Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2020)Volume 10, Issue 3
A study was conducted to determine mainly the n 3 fatty acids enrichment and decreased oxidation of broiler meat using the plant Moringa oleifera and a blue green algae Spirulina platensis. The effects of the dietary treatments were evaluated in terms of growth performance, carcass and meat yields, oxidative stability and fatty acids modulation. Two hundred and forty (240) one day old Cobb 500 broiler chicks were assigned to 5 dietary treatments for 5 weeks, with 4 replications having 12 chicks per replication. The diets of the treatments were formulated from the basal feed as follows: control (T1), including 2 different levels of M. oleifera leaf meal 1% (T2) and M. oleifera leaf meal 1.5%, (T3) and 2 different levels of S. platensis 1% (T4) and S. platensis 1.5% (T5). The birds were randomly distributed in each pen, and the data were analyzed using the SPSS statistical package. The final body weight (BW) gain was significantly higher in the T2 and T5 groups, and the feed conversion ratio improved in the T2 group (1.68). The lowest (p ≤ 0.05) thiobarbituric acid-reactive substances values (TBARS) of breast and thigh were obtained in T2 groups compared to T3-T5 group after the second week of preservation. Regarding fatty acid profile of breast and thigh meat, the omega-3 fatty acid levels, such as those of linolenic and docosahexaenoic acid (DHA), were increased in the additives groups. The results of the present study elucidated that dietary inclusion of the 2 medicinal plants in the T2 and T5 groups could be promising functional ingredients to produce value-added broiler meat in terms of oxidative stability and omega-3 fatty acids enhancement.
Broiler chicken; Moringa oleifera; Spirulina platensis; Oxidation; Fatty acid
At present, consumer demand is increasing for natural, safe, and eco-friendly products that can improve well-being, limit the risk of some chronic diseases, and promote health benefits beyond their nutritional value. These foods are called functional foods or nutraceuticals, a concept that was born in Japan early in 1980 [1,2] This consumer attitude applies to poultry products as it does to other value-added products. The advancements in poultry research have pooled knowledge of the biochemical and physiological mechanisms that increase the efficiency of feed utilization and desired carcass attributes through dietary manipulation [3]. Another reason for the broiler meat industry’s success has been the consumer perception of a healthy product that contains less fat (< 5%), less cholesterol (<50 mg/100 g), and most predominantly unsaturated fatty acids as compared to beef or pork products [4]. However, Sirri et al. [5] reported that bird genotype might strongly influence meat functional properties as well as nutritional characteristics. The feeding and rearing conditions under which the broilers are produced and slaughtered may also influence the meat’s oxidative stability [6]. Lipid oxidation is a primary cause of quality deterioration in meat products through adverse changes in undesirable odors and flavors, which lowers the functional, sensory, and nutritive values of meat products [7].
To date, various plants and algae have been researched, and many are reported to contain functional properties that have an impact on the meat quality of poultry [8]. M. oleifera, a plant from the family Moringacea is a major crop in Asia and Africa. For centuries, people in many countries have used moringa leaves as traditional medicine for common ailments. The most used parts of the plant are the leaves, which are rich in vitamins, carotenoids, polyphenols, phenolic acids, alkaloids, glucosinolates, isothiocyanates, tannins and saponins [9]. These bioactive compounds might explain the pharmacological properties with having antioxidant, antiseptic [10], antimicrobials properties and can reduce microbial growthon the food and food products [11,12]. On the other hands, among edible algae, Spirulina platensis, blue-green microalgae, has recently served as an important source of valuable bioactive compounds. Dried spirulina is a good nutritional source with high protein and significant polyunsaturated fatty acids content, such as oleic, linoleic, gamma-linolenic, and docosahexaenoic (DHA) acid [12]. In addition, M. oleifera leaves and S. platensis act as good natural antioxidant sources due to the presence of various types of antioxidant compounds, such as ascorbic acid, flavonoids, phenolics, and carotenoids [13,14]. Antioxidants have the potential to maintain meat quality and protect body cells against the damaging effects of reactive oxygen species [15]. According to World Health Organization (WHO) developing countries populations, about 80% depends on the medicinal plants for their health care due to their beneficial properties [2,3]. However, information is limited concerning the oxidative stability and fatty acid profiles of broiler meatusing M. oleifera and S. platensis as a natural functional ingredient in Bangladesh. This is an ongoing search for natural feed additives with excellent physiological activity for broiler meat. Therefore, the objectives of the present study were to evaluate the feeding effect of M. oleifera leaf meal and S. platensis on their performance, oxidative stability and fatty acid profiles of broiler meat.
Preparation of M. oleifera leaf and S. platensis meal Fresh M. oleifera leaves were collected and air-dried during the daytime. After 4-5 d of drying, the leaves were grinded to a fine powder to pass through a 0.15 mm sieve. The leaf meal was tightly packaged in polythene plastic bags and kept at room temperature until required. The blue-green alga S. platensis was collected from the Bangladesh Council of Scientific and Industrial Research (BCSIR) in Dhaka, Bangladesh. M. oleifera and S. platensis were analyzed in triplicate for Crude Protein (CP), Ether Extract (EE), moisture and ash as described by the Association of Official Analytical Chemists [16].
