
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2020)Volume 10, Issue 3
Background and Aims: The incidences of end-stage liver disease and hepatocellular carcinoma arising from
nonalcoholic steatohepatitis (NASH) are rapidly increasing. Although weight reduction is recommended as an initial
treatment, no pharmacologic treatments for this condition are presently available. The aims of the current study
were(1) to develop a smartphone application-based intervention for weight reduction in NASH patients, and (2) to
evaluate the feasibility of using this application in daily clinical practice.
Methods: We developed a mobile phone application for NASH patients (NASH App). We then prospectively
enrolled 9 clinically diagnosed NASH patients and applied a 24-week intervention using the NASH App in addition
to the usual follow-up with laboratory and imaging studies. The use of the NASH App by the patients and the
changes in weight and biomarkers were evaluated after the 24-week intervention.
Results: The mean patient age was 37.67 years, and 7 patients (77.78%) were male. The mean BMI was 29.63 kg/m2.
Seven patients completed the counseling provided by the NASH App. Since one patient was lost to follow-up, the
comparison between pre- and post-intervention values was performed for 8 patients. A post-intervention weight
reduction was observed in 7 patients, and this weight reduction was statistically significant (p=0.02).Normalization of
the ALT level (<30 U/L) was observed in two patients.
Conclusion: The NASH App intervention for NASH patients is feasible and acceptable. Further studies using a
control arm and a larger population are needed to confirm the efficacy of the NASH App.
Nonalcoholic Steatohepatitis; Smartphone; NASH App; Application; Intervention
ALP: Alkaline Phosphatase; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; BMI: Body Mass Index; GGT: Gamma-Glutamyltransferase; HCC: Hepatocellular Carcinoma; LS: Liver Stiffness; mBaaS: Mobile Backend as a Service; NAFLD: Nonalcoholic Fatty Liver Disease; NASH: Nonalcoholic Steatohepatitis; TB: Total Bilirubin
Because of the global epidemic of obesity, nonalcoholic fatty liver disease (NAFLD) is rapidly becoming the most common liver disorder worldwide [1-4]. Nonalcoholic steatohepatitis (NASH) is a severe form of NAFLD characterized by the accumulation of fat in the liver, lobular inflammation, hepatocellular ballooning, and insulin resistance [5,6]. An effective treatment for NASH is clearly needed because NASH can progress to cirrhosis and hepatocellular carcinoma (HCC); however, no pharmacologic therapy has been approved for this condition [7].
Because obesity is reported to be one of the most important risk factors for this condition [8], weight reduction is frequently recommended as an initial treatment for NASH patients [9]. Although obese patients with NASH rarely achieve or maintain a sustained reduction in body weight, previous studies showed that an intensive lifestyle program could successfully produce a 7% to 10% weight reduction in patients with NASH, and weight reduction was associated with a significant improvement in NASH histological activity [10,11]. However, not all hospitals can provide lifetime intervention services because of the cost and/or availability of trained counselors.
A number of mobile phone applications that provide healthcare services to individuals who live at a distance from healthcare providers have been developed and tested [12-15]. In 2013, the FDA approved Bluestar (WellDoc Communications Inc., USA), an application that enables users to track blood-glucose levels, exercise regimes, and diet for adults with type 2 diabetes. Bluestar became the world’s first prescription-only application [16]. Compared with traditional care, technology can provide continuous personalized care without the need of specialized human counselors. This mobile phone application-based intervention could potentially become a novel solution for NASH patients. The aims of the current study were (1) to develop a smartphone application designed to provide continuous curative intervention or to motivate NASH patients to comply with treatment and (2) to evaluate the feasibility of using the current application in daily clinical practice.
Study design and participants
The current study was at a single center, and consisted of a single arm, 24-week trial of a smartphone application-based intervention. Between October 1, 2016, and September 30, 2017, we prospectively enrolled patients who visited our liver clinic. Patients who met the following criteria were enrolled in the current study: body mass index (BMI) of 25 kg/m2 or more at the time of screening, the presence of fatty liver on ultrasonography, a liver stiffness (LS) value of more than 7.0 kPa as evaluated using transient elastography, and the use of a smartphone in their daily life. Patients with other causes of liver disease, substantial alcohol consumption (>20 g/day for women or >30 g/day for men), or a clinical suspicion of cirrhosis (platelet count less than 16 × 104/μL or LS value more than 17.0 kPa) or who were pregnant were excluded. This project was approved by the ethical committee of the University of Tokyo (No. P2016008), and all patients provided written informed consent.
