Angiology: Open Access
Open Access
ISSN: 2329-9495
ISSN: 2329-9495
Research Article - (2024)Volume 12, Issue 12
Objective: This study extensively evaluated the therapeutic effects of Dihuang Yinzi (DHYZ) decoction on Vascular Dementia (VaD), providing evidence-based support for the clinical use of Traditional Chinese Medicine (TCM).
Methods: A thorough search was carried out across several databases, including PubMed, Cochrane Library, Web of Science (WOS), China National Knowledge Infrastructure (CNKI), China Biology Medicine Disc (CBM), and the VIP Chinese Journal Database (VIP), to collect clinical randomized controlled trials investigating the use of DHYZ decoction for treating VaD. Literature screening was meticulously performed based on predefined inclusion and exclusion criteria. The quality of the included studies was evaluated using the Cochrane Collaboration's risk-of-bias tool and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system. Data synthesis and analysis were performed using Review Manager 5.4 and Stata 17 software, while GRADE Profiler 3.6 was utilized to assess the quality of evidence for the outcome indicators (risk ratio, standardized mean difference, and weighted mean difference).
Results: A total of 23 studies involving 1,829 patients met the inclusion criteria. DHYZ decoction, either as a standalone treatment or in combination with conventional Western medicine, showed greater efficacy compared to conventional Western medicine alone in improving the overall clinical effectiveness rate, TCM syndrome score, activities of daily living score, Mini-Mental State Examination score and Hasegawa Dementia Scale score in the treatment of VaD. Additionally, DHYZ decoction demonstrated a favorable safety profile.
Conclusion: Current clinical evidence indicates that DHYZ decoction is effective and safe for treating vascular dementia, supporting its integration with Western medicine in treatment. However, the quality of the research methodologies needs improvement and these conclusions should be validated through further high-quality studies.
Dihuang Yinzi decoction; Vascular dementia; Efficacy; Systematic review; Meta-analysis
VaD is a clinical syndrome characterized primarily by brain tissue damage and dementia, resulting from cerebrovascular disease [1]. Common conditions associated with VaD include hemorrhagic cerebrovascular disease, ischemic cerebrovascular disease and both acute and chronic hypoxic cerebrovascular disease [2,3].
All patients with VaD have a history of stroke, with the main clinical manifestations including dementia, confusion, cognitive impairment, aphasia, memory loss, and disability [1,4,5]. VaD is generally considered the second most common subtype of dementia after Alzheimer's disease, representing about 15%-20% of dementia cases in North America and Europe and a higher proportion in Asia and developing countries, at approximately 30%, with an increasing incidence year by year [6,7]. Similar to Alzheimer's disease, a large proportion of VaD patients are elderly. As society continues to age, the incidence of VaD has sharply increased, contributing to a rising prevalence among the global aging population. Consequently, VaD is becoming a more serious public health issue in the 21st century [8,9]. However, there is insufficient convincing evidence to support the widespread use of drugs like cholinesterase inhibitors or memantine in the treatment of VaD [1,10,11].
TCM has a long-standing history in the treatment of dementia [12,13]. The TCM prescription DHYZ decoction has been shown to be effective in treating stroke and its related sequelae, such as dementia [14,15]. DHYZ is derived from Di Huang Yin, which is selected from Sheng Ji Zong Lu (AD 1117). It contains 15 common herbs (Rehmannia, Morinda, Dogwood, Cistanche, Dendrobium, Aconitum, Schisandra, Cinnamomi, Poria, Radix, Shichangpu, Polygala, Zingiber, Jujube, and Mint). The function is to nourish kidney yin and kidney yang, Huatan Kaiqiao. DHYZ has been a traditional treatment for nervous system diseases since the Song Dynasty. Its enduring application in traditional Chinese medicine highlights its importance and the recognition of its healing properties for ailments related to the nervous system.
DHYZ reportedly improves neurological function and may enhance functional recovery due to its anti-dementia properties [16]. However, a systematic summary of the evidence on the effectiveness of DHYZ in treating VaD has not been conducted. Therefore, we undertook this systematic review to critically assess the efficacy and safety of DHYZ for this particular condition.