Experimental design, dietary treatments and bird management
A total of 240 one day old Cobb 500 broiler chicks were purchased from Nourish Poultry and Hatchery Ltd., a commercial hatchery. The chicks were randomly weighed and allocated to 20 floor pens (100 cm long × 90 cm wide) in shed containing fresh wood shavings at a depth of 5 cm. The internal temperature of the broiler house was set and maintained at 34°C for the first week, after which it was gradually reduced to 23°C at 3°C per week, and then maintained at this temperature until the end of the total experimental period. The experiment was divided into 5 dietary treatments, with 4 replications having 48 chicks in each group. Each pen served as an experimental unit. The chicks were vaccinated against commercial Newcastle disease virus (NDV) through eye drops and drinking water at days 4 and 18 during the experiment period, respectively. The chicks were inspected daily, and dead birds were removed following mortality recording (pen, date, and body weight [BW]). Feed and fresh water were offered ad libitum feed intake and free access to water throughout the period (35 d rearing). The birds’ care and basal diet was formulated to meet the Nutrient Requirements of Poultry [17] and applied for a total of 5 weeks in two stages: starter (0-3 weeks) and finisher (4-5 weeks), the diet and chemical composition of additives are shown in supplemental information (Supplementary Tables 1 and 2). All diets were in mashed form. Five dietary treatment groups were produced from the basal feed as follows: control (T1), including 2 different levels of M. oleifera leaf meal 1% (T2) and M. oleifera leaf meal 1.5%, (T3) and 2 different levels of S. platensis 1% (T4) and S. platensis 1.5% (T5). A proximate analysis for moisture, crude protein, ash, and ether extract was performed on the experimental diets (supplementary information (Tables 1 and 2) according to the Association of Official Analytical Chemists’ (AOAC, 2000) methods. The neutral detergent fiber (NDF) concentration was analyzed according to the methods described by Van Soest et al. [18]. The diets’ metabolizable energy (ME) contents were calculated on the basis of the determined nutrients in the proximate analyses. The BW per pen were recorded at placement, as well as at weekly intervals (i.e., at 7, 14, 21, 28, and 35 d of age, respectively). The BW (g) was calculated as final body weight (ΔBW) minus initial BW; and average daily gains (ADG, g) were calculated accordingly. The feed intake (FI, g) was calculated as feed allocated minus feed refused; whereas, the feed conversion ratio (FCR) was calculated as the FI (g) per ΔBW (g) on a pen weight basis. Experimental birds were reared in the Bangladesh Livestock Research Institute Savar, in research shed. The experiment was also subjected to an assessment of broiler chicken health status for its ethical acceptability and was approved by the Ethics Committees of Bangladesh Livestock Research Institute in Savar, Dhaka.
Table 1: Effect of dietary added M. oleifera leaf and S. platensis on the body weight, average daily gain, feed intake, and feed conversion ratio (Feed: Gain) of broiler chickens.
| Experimental period (d) and parameters | Dietary treatments | ||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | |
| BW (g) | |||||
| 0 d | 46.42 ± 0.34 | 46.38 ± 0.43 | 47.54 ± 0.88 | 47.06 ± 0.15 | 48.46 ± 1.4 |
| 21 d | 779.50 ± 11.97a | 786.25 ± 5.06a | 751.75 ± 6.61b | 768.50 ± 11.25b | 778.75 ± 75a |
| 35 d | 1512.65 ± 29.47b | 1632.28 ± 25.66a | 1521.71 ± 23.96b | 1518.31 ± 30.07b | 1629.47 ± 29.01a |
| ADG (g) | |||||
| 21 d | 37.11 | 37.44 | 35.77 | 36.60 | 37.08 |
| 35 d | 43.21b | 46.07a | 43.48b | 43.39b | 46.56a |
| FI (g/bird) | |||||
| 21d | 1004.12 ± 3.74 | 1003.75 ± 4.66 | 1008.10 ± 4.280 | 1000.66 ± 5.310 | 1007.50 ± 4.247 |
| 35d | 2714.37 ± 9.41a | 2716.54 ± 6.61a | 2661.14 ± 20.19b | 2694.08 ± 16.98ab | 2709.66 ± 7.49a |
| FCR (Feed: gain) | |||||
| 21d | 1.66 ± 0.09 | 1.62 ± 0.09 | 1.63 ± 0.07 | 1.61 ± 0.08 | 1.64 ± 0.09 |
| 35d | 1.79 ± 0.02b | 1.68 ± 0.03a | 1.75 ± 0.04b | 1.77 ± 0.02b | 1.72 ± 0.11ab |
a,b,cMeans with different superscripts within same raw are significantly different (p<0.05). BW - Body weight (g), ADG - Average Daily Gain, FI - Feed intake (g/bird), FCR - Feed Conversion Ratio; T1 - Control (basal diet); T2 - M. oleifera leaf meal 1% ; T3 - M. oleifera leaf meal 1.5% ; T4 - S. platensis 1%; and T5 - S. platensis 1.5%. The values are means ± SD (n=4).