Development of smartphone application
In collaboration with CureApp, Inc., we developed a smartphone application for the treatment of NASH (NASH App). Figure 1 shows the conceptual structure of the NASH App. Mobile backend as a Service (mBaaS) is used to connect the patient ’ s mobile application, the doctor ’ s site, a data processing system, and cloud storage. This mBaaS-based online framework enables real-time personalized guidance for lifestylemodifications based on a cognitive behavioral approach for patients and information sharing with the doctor’s site. The NASH App has three components. The first component is to motivate patients to comply with treatment for NASH through guidance on correct disease recognition and an understanding of the significance of treatment (weight reduction). The second component is to coach patients regarding concrete behavioral changes to promote weight loss; this component also functions to support behavior modification. The third component isto provide ongoing support to maintain acquired habits that are advantageous to realizing NASH improvements, such as providing support that will help patients to maintain an appropriate weight.
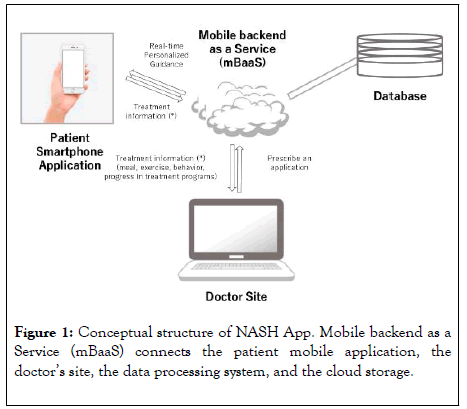
Figure 1: Conceptual structure of NASH App. Mobile backend as a Service (mBaaS) connects the patient mobile application, the doctor’s site, the data processing system, and the cloud storage.
Procedures
After a 4-week run-in period, the patients returned for study visits at weeks 4, 8, 12, 16, 20, and 24. During the study period, all the patients received regular follow-up care with laboratory tests and imaging studies such as ultrasonography, in addition to the NASH App intervention, for 24 weeks. Blood samples were obtained for routine clinical blood tests at all the hospital visits.
Outcomes
The primary outcome measure was the use of the NASH App by patients and its availability. We assessed the proportion of patients who completed the counseling component (the first component) of the application.
The secondary outcome measure was the change in weight after the 24-week NASH App intervention. We also compared baseline and post-intervention biochemical markers related to liver disease (aspartate aminotransferase [AST], alanine aminotransferase [ALT], gamma-glutamyltransferase [GGT], alkaline phosphatase [ALP], total bilirubin [TB], platelet count, and prothrombin time).
Statistical analysis
Unless otherwise specified, continuous variables are presented as the meanwith the 95% confidence interval, whereas categorical variables are expressed as frequencies (%). We used the Wilcoxon rank-sum test to compare continuous variables. Paired t-tests (two-tailed) were used to compare the baseline and follow-up measurements. All the statistical analyses were performed using R 3.4.3 software (http://www.r-project.org).
Patient characteristics
The baseline characteristics of the 9 patients are shown in Table 1. The mean patient age was 37.67 years, and 7 patients (77.78%) were male. All the patients had a BMI of 25 kg/m2 or more, and the mean BMI was 29.63 kg/m2. The mean ALT level was 110.89 U/L, and all the patients had an ALT level of more than 30 U/L.