Search strategy
A literature search was conducted across multiple databases, including three English-language medical databases (PubMed, Cochrane Library, Web of Science) and four Chinese-language medical databases, (CNKI, China Biology Medicine disc (CBM), VIP chinese journal database (VIP), and Wanfang Database). This search encompassed articles published from the inception of each database up to 1st September, 2024. The objective was to identify clinical research literature on the use of DHYZ decoction for treating VaD. The search strategy incorporated a mix of subject headings and keywords.
The Chinese-language literature search in CNKI used the following formula: SU='地黄饮子' AND (SU='血管性痴呆' OR SU='痴呆') AND AB='随机'
The English-language literature search used the following formula:
#1: "Dementia, Vascular"[Mesh]
#2: ("Vascular Dementia"[Title/Abstract] OR "Subcortical Vascular Dementia"[Title/Abstract] OR "Arteriosclerotic Dementia"[Title/Abstract] OR "Binswanger Disease"[Title/Abstract] OR "Chronic Progressive Subcortical Encephalopathy"[Title/Abstract] OR "Subcortical Leukoencephalopathy"[Title/Abstract] OR "Subcortical Arteriosclerotic Encephalopathy"[Title/Abstract] OR "Binswanger Encephalopathy"[Title/Abstract])
#3: #1 OR #2
#4: ("Dihuang Yinzi"[Title/Abstract] OR "Dihuang Yinzi Decoction"[Title/Abstract])
#5: #3 AND #4
Inclusion and exclusion criteria: All Randomized Controlled Trials (RCTs) evaluating the treatment of VaD with DHYZ were included in the review. Additionally, the included RCTs met the diagnostic criteria established by authoritative neurological and mental health associations, were published in reputable journals, or were part of teaching materials from National Higher Medical Colleges. Articles that were duplicates, of poor quality, or lacking sufficient information were excluded, while the most recent research reports were retained. Studies that combined participants with other types of dementia or included individuals with severe primary diseases, mental illnesses, severe infections, or toxic encephalopathy were also excluded.
Data extraction and quality evaluation
Data Extraction: Two authors (Li Tingting and Lu Tong) independently evaluated the literature according to the established inclusion and exclusion criteria, extracted pertinent data and verified their findings. The process began with a review of the titles and abstracts of each article for preliminary screening. After removing studies that were clearly irrelevant, the full texts were analyzed to assess their eligibility for inclusion. In cases where there was disagreement, discussions were held with a third author (Zhan Libin) to come to a consensus about whether to include the article. An Excel spreadsheet was created to capture important details such as the authors of the studies, publication year, demographics of participants, interventions, therapeutic efficacy indicators, and any adverse reactions reported.
Quality assessment: Methodological quality was assessed according to the Cochrane Handbook using Revman 5.4 software. Studies were evaluated based on the following parameters: Random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. All test results were classified as either high risk of bias, unclear risk of bias, or low risk of bias.
Data analysis
Data analysis was conducted using Cochrane Revman 5.4 software. Dichotomous outcomes were expressed as Relative Risk (RR) with a 95% Confidence Interval (CI), while continuous outcomes were presented as Weighted Mean Difference (WMD) with a corresponding 95% CI. The I² test was used to evaluate statistical heterogeneity, with values of 50% or greater indicating significant heterogeneity. When heterogeneity was not significant (I²<50%), a fixed-effect model was used for data pooling. Otherwise, a random effects model was applied when substantial heterogeneity was present (I² ≥ 50%). Additionally, publication bias was assessed through funnel plot analysis to visually examine the distribution of the data.