Table 2: Effect of M. oleifera and S. platensis on broiler meat composition.
| Parameters | Treatments | ||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | |
| Breast meat (%) | |||||
| Moisture | 74.75 ± 0.24 | 75.63 ± 0.09 | 74.62 ± 0.04 | 73.89 ± 0.25 | 73.93 ± 0.43 |
| Crude ash | 1.45 ± 0.12 | 0.90 ± 0.12 | 0.80 ± 0.43 | 1.50 ± 0.04 | 1.20 ± 0.22 |
| Crude protein | 22.36 ± 0.31 | 24.88 ± 0.06 | 23.99 ± 0.31 | 23.58 ± 0.24 | 23.01 ± 0.14 |
| Crude fat | 1.61 ± 0.12bc | 1.01 ± 0.13b | 1.20 ± 0.24b | 1.20 ± 0.41b | 2.42 ± 0.11a |
| Thigh meat (%) | |||||
| Moisture | 76.02 ± 0.42 | 76.02 ± 0.32 | 75.99 ± 0.22 | 75.21 ± 0.32 | 76.34 ± 0.32 |
| Crude ash | 1.01 ± 0.11 | 0.65 ± 0.43 | 0.85 ± 0.14 | 1.30 ± 0.41 | 0.85 ± 0.12 |
| Crude protein | 20.68 ± 0.33 | 20.25 ± 0.17 | 20.82 ± 0.32 | 20.28 ± 0.34 | 20.39 ± 0.42 |
| Crude fat | 2.80 ± 0.08 | 2.65 ± 0.21 | 2.60 ± 0.13 | 2.41 ± 0.15 | 2.53 ± 0.16 |
a,bValues with different superscripts in the same row differ significantly (p<0.05), T1 - Control (basal diet); T2 - M. oleifera leaf meal 1% ; T3 - M. oleifera leaf meal 1.5% ; T4 - S. platensis 1%; and T5 - S. platensis 1.5%. The values are means ± SD (n=8).
Slaughter procedure
Eight birds were randomly selected per treatment, 2 per replicate pen, and were individually weighed at the age of 35 d. Birds were then fasted for 8 h with water offered ad libitum consumption and were reweighed before they were slaughtered. After bleeding, scalding, plucking, and washing, the feet, head, neck and skin were removed. Then, the carcasses were manually eviscerated and cut into breast, drumsticks (legs), wings, and thighs. The visceral organs and cuts were then weighed individually, and the yields and carcass dressing percentage (CW/BW) were calculated. The breast muscles and thigh muscles was separated from the bones and skin, and was trimmed of external/adjacent fat and connective tissue. The breast and thigh meat samples from each bird were ground separately using a meat grinder. The samples were subsequently divided into three parts, one for the oxidative stability analysis and another two for the proximate and fatty acid composition analysis. Finally, the samples were kept into zipper bag, after which those for oxidative rancidity analysis were refrigerated at 4°C and samples for other analyses were stored at -20°C.
Determination of oxidative stability and fatty acids profile
The lipid oxidation of the broiler meat was determined according to the method described by Sarker et al. [19], with slight modification. For this analysis, 4 g of the thigh and breast meat samplewere blended at full speed for 1.5 min in a chilled stainless watering blender cup with 10 mL of extracting solution containing 20% trichloroacetic acid (TCA) in 2 M phosphoric acid. The resulting sediment was quantitatively transferred to a 50 mLconical tube with 10 mL of distilled water, homogenized and diluted by shaking. After, the aliquot was filtered through Whatman No.6 filter paper, then 5 mL of filtrate was transferred to a test tube, and 5 mL of 2-thiobarbituric acid (0.005 M in DW) was added. The solution was then subsequently shaken in a water bath at 80°C (HB-205 SW Hanbaek Scientific Co., Korea) for 30 min. After cooling, the color development was measured at 530 nm in a Jenway 6305 spectrophotometer (Bibby Scientific Ltd., Staffordshire, United Kingdom). TBARS values were expressed as micromoles of malondialdehyde (MDA) per 100 g of meat sample. The fatty acids compositions of breast and thigh meat were conducted at the BCSIR’s Institute of Food Science and Technology in Dhaka, Bangladesh. The Fatty Acid Methyl Ester (FAME) was determined using a gas chromatograph (Shimadzu 14b, JAPAN) equipped with a flame ionization detector and identified by matching their retention times with those of their relative standards (Polyunsaturated Fatty Acid-2, Animal Source, SUPELCO, Bellefonte, PA, USA) as well as with the Food Composition Table (NRLSI, 2002).
Statistical analysis
The analytical measurements were done in triplicates, and the results were presented as the average of 3 analyses ± the standard deviation (SD). The statistical analysis was done using the SPSS statistical package (IBM Corp., IBM SPSS Statistics for Windows, Version 16.0, Armork, NY, USA) with a one-way ANOVA. A p value of <0.05 was taken as statistically significant based on Tukey’s tests.
Broilers’ growth performance and meat composition
The effects of diets with added M. oleifera leaf meal and S. platensis on the FI, ΔBW, and FCR are shown in Table 1. The dietary addition of M. oleifera leaf meal and S. platensis affected the broilers’ growth performance. The final ΔBW and BW gain were significantly higher (p<0.05) in the T2 and T5 groups compared to the T3 andT5 groups and the T1 control group. Similarly, on d 35, birds fed diets with M. oleifera 1% and S. platensis 1.5%, in particular those in T2 and T5, had the highest ADG, while those in T1 and T3 had the lowest (p < 0.05). T2 group was observed decreased feed consumption ratio as compared to control (T1) group of broilers for the total period. No mortality was observed in the experimental period.
Addition of feed additives had an effect on the composition of breast and thigh meats (Table 2). Dietary addition of M. oleifera and S. platensis groups were increased (p<0.05) crude protein content in breast meat compared to the control group. In the breast meat crude fat content was decreased in T2-T4 group whereas increased in T5 group in compared to control group (P<0.05). Moisture content did not differ with control group; but there was found higher moisture content in the T2 group compared to other group (P<0.05). The crude ash content in thigh meat was lower in T2 group (0.65%) than the control groupT1 (1.01%); however crude protein and crude fat content did not differ with the control group. In case of thigh and breast meat moisture content did not differ with the control group. Table 3 represents the data on internal organs of broiler chicks. Results showed that heart, liver, and small intestine weight were significantly different (p<0.05) among the treatments. However, other internal organs of birds there were no significant differences observed in this study. Abdominal fat pad was decreased in the additives group compared to control.