| Parameter | Values |
|---|---|
| Age (years) | 37.67 (26.75-48.58) |
| Male sex | 7 (77.78%) |
| Weight (kg) | 83.41 (70.77-96.05) |
| Body-mass index | 29.63 (26.50-32.75) |
| Liver stiffness value (kPa) | 9.65 (7.44-11.87) |
| Aspartate aminotransferase (U/L) | 59.89 (32.92-86.86) |
| Alanine aminotransferase (U/L) | 110.89 (59.41-162.37) |
| Gamma-glutamyltransferase (IU/L) | 67.56 (41.81-93.30) |
| Alkaline phosphatase (IU/L) | 250.78 (212.40-289.16) |
| Total bilirubin (mg/dL) | 0.911 (0.667-1.156) |
| Platelet count (× 104/μL) | 26.11 (21.41-30.81) |
| Prothrombin time (%) | 99.0 (97.46-100.54) |
| Cholesterol (mg/dL) | |
| Total | 203.67 (185.64-221.69) |
| Low-density lipoprotein | 124.0 (102.93-145.07) |
| High-density lipoprotein | 48.6 (37.11-60.09) |
| Triglycerides (mg/dL) | 155.78 (97.11-214.45) |
| Fasting plasma glucose (mg/dL) | 90.78 (81.86-99.70) |
Continuous variables are presented as the mean and 95% confidence interval, while categorical variables are presented as the number and frequency (%).
Table 1: Baseline Clinical Characteristics (n=9).
Study endpoints
Table 2 shows the profile and outcome of each patient enrolled in the current study. Seven patients (77.8%) completed the counseling provided by the NASH App. Since one patient was lost to follow-up one month after enrollment, the comparisons between pre- and post-intervention values were performed for 8 patients. Table 3 shows the baseline and 24-week follow-up changes in the patient characteristics. The 24-week intervention enabled a statistically significant weight reduction (p=0.02). The mean post-intervention gamma-glutamyltransferase level tended to be lower than that at baseline (p=0.058). Figure 2 shows the body weight and BMI changes expressed as the rates of change (%) for each patient between baseline and at 24 weeks. Weight reductions were observed in 7 patients. The mean weight reduction of these 7 patients was 3.4 kg (range, 1.0-6.4 kg). We also compared the weight reduction between the patients who completed the counseling provided by the NASH App and those who did not. Although the difference did not reach a significant level (p=0.09), the mean weight reduction in the patients who completed the counseling was greater than that in those who did not complete the counseling (3.85 kg vs. -0.40 kg [average gain of 0.40 kg]). Normalization of the ALT level (<30 IU) was observed in two patients who achieved weight reductions of 3.6 kg and 6.4 kg, respectively.
| Patient | Sex | Age | Completion of 24-week | Completion of counselling |
|---|---|---|---|---|
| number | follow up | provided by application | ||
| 1 | Male | 52 | No | Yes |
| 2 | Female | 67 | Yes | No |
| 3 | Male | 36 | Yes | Yes |
| 4 | Female | 25 | Yes | Yes |
| 5 | Male | 27 | Yes | Yes |
| 6 | Male | 43 | Yes | Yes |
| 7 | Male | 25 | Yes | No |
| 8 | Male | 35 | Yes | Yes |
| 9 | Male | 29 | Yes | Yes |
Table 2: Profile and outcome of each patient enrolled in the current study.
| Parameters | Values | p value | |
|---|---|---|---|
| Baseline | Post-intervention | ||
| Weight (kg) | 85.15 (71.22-99.08) | 82.36 (69.00-95.72) | 0.02 |
| Aspartate aminotransferase (U/L) | 59.5 (28.16-90.84) | 48.0 (28.52-67.48) | 0.32 |
| Alanine aminotransferase (U/L) | 108.13 (48.73-167.52) | 85.63 (38.38-132.87) | 0.22 |
| Gamma-glutamyltransferase (IU/L) | 66.63 (36.80-96.45) | 55.63 (22.95-88.30) | 0.058 |
| Alkaline phosphatase (IU/L) | 258.5 (218.97-298.03) | 251.38 (219.47-28..28) | 0.43 |
| Total bilirubin (mg/dL) | 0.888 (0.610-1.165) | 0.975 (0.809-1.141) | 0.6 |
*Data were expressed as the mean and 95% confidence interval.
Table 3: Changes in clinical parameters after 24 weeks of intervention (n=8).