Results of the literature search
A total of 196 articles were initially identified. After removing 88 duplicate records, the remaining articles were screened by reviewing their titles and abstracts, leading to the exclusion of 45 documents that did not clearly meet the inclusion criteria. This thorough screening process continued based on the predefined inclusion and exclusion criteria, leading to the final selection of relevant studies, 23 articles were ultimately included, all of which were in Chinese was showed in Table 1. The retrieval process and results are illustrated in Figure 1.
| Study ID | Sample E/C | Age(years) E/C | Sex(Male/Female) | Intervention | Therapy duration (week) | Outcome measure | ||
|---|---|---|---|---|---|---|---|---|
| E | C | E | C | |||||
| Chen L et al. [17] | 44/44 | (64.86 ± 4.54)/64.79 ± 4.42) | 25/19 | 24/20 | DHYZ+CWM | CWM | 8 | 1,3,4,6,7 |
| Li DL et al. [18] | 36/20 | Unclear | 43/13 | DHYZ | CWM | 12 | 1 | |
| Shang FK et al. [19] | 30/30 | 60-82 | 48/12 | DHYZ+CWM | CWM | 4 | 2,4 | |
| Li HL et al. [20] | 30/29 | (67.03 ± 4.28)/69.38 ± 4.74) | 20/10 | 18/11 | DHYZ+CWM | CWM | 12 | 3,4,5,7 |
| Hu CC et al. [21] | 50/50 | (67.83 ± 8.03)/66.99 ± 9.77) | 25/25 | 26/24 | DHYZ | CWM | 8 | 1,3,6 |
| Hu SX et al. [22] | 36/34 | 66.58/67.86 | 27/8 | 28/6 | DHYZ | CWM | 6 | 1 |
| Huang JH et al. [23] | 32/32 | (66.1 ± 6.62)/(67.8 ± 5.56) | Unclear | DHYZ+CWM | CWM | 8 | 1,2,3,4 | |
| Huang XF et al. [24] | 42/42 | (39.1 ± 4.15)/68.52 ± 4.65) | 22/20 | 23/19 | DHYZ+CWM | CWM | 12 | 4 |
| Jia JX et al. [25] | 38/16 | (67.1 ± 6.8)/(65.2 ± 7.1) | 28/10 | 09-Jul | DHYZ+CWM | CWM | 16 | 1 |
| Xie JH et al. [26] | 39/39 | 58.63/60.03 | 28/11 | 29-Oct | DHYZ+CWM | CWM | 6 | 1,2 |
| Li SG et al. [27] | 57/53 | (70.4 ± 5.7)/(69.5 ± 6.2) | 35/22 | 32/21 | DHYZ+CWM | CWM | 24 | 3,5 |
| Li XR et al. [28] | 36/36 | (67.45 ± 5.47)/68.54 ± 4.42) | 24/12 | 23/13 | DHYZ+CWM | CWM | 16 | 1,2 |
| Liao F et al. [29] | 59/57 | 50-85 | 21/38 | 23/34 | DHYZ | CWM | 8 | 2 |
| Lu LJ et al. [30] | 30/29 | (50-79)/50-78) | 21/12 | 22/13 | DHYZ+CWM | CWM | 12 | 4,5,7 |
| Ma YZ et al. [31] | 42/38 | (60.4 ± 13.7)/(56.9 ± 15.3) | 28/14 | 22/16 | DHYZ+CWM | CWM | 8 | 1,5,7 |
| Qin H et al. [32] | 48/48 | (67.1 ± 4.5)/67.3 ± 4.3) | 32/16 | 30/18 | DHYZ+CWM | CWM | 12 | 1,3,4 |
| Wang SF et al. [33] | 30/30 | 50-75 | 40/20 | DHYZ+CWM | CWM | 12 | 1,3,7 | |
| Sun Q et al. [34] | 42/38 | (55-80)/(56-80) | 28/14 | 25/13 | DHYZ | CWM | 4 | 1,2 |
| Wang LJ et al. [35] | 60/56 | 63.2/63.8 | 50/10 | 44/12 | DHYZ | CWM | 8 | 1 |
| Wu JX et al. [36] | 68/36 | (63.85 ± 4.29)/(64.08 ± 3.39) | 44/24 | 23/13 | DHYZ | CWM | 12 | 1,2 |
| Diao ZY et al. [37] | 49/47 | (66.1 ± 3.4)/(65.1 ± 3.7) | 23/27 | 24/26 | DHYZ+CWM | CWM | 4 | 1,3,4,5 |
| Yang C et al. [38] | 38/15 | (67.3 ± 7.6)/(65.2 ± 7.6) | 26/12 | 08/07 | DHYZ | CWM | 12 | 1,3,4,7 |
| Zhao ZL et al. [39] | 40/20 | (62-78)/(64-80) | 21/19 | 13/7 | DHYZ | CWM | 8 | 1,2,4 |
Note: E: Individual sample in the analysis; C: Sample for comparison against E; DHYZ: Dihuang Yinzi; CWM: Cerebral White Matter
Table 1: Baseline data for the included studies.