Table 3: Effect of M. oleifera and S. platensis meal as feed additives on meat yield traits of broiler.
| Parameters (g/bird) | T1 | T2 | T3 | T4 | T5 | SEM |
|---|---|---|---|---|---|---|
| Head | 38.30 ± 4.32ab | 41.50 ± 2.51a | 39.00 ± 3.46ab | 39.50 ± 3.78ab | 40.50 ± 3.41a | 0. 75 |
| Neck | 44.52 ± 3.21ab | 46.31 ± 1.63a | 44.50 ± 3.46b | 44.43 ± 9.38ab | 44.51 ± 4.12ab | 1.08 |
| Liver | 37.50 ± 3.78a | 31.43 ± 3.46b | 32.50 ± 3.78b | 31.50 ± 1.00b | 31.00 ± 4.76b | 0. 91 |
| Gizzard | 58.09 ± 1.02 | 57.32 ± 2.11 | 57.11 ± 3.10 | 58.01 ± 0.22 | 57.53 ± 2.41 | 0.87 |
| Small intestine | 73.21 ± 5.21a | 62.23 ± 11.43b | 66.50 ± 5.74b | 68.50 ± 5.03ab | 71.21 ± 4.76b | 1.64 |
| Large intestine | 9.37 ± 2.49 | 9.25 ± 0.95 | 10.75 ± 2.21 | 10.52 ± 1.29 | 11.02 ± 1.41 | 0. 38 |
| Abdominal fat | 18.23 ± 2.82 | 17.02 ± 1.15 | 16.20 ± 3.26 | 15.51 ± 3.02 | 15.50 ± 2.51 | 0. 57 |
| Heart | 6.11 ± 3.46 | 7.50 ± 1.31 | 7.50 ± 1.08 | 7.02 ± 1.15 | 6.50 ± 1.02 | 0.42 |
a,b,cMean with different superscripts within same raw are significantly different (p<0.05); T1 - Control (basal diet); T2 - M. oleifera leaf meal 1% ; T3 - M. oleifera leaf meal 1.5%; T4 - S. platensis 1%; and T5 - S. platensis 1.5%. The values are means ± SD (n=8)
Oxidative stability of broiler meat
The TBARS test, of broiler breast and thigh meats which determine the amount of malondialdehyde (MDA), a major secondary lipid oxidation byproduct was shown in Figures 1 and 2. Additives groups had lower (p<0.05) TBARS values after weeks 1 and 2 of preservation as well as 3rd weeks values in breast and thigh meat compare to control group, T1. The lowest TBARS values obtained in breast and thigh meat were 9.95 and12.88 μmol MDA/100g, respectively, for T2 group in which other group’s value were significantly higher in 2nd weeks. Similarly, the TBARS in preserved both thigh and breast meat were found to be significantly lowest for the T3, T4, and T5 dietary groups, with values of 12.55, 13.47, and 14.67 μmol MDA/100 g, for thigh and 14.21, 12.27 and 13.47 μmol MDA/100 g for breast meat compared to the T1 (25.09 and27.19 μmol MDA/100 g) group.
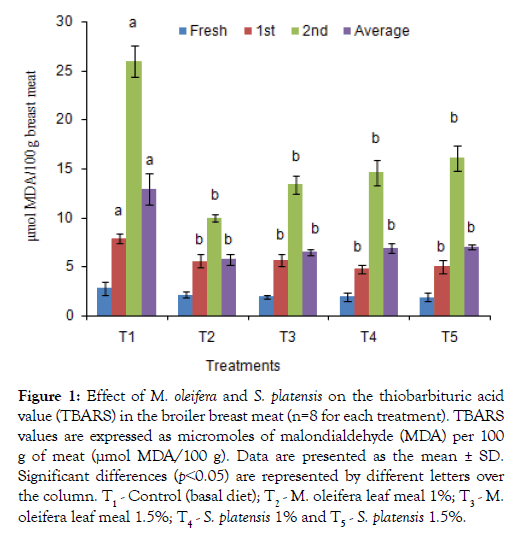
Figure 1: Effect of M. oleifera and on the thiobarbituric acid value (TBARS) in the broiler breast meat (n=8 for each treatment). TBARS values are expressed as micromoles of malondialdehyde (MDA) per 100 g of meat (μmol MDA/100 g). Data are presented as the mean ± SD. Significant differences (p˂0.05) are represented by different letters over the column. T1 - Control (basal diet); T2 - M. oleifera leaf meal 1%; T3 - M. oleifera leaf meal 1.5%; T4 - 1% and T5 - 1.5%.