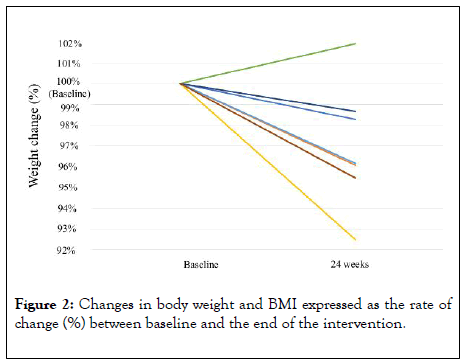
Figure 2: Changes in body weight and BMI expressed as the rate of change (%) between baseline and the end of the intervention.
NASH is the most common cause of chronic liver disease worldwide, and the incidences of end-stage liver disease and HCC resulting from NASH are rapidly increasing [17,18]. However, no approved pharmacologic treatment for NASH currently exists, and there is an urgent need for novel treatment options that can help patients to overcome this condition [7].
Although weight reduction through life modification is recommended as an initial treatment for NASH [7], an interventional approach for promoting weight reduction has not been established. To the best of our knowledge, the current study is the first trial of a mobile phone application-based intervention for patients with clinically diagnosed NASH. A statistically significant weight reduction was achieved after a 24- week intervention using the NASH App. Although the mean weight reduction in patients who completed counseling was greater than that of the patients who did not complete counseling, the difference was not significant, probably because of the small sample size. These results suggest that the NASH App-based intervention was effective. Even though the current study was a single-arm trial and nonrandomized, the NASH App may be a feasible and acceptable intervention for NASH patients.
Smartphones are ubiquitous, highly personalized, and easy-touse devices. The widespread distribution of smartphones, which can process and communicate data in real time, makes them an ideal platform for therapeutic interventions. Computerized interventions have been shown to have a significant effect in some fields, such as type 2 diabetes and depression [15,19]. A previous study from New Zealand showed a significantly higher remission rate for a computerized cognitive behavioral therapy intervention for patients with depression, compared with conventional face-to-face therapy provided by trained counselors or psychologists [19].
Since the cost of computerized therapy was shown to be substantially lower than that of traditional therapy [20], mobile applications and discount programs have arisen in response to the problem of rising medication costs [21]. Previous studies have shown a cost-saving effect of mobile phone-supported interventions for smoking cessation [22] and diabetes [23]. Obesity-related medical costs are increasing [24-26]. Computerized cognitive behavioral interventions using the NASH App may lead to future reductions in obesity-related medical costs through patient cognitive reconstruction as well as the amelioration of liver conditions.
A major limitation of the present study is the lack of pre- and post-intervention histological assessments. Liver biopsy is the current gold standard for NASH diagnosis. However, biopsies are invasive and can result in major or minor complications. Since the current study was originally designed as an exploratory, feasibility study, we used only non-invasive biomarkers for the clinical diagnosis of NASH and the assessment of outcome in consideration of ethical aspects. However, since body weight changes and changes in liver-related enzyme levels have been shown to be reliable surrogates for histological alterations, the reliability of the current study can be assured. Further study including histological assessments using paired biopsy specimens are presently underway.
In conclusion, a smartphone application-based intervention for NASH patients is both feasible and acceptable. Further studies using a control arm and a larger patient population are needed to confirm the efficacy of the NASH App.
This research was supported by Support Projects for Seed-stage Technology-based Startups of NEDO and AMED under Grant number JP20fk0210040.
The English in this document has been checked by professional editor, native speakers of English.
Citation: Sato M, Suzuki S, Tateishi R, Kinoshita MN, Nakatsuka T, Ogawa T, et al. (2020) Effect of Smartphone Application-Enabled Daily Intervention for Patients with Nonalcoholic Steatohepatitis: A Feasibility Study. J Clin Trials 10:412. doi: 10.35248/2167-0870.20.10.412
Received: 01-May-2020 Accepted: 15-May-2020 Published: 22-May-2020 , DOI: 10.35248/2167-0870.20.10.412
Copyright: © 2020 Sato M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This research was supported by Support Projects for Seed-stage Technology-based Startups of NEDO and AMED under Grant number JP20fk0210040.