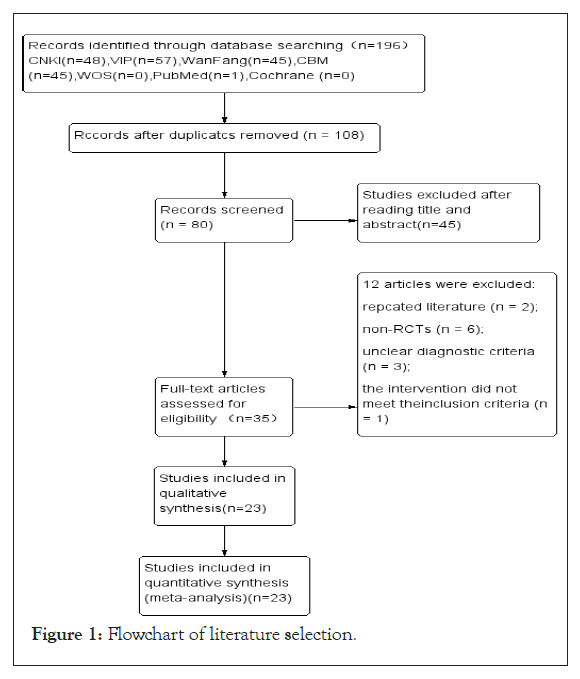
Figure 1: Flowchart of literature selection.
Fundamental characteristics of the selected literature
This study included 23 RCTs conducted in China, encompassing a total of 1,829 patients, with 980 in the experimental group and 849 in the control group. The interventions in these trials involved the administration of DHYZ decoction either alone or in combination with conventional Western medicine. Of the 23 included studies, 9 used DHYZ decoction as a standalone intervention, whereas the remaining studies investigated the combined use of DHYZ decoction and conventional Western medicine.
Evaluation of the quality of the selected literature
Of the 23 studies included, 4 studies mentioned the use of random number tables for generating allocation sequences, whereas the other 19 studies indicated that random allocation was used but did not detail the method used for sequence generation [20,21,23,30]. None of the studies provided sufficient information to determine whether allocation concealment was adequately implemented or whether the outcome assessors were blinded. Although the studies were not blinded for participants due to the nature of TCM prescriptions, the outcome measures were not influenced by the unblinded treatment in open clinical trials, resulting in a low risk of bias for all studies. The data from each study were complete and also considered to have a low risk of bias.
Two studies failed to report all the outcome measures that were initially planned, resulting in a high risk of bias for those studies [18,25]. In contrast, the other 21 studies lacked sufficient detail, making it challenging to evaluate the risk of reporting bias effectively. One study exhibited a high risk of other types of bias due to inconsistencies in baseline characteristics, while none of the other 22 studies presented additional potential sources of bias [29]. A risk of bias assessment map was created using RevMan 5.4 software, which is illustrated in Figures 2 and 3.
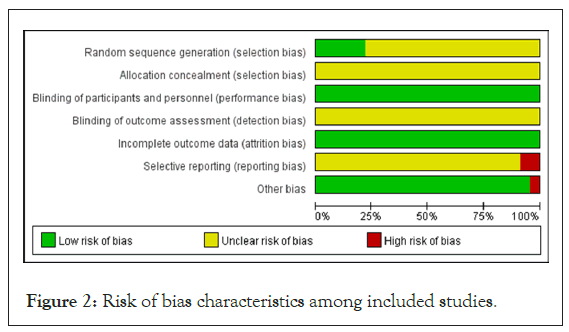
Figure 2: Risk of bias characteristics among included studies.