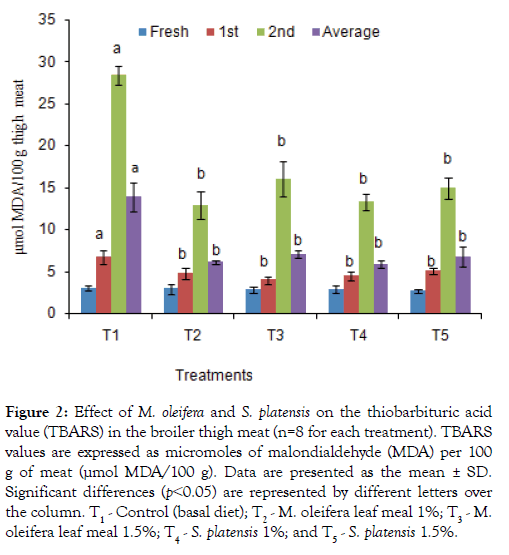
Figure 2: Effect of M. oleifera and on the thiobarbituric acid value (TBARS) in the broiler thigh meat (n=8 for each treatment). TBARS values are expressed as micromoles of malondialdehyde (MDA) per 100 g of meat (μmol MDA/100 g). Data are presented as the mean ± SD. Significant differences (p˂0.05) are represented by different letters over the column. T1 - Control (basal diet); T2 - M. oleifera leaf meal 1%; T3 - M. oleifera leaf meal 1.5%; T4 - 1%; and T5 - 1.5%.
Fatty acid profile in broiler meat
The dietary treatments effect on the composition of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFAs) in the meat is shown in Tables 4 and 5. A tendency of breast meat SFA (38.47 and 37.99 for T2 and T3, respectively) to be higher from M. oleifera added birds was found, whereas S. platensis added birds had a lower SFA (37.22 and 35.46 for T4and T5, respectively) composition. Eicosatrienoic acid with values of 0.50 ± 0.11, 0.42 ± 0.03, 0.40 ± 0.08 and 0.52 ± 0.04 were found higher in the additives groups’ meats than the T1 control group’s 0.36 ± 0.03. Similar trends were observed in case of both EPA and DHA. Linolenic acid was found higher in T2 group both breast and thigh meat. The sum of omega -3 fatty acids content in breast meat was found higher in all additives groups except T3 and control (T1) (Figure 3). In Table 5, for the thigh meat, it was observed that sum of SFA differ in T2-T5 groups whereas sum of MUFA slightly differ in comparison to the control, T1. In thigh meat, T4 group also decreased stearic acid content whereas arachidic and oleic acid contents were increased in T2 groups, compared to that of the T3 group. The sums of n-3 fatty acid in broiler thigh meat were observed significantly higher in all dietary additive groups (Figure 4) compared to control.
Table 4: Effect of M. oleifera and S. platensis as natural feed additives on fatty acid profile of broiler breast meat (g/100 g fatty acids).
| Parameters (g/100 g) | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| Myristic acid | 0.43 ± 0.12 | 0.50 ± 0.31 | 0.58 ± 0.10 | 0.56 ± 0.55 | 0.65 ± 0.76 |
| Palmitic acid | 25.75 ± 1.55 | 25.24 ± 1.64 | 29.45 ± 1.48 | 24.33 ± 1.04 | 27.44 ± 1.35 |
| Stearic acid | 11.29 ± 1.69 | 12.58 ± 0.95 | 7.87 ± 1.01 | 12.24 ± 1.83 | 7.05 ± 0.88 |
| Arachidic acid | 0.03 ± 0.11 | 0.16 ± 0.56 | 0.09 ± 0.02 | 0.09 ± 0.12 | 0.31 ± 0.28 |
| Myristoleic acid | 0.03 ± 0.20 | 0.11 ± 0.56 | 0.03 ± 0.21 | 0.18 ± 0.21 | 0.16 ± 0.34 |
| Palmitoleic acid | 5.23 ± 0.37 | 3.61 ± 0.57 | 4.84 ± 0.17 | 3.14 ± 0.47 | 4.59 ± 0.24 |
| Oleic acid | 36.87 ± 0.91 | 33.64 ± 0.46 | 39.39 ± 0.43 | 36.30 ± 0.23 | 40.08 ± 0.74 |
| Eicosenoic acid | 0.09 ± 0.17 | 0.19 ± 0.22 | 0.31 ± 0.35 | 0.10 ± 0.11 | 0.25 ± 0.22 |
| Eicosadienoic acid | 0.03 ± 0.06 | 0.33 ± 0.69 | 0.10 ± 0.05 | 0.32 ± 0.45 | 1.65 ± 0.31 |
| Eicosatrienoic acid | 0.36 ± 0.03 | 0.50 ± 0.11 | 0.42 ± 0.03 | 0.40 ± 0.08 | 0.52 ± 0.04 |
| Linolenic acid | 0.43 ± 0.21 | 0.52 ± 0.13 | 0.29 ± 0.41 | 0.43 ± 0.14 | 0.64 ± 0.22 |
| Eicosapentaenoic acid | ND | 0.04 ± 0.34 | 0.02 ± 0.24 | 0.03 ± 0.41 | 0.05 ± 0.22 |
| Docosahexaenoic acid | ND | 0.06 ± 0.18 | 0.04 ± 0.11 | 0.07 ± 0.14 | 0.09 ± 0.14 |
| Linoleic acid | 18.06 ± 0.56 | 20.52 ± 0.51 | 15.91 ± 0.21 | 18.94 ± 0.09 | 16.41 ± 0.41 |
| Arachidonoic acid | 1.19 ± 0.34 | 1.50 ± ± 0.15 | 2.02 ± 0.07 | 1.75 ± 0.23 | 1.81 ± 0.14 |
| ΣSFA | 37.50 ± 0.05 | 38.47 ± 0.33 | 37.99 ± 0.41 | 37.22 ± 0.36 | 35.46 ± 0.33 |
| ΣMUFA | 42.22 ± 0.33 | 37.54 ± 0.44 | 44.57 ± 0.21 | 39.73 ± 0.39 | 45.08 ± 0.57 |
| ΣPUFA | 20.99 ± 0.04 | 23.33 ± 0.24 | 18.21 ± 0.36 | 21.91 ± 0.28 | 21.17 ± 0.25 |
| Σn - 6 | 19.25 ± 0.32 | 22.02 ± 0.18 | 17.92 ± 0.26 | 20.69 ± 0.15 | 18.22 ± 0.09 |
Saturated Fatty Acids (SFA); Mono Unsaturated Fatty Acids (MUFA); Poly Unsaturated Fatty Acids (PUFA) ); T1 - Control (basal diet); T2 - M. oleifera leaf meal 1%; T3 - M. oleifera leaf meal 1.5%; T4 - S. platensis 1%; and T5 - S. platensis 1.5%. The values are means ± SEM (n=4).
