 1-8.
- Stams AJM, Sousa DZ, Kleerebezem R, Plugge CM (2012) Role of syntrophic microbial communities in high-rate methanogenic bioreactors. Water Sci Technol 66: 352-362.
- McGuinness LM, Salganik M, Vega L, Pickering KD, Kerkhof LJ (2006) Replicability of bacterial communities in denitrifying bioreactors as measured by PCR/T-RFLP analysis. Environ Sci Technol 40: 509-515.
- Gentile ME, Nyman JL, Criddle CS (2007) Correlation of patterns of denitrification instability in replicated bioreactor communities with shifts in the relative abundance and denitrification patterns of specific populations. ISME J 1: 714-728.
- Weiss A, Jerome V, Freitag R, Mayer HK (2008) Diversity of the resident microbiota in a thermophilic municipal biogas plant. Appl Microbiol Biotechnol 81: 163-173.
- Werner JJ, Knights D, Garcia ML, Scalfone NB, Smith S, et al. (2011) Bacterial community structures are unique and resilient in full-scale bioenergy systems.Proc Natl Acad Sci U S A 108: 4158-4163.
- Nelson MC, Morrison M, Yu Z (2011) A meta-analysis of the microbial diversity observed in anaerobic digesters. Bioresour Technol 102: 3730-3739.
- Li S, Li A, Chu Y, Wang X, Ren L, et al. (2013) A pyrosequencing-based metagenomic study of methane-producing microbial community in solid-state biogas reactor. Biotechnol Biofuels 6: 3-21.
- Alvarado A, Montanez-Hernandez LE, Palacio-Molina SL, Oropeza-Navarro R., Luévanos-Escareño MP, et al. (2014) Microbial trophic interactions and mcrA gene expression in monitoring of anaerobic digesters. Front Microbiol 5: 597.
- Bartell RD, Matson E, Mueller-Spitz S, Kleinheinz GT (2015) Investigation of Methanosarcinales and Methanomicrobiales Presence within a Dry Anaerobic Digester. J Microbiol Res 5: 101-108.
- Stanley HO, Ogbonna CB, Abu GO (2017) Dynamics of Micro/Macro Elements and Heavy Metals during Anaerobic Treatment of Organic Fraction of Municipal Solid Waste (OFMSW). Asian J Biotechnol Bioresour Technol 2: 1-17.
- APHA (2005) Standard Methods for the Examination of Water and Wastewater (21stedn), American Public Health Association, Washington.
- Holdeman LV, Moore WEC (1972) Roll-Tube Techniques for Anaerobic bacteria. Am J Clin Nutr 25: 1314-1317.
- Wolfe RS (2011) Techniques for Cultivating Methanogens. Methods Enzymol 494: 1-22
- Lozano CJ, Mendoza MV, de Arango MC Monroy EF (2009) Microbiological characterization and specific methanogenic activity of anaerobe sludges used in urban solid waste treatment. Waste Manag 29: 704-711.
- Oblinger JL, Koburger JA (1975) Understanding and Teaching the Most Probable Number Technique. J Milk Food Technol 38: 540-545.
- Adachi K (1999) Isolation of hydrogenotrophic methanogenic archaea from a subtropical paddy field. FEMS Microbiol Ecol 30: 77-85.
- Moissl-Eichinger C, Stieglimeier M, Wirth R, Kminek G (2009) Cultivation of anaerobic and facultatively anaerobic bacteria from spacecraft associated clean rooms. Appl Environ microbiol 75: 3484-3491.
- Labat M, Garcia JL (1986) Study on the development of methanogenic microflora during anaerobic digestion of sugar beet pulp. Appl Microbiol Biotechnol 25: 163-168.
- Rodriguez-Martinez J, Garza-Garcia Y, Aguilera-Carbo A, Martinez-Amador SY, Sosa-Santillan GJ (2005) Influence of nitrate and sulfate on the anaerobic treatment of pharmaceutical wastewater. Eng Life Sci 5: 568-573.
- Sponza DT, Atalay H (2005) Influence of nitrate and COD on phosphorous and nitrogen removals under batch methanogenic and denitrifying conditions. Environ Eng Sci 22: 145-155.
- Dai X, Yi J, Dong B, Xue Y, Li N, et al. (2014) Microbial Community Dynamics in Batch High-Solid Anaerobic Digestion of Food Waste under Mesophilic Conditions. J Microbiol Biotechnol 24: 270-279.
- Nopharatana A, Pullammanappallil PC, Clarke WP (2007) Kinetics and dynamic modeling of batch anaerobic digestion of municipal solid waste in a stirred reactor. Waste Manag 27: 595-603.
- Khalid A, Arshad M, Anjum M, Mahmood T, Dawson L (2011) The anaerobic digestion of solid organic waste. Waste Manag 31: 1737-1744.
- McMahon KD, Stroot PG, Mackie RI, Raskin L (2001) Anaerobic co-digestion of municipal solid waste and biosolids under various mixing conditions-II: Microbial population dynamics. Water Res 35: 1817-1827.
- Liu Y, Whitman WB (2008) Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann N Y Acad Sci 1125: 171-189.
- Charles W, Walker L, Cord-Ruwisch R (2009) Effect of pre-aeration and inoculum on the start-up of batch thermophilic anaerobic digestion of municipal solid waste. Bioresour Technol 100: 2329-2335.
- Liu FH, Wang SB, Zhang JS, Zhang J, Yan X, et al. (2009) The structure of the bacterial and archaeal community in a biogas digester as revealed by denaturing gradient gel electrophoresis and 16S rDNA sequencing analysis. J Appl Microbiol 106: 952-966.
- Lee C, Kim J, Hwang K, O’Flaherty V, Hwang S (2009) Quantitative analysis of methanogenic community dynamics in three anaerobic batch digesters treating different wastewaters. Water res 43: 157-165.
- Trzcinski AP, Ray MJ, Stuckey DC (2010) Performance of a three-stage membrane bioprocess treating the organic fraction of municipal solid waste and evolution of its archaeal and bacterial ecology. Bioresour Technol 101: 1652-1661.
- Gowdaman V, Srikanth M (2015) Identification of metabolically active methanogens in anaerobic digester by DNA Stable-Isotope Probing using 13C-acetate. Carbon-Sci Technol 2: 8-16.
- Jeong TY, Cha GC, Seo YC, Jeong C, Choi SS (2008) Effect of COD/Sulphate ratios on batch anaerobic digestion using waste-activated sludge. J Ind Eng Chem 14: 693-697.
- Xie L, Chen J, Wang R, Zhou Q (2012) Effect of carbon source and COD/NO3--N ratio on anaerobic simultaneous denitrification and methanogenesis for high-strength wastewater treatment. J Biosci Bioeng 113: 759-764.
- Akunna JC, Bizeau C, Moleta R (2008) Denitrification in anaerobic digesters: Possibilities and influence of wastewater COD/N-NOx ration. Environ Technol 13: 825-836.
- Sibiya NT, Muzenda E, Tesfagiorgis HB (2014) Effect of Temperature and pH on The Anaerobic Digestion of Grass Silage.
- Ghasimi DSM, Tao Y, de Kreuk M, Zandvoort MH, van Lier JB (2015) Microbial population dynamics during long‑term sludge adaptation of thermophilic and mesophilic sequencing batch digesters treating sewage fine sieved fraction at varying organic loading rates. Biotechnol Biofuels 8: 1-15.
Copyright: © 2018 Ogbonna CB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.