Table 5: Effect of M. oleifera and S. platensis as natural feed additives on fatty acid profile of broiler thigh meat (g/100 g fatty acids).
| Parameters (g/100 g) | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| Myristic acid | 0.46 ± 0.67 | 0.71 ± 0.31 | 0.56 ± 0.10 | 0.60 ± 0.55 | 0.52 ± 0.76 |
| Palmitic acid | 23.05 ± 1.55 | 26.97 ± 1.64 | 28.03 ± 1.48 | 25.61 ± 1.04 | 23.86 ± 1.35 |
| Stearic acid | 11.56 ± 1.69 | 14.53 ± 0.95 | 13.71 ± 1.01 | 11.53 ± 1.83 | 13.02 ± 0.88 |
| Arachidic acid | 0.05 ± 0.20 | 0.32 ± 0.56 | 0.29 ± 0.17 | 0.26 ± 0.12 | 0.32 ± 0.28 |
| Myristoleic acid | 0.06 ± 0.37 | 0.10 ± 0.56 | 0.18 ± 0.43 | 0.11 ± 0.21 | 0.10 ± 0.34 |
| Palmitoleic acid | 7.00 ± 0.91 | 8.59 ± 0.57 | 8.15 ± 0.35 | 7.52 ± 0.47 | 6.42 ± 0.24 |
| Oleic acid | 35.10 ± 0.81 | 35.95 ± 0.46 | 36.96 ± 0.41 | 36.38 ± 0.23 | 35.66 ± 0.74 |
| Eicosenoic acid | 0.03 ± 0.11 | 0.12 ± 0.22 | 0.06 ± 0.31 | 0.04 ± 0.11 | 0.05 ± 0.22 |
| Eicosadienoic acid | ND | 0.05 ± 0.69 | 0.15 ± 0.12 | 0.04 ± 0.11 | 0.12 ± 0.31 |
| Eicosatrienoic acid | 0.15 ± 0.17 | 0.48 ± 0.11 | 0.47 ± 0.46 | 0.34 ± 0.45 | 0.39 ± 0.22 |
| Linolenic acid | 0.37 ± 0.64 | 0.53 ± 0.13 | 0.38 ± 0.21 | 0.46 ± 0.05 | 0.70 ± 0.41 |
| Eicosapentaenoic acid | ND | 0.05 ± 0.34 | 0.03 ± 0.11 | 0.05 ± 0.08 | 0.04 ± 0.25 |
| Docosahexaenoic acid | 0.02 ± 0.14 | 0.07 ± 0.18 | 0.03 ± 0.14 | 0.03 ± 0.11 | 0.08 ± 0.16 |
| Linoleic acid | 14.68 ± 0.03 | 18.91 ± 0.51 | 16.97 ± 0.31 | 17.19 ± 0.15 | 18.74 ± 0.11 |
| Arachidonoic acid | 0.05 ± 0.23 | 0.08 ± 0.14 | 0.06 ± 0.24 | 0.09 ± 0.24 | 0.11 ± 0.31 |
| ΣSFA | 32.74 ± 0.06 | 42.52 ± 0.15 | 35.30 ± 0.21 | 37.99 ± 0.14 | 37.76 ± 0.09 |
| ΣMUFA | 42.25 ± 0.21 | 44.72 ± 0.33 | 45.35 ± 0.07 | 44.02 ± 0.09 | 42.22 ± 0.57 |
| ΣPUFA | 15.28 ± 0.34 | 20.08 ± 0.44 | 18.00 ± 0.41 | 18.20 ± 0.23 | 20.20 ± 0.33 |
| Σn - 6 | 14.73 ± 0.05 | 18.99 ± 0.18 | 16.97 ± 0.36 | 17.28 ± 0.39 | 18.85 ± 0.19 |
Saturated Fatty Acids (SFA); Mono Unsaturated Fatty Acids (MUFA); Poly Unsaturated Fatty Acids (PUFA) ); T1 - Control (basal diet); T2 - M. oleifera leaf meal 1%; T3 - M. oleifera leaf meal 1.5%; T4 - S. platensis 1% and T5 - S. platensis 1.5%. The values are means ± SEM (n=4).
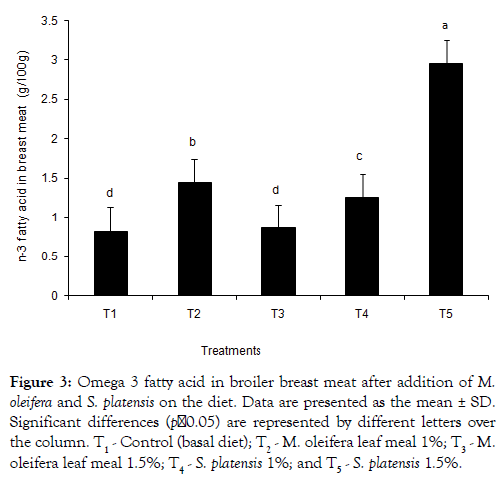
Figure 3: Omega 3 fatty acid in broiler breast meat after addition of M. oleifera and on the diet. Data are presented as the mean ± SD. Significant differences (p˂0.05) are represented by different letters over the column. T1 - Control (basal diet); T2 - M. oleifera leaf meal 1%; T3 - M. oleifera leaf meal 1.5%; T4 - 1%; and T5 - 1.5%.
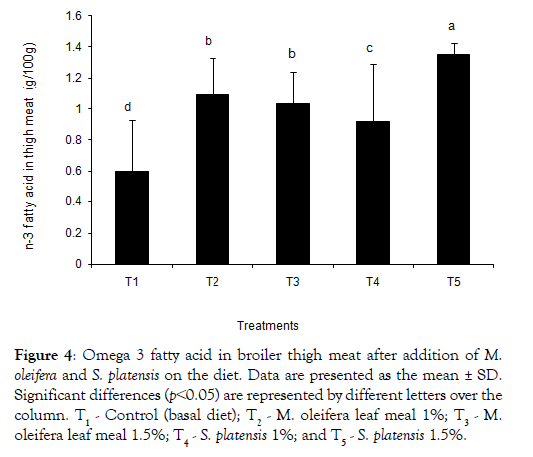
Figure 4: Omega 3 fatty acid in broiler thigh meat after addition of M. oleifera and on the diet. Data are presented as the mean ± SD. Significant differences (p˂0.05) are represented by different letters over the column. T1 - Control (basal diet); T2 - M. oleifera leaf meal 1%; T3 - M.oleifera leaf meal 1.5%; T4 - 1%; and T5 - 1.5%.
Medicinal plants contain a wide range of bioactive components and can play important role in the biological reactions through their specific mode of action. M. oleifera and S. platensis has been recognized as containing a great number of bioactive compounds [3,4,20]. The dried leaves of M. oleifera are a great source of polyphenol compounds, such as flavonoids and phenolic acids. A number of animal studies documented the effects of M. oleifera leaves in protecting against cardiovascular disease, diabetes, hypertension and others, due the actions of the bioactive components in preventing lipid accumulation, reducing insulin resistance and inflammation [9,21]. Besides M. oleifera, the benefits of S. platensis has already been evaluated through a number of studies on the growth performances and carcass percentage of broiler chickens [20,22,23]. There is paucity of scientific reports on the antioxidant properties and fatty acid profile of broiler meat after addition of M. oleifera and S. platensis as feed additives.
Commonly, BW is used to monitor animals’ nutritional status and growth [24]. Nkukwana et al. [25] described the fact that broiler ∆BW and ADG were increased in birds fed diets with M. oleifera. It is possible that depending on the amount of M. oleifera addition, the concentration of anti-nutritional factors in these diets could have been relatively low to have a growth depression effect [25].
In contrast, numerous studies have shown that S. platensis dietary addition can improve the growth performance of poultry [18,26]. In this present study, a 1.5% addition (T5) of S. platensis recorded a higher BW compared to the 1% addition (T4). It should be mentioned that a low FCR means chickens gain more weight when eating less feed. In our study significantly lowest FCR was observed in the T2 group at the finisher period in d 35 but, when considering the T5 group, there were no significant differences between the groups (p<0.05). The level of feed consumption is a basic and important factor that determines the rate of growth and body composition achieved by animals throughout their life cycles. The increase in FI as well as in weight gain could be due to the experimental diets’ reduced palatability, following the T2 andT5 group. These results are in agreement with those of previous researchers, who recorded nonsignificant effects of dietary S. platensis addition on performance parameters. Furthermore, Bellof and Alarcon [27] reported that under organic farming, dietary S. platensis supplementation (5 g/kg or 10 g/kg) significantly improved broilers’ growth and carcass performance parameters. Contradictory results are possibly due to the different inclusion levels and quality of M. oleifera and S. platensis in the present trials. In summary, it can be stated that natural additives have great potential, with the right combination and doses.
The crude protein content in feed additives group was increased and decreased crude fat content in thigh meat except M. oleifera 1.5% group in breast meat. This finding was consistent with the studies by Sarker et al. [19] and Hossain et al. [28], they reported crude protein content in breast meat was increased in the addition ofnatural herbs Alismacanaliculatum with probiotics. Abdominal fat were slightly decreased in feed additives groups compared to control. The health condition of experimental birds did not show any difference on addition of M. oleifera and S. platensis in diets. Du et al. [29] reported that dietary addition of M. oleifera and S. platensis might increase immunity of broilers.
Lipid oxidation causes the loss of nutritional and sensory values as well as the formation of potentially toxic compounds that compromise meat quality and reduce shelf life. Enhancing the antioxidant capability in muscle tends to improve meat quality and extend shelf life [7]. TBARS values of the additives groups were significantly different from the T1 group. The result of the current study is consistent with those of Aksu et al. [30]. M. oleifera leaves and S. platensis were revealed to have significantly high oxidative stabilities, indicating the presence of natural antioxidants [31] and phytochemicals. Predominantly, M. oleifera leaves contain flavonols, quercetin, and kaempferol in their 30-O-glycoside forms, which are well-known compounds with radical scavenging properties [32]. Surprisingly, in the second week, breast and thigh meat from birds fed T1 diets had found the highest TBARS value (p<0.05), about twofold higher than the first week’s TBARS value. On the other hand, S. platensis has antioxidants compounds, such as tocopherol, butylated hydroxyanisole and carotene, which possess antioxidant properties beneficial in the prevention of cancer and cardiovascular diseases [6]. These antioxidant compounds also assist in the prevention of meat degradation by oxidation. Several researchers concluded that the percentage of lipid oxidation inhibition observed in the meat from M. oleifera and S. platensis supplemented animals stimulated the defense mechanism in the animals’ systems to prevent the formation of excessive free radicals [6,33]. The results from the TBARS test provided evidence that the additives groups could improve the oxidative stability in broiler meat. These 2 substances, M. oleifera and S. platensis, may intercept and neutralize free radicals, preventing the oxidation process propagation.
Consumers are becoming more conscious of their health and are particularly interested in reducing the risk of cardiovascular and other diseases by consuming more PUFA, especially omega-3 fatty acids [34]. Due to enrichment of energy and fatty acid pattern of poultry, the composition of meat can be modified by dietary manipulation, in which fat and oil sources are important dietary ingredients [35]. Therefore, the fatty acid composition of meat products is an important parameter of meat quality. Noteworthy is the relatively high PUFA content observed in meat from birds fed M. oleifera and S. platensis added diets both breast and thigh meat. The most important omega-3 fatty acids in human nutrition are eicosapentaenoic acid (EPA) (20:5n-3), DHA (22:6n-3), and α-linolenic acid (ALA; 18:3n3), which serve as precursors for EPA and DHA synthesis [36]. However, in the present study, EPA was found to be lower than DHA in all the dietary groups. Herein, DHA was found to be higher in the T2 and T5 groups of breast meat, with values of 0.06 ± 0.18 and 0.09 ± 0.14, respectively. The addition of M. oleifera and S. platensis may have the potential to improve the fatty acids due to the phenolic compounds and organic acids because they exert the antioxidant potential and consequently prevent PUFA oxidation [34]. The antihyperlipidemic activity due to the phenolic acids of M. oleifera and S. platensis as well as medicinal plants might beattributed in the improvement of the fatty acid content of broiler meat through the lipid homeostasis and fatty acid synthase enzyme [36].
The fatty acid profile of the thigh meat was enriched in PUFA, especially eicosapentaenoic acid and docosahexaenoic acid. The elevation of PUFA in the both breast and thigh meat of additives group mainly upgradation of linolenic and linoleic acid. However, in breast meat T3 group the content of linoleic acid was found lower compared to control group. Reduction of linoleic acid content led to decreased levels of PUFA. Linoleic acid is an essential fatty acid that acts as the primary precursor of n6 PUFAs [37]. Linoleic acid in the diet can suppress lymphocyte proliferation in rats [38]; and linolenic acid can prevent cardiovascular disease [39], which all could be beneficial for human health through consumption of broiler meat. A total of n3 fatty acid content in broiler breast meat was found to be higher than thigh meat. The fatty acid composition can be different between these different muscle tissues possibly due to their different phospholipid contents [40]. The n3 PUFA of fatty acids are important to human health since they are precursors for the biosynthesis of eicosanoids, which are considered as an important bio-regulator of many cellular metabolic processes, blood pressure and clotting, tissue growth and immune system modulation [41]. The enhancement of fatty acids especially omega 3 fatty acids in thigh meat after addition of feed additives might be due to the dietary active components. Although this trend was supportive in thigh meat perfectly as dose-response relationship, which was not followed for breast meat in T3 i.e., M. oleifera 1.5% group. Actually, many factors are responsible for the mechanism of PUFA modulation and also lipid oxidation. These results provide valuable information on screening of two additives for enriching broiler meat with n3 fatty acids and reducing oxidation using M. oleifera and S. platensis.
The dietary inclusion of M. oleifera leaf 1% and S. platensis 1.5% meal in the starter and finisher diets did not alter the diets’ nutrient compositions. The growth performance and yield results indicate that T2group significantly improved the feed utilization efficiency in the broiler chickens used in this study. The current study results demonstrated that the addition of M. oleifera leaf meal and S. platensis at the levels of 1.5% of the birds’ reduced TBARS value with influenced the fatty acid composition in broiler meat. The tendency to have lower TBARS in meats from the additives groups’ birds was a clear indication that it is capable of reducing the lipid oxidation in broiler meat. Furthermore, additives groups increased the ω3 fatty acid in breast and thigh meat. Therefore, M. oleifera leaf meal and S. platensis could be promising functional ingredients in broiler chicken meat production.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. This work has been funded by the Bangladesh Livestock Research Institute (BLRI) in Savar, Dhaka, through an internal Core Project. The authors also thank the Ministry of Science and Technology for kindly providing the Bangabandhu Science and Technology Fellowship to conduct the research work as a Post-Doctoral Fellow at this institute.
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Citation: Sharmin F, Sarker NR, Sarker SK (2020) Effect of Using Moringa oleifera and Spirulina platensis as Feed Additives on Performance, Meat Composition and Oxidative Stability and Fatty Acid Profiles in Broiler Chicken. J Nutr Food Sci 10:772. doi: 10.35248/2155-9600.20.10.772.
Received: 19-Mar-2020 Accepted: 26-Apr-2020 Published: 04-May-2020 , DOI: 10.35248/2155-9600.20.10.1000772
Copyright: © 2020 Sharmin F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.