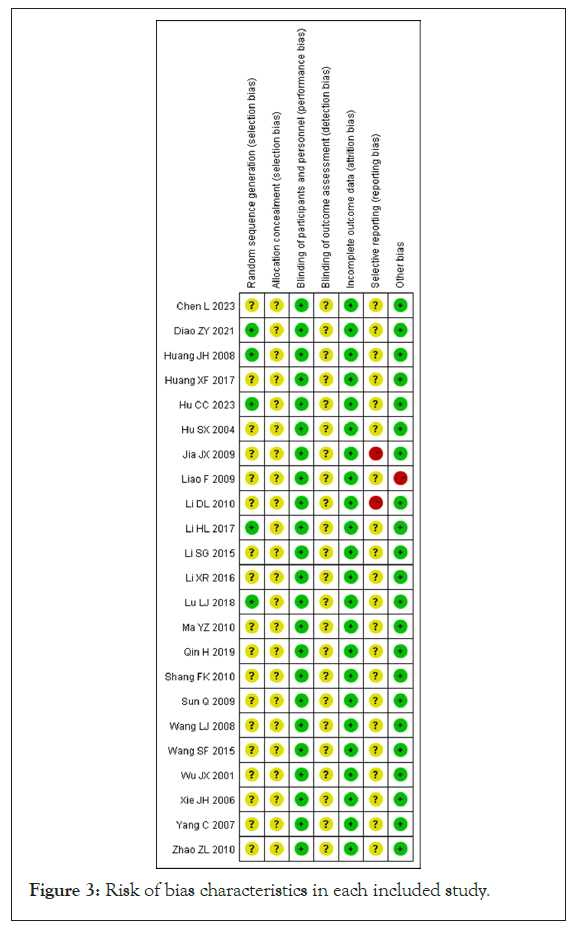
Figure 3: Risk of bias characteristics in each included study.
Meta-analysis results
Clinical effective rate: Seventeen among those included reported total clinical response rates, encompassing a sample size of 1,330 participants, with 728 patients in the experimental group and 602 in the control group. Since the data from these studies were dichotomous, RR was utilized for analysis. The heterogeneity test results indicated no significant variation between the studies (p=0.67, I²=0%), allowing for the use of a Fixed-Effect Model (FEM) to summarize the effect indicators. The meta-analysis revealed a higher total effective rate for VaD, with a statistically significant difference (RR=1.37, 95% CI [1.29, 1.46], p<0.00001), as illustrated in Figure 4.
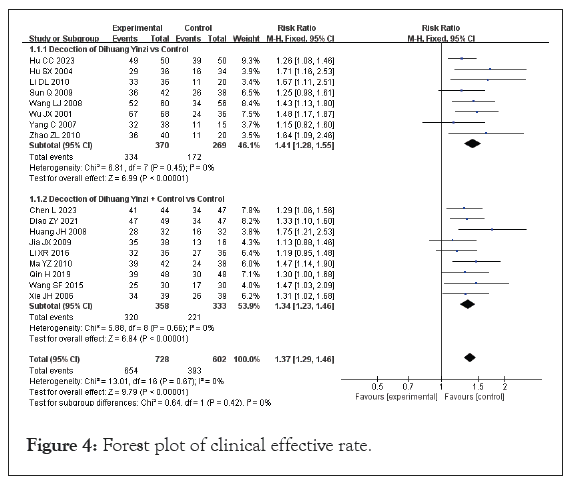
Figure 4: Forest plot of clinical effective rate.
With regard to combined use of Western medicine treatment, 17 studies were used for the subgroup analysis. Among these studies, 7 used DHYZ decoction as a standalone treatment for VaD [18,22,34-36,38]. The heterogeneity test results indicated no significant variation among the studies (P=0.45, I²=0%), which led to the selection of a FEM for analysis. The meta-analysis demonstrated a RR of 1.41, with a 95% CI ranging from 1.28 to 1.55 (p<0.00001), indicating a statistically significant improvement in outcomes associated with the use of DHYZ decoction.
Ten studies used the combination of modified DHYZ prescription and Western medicine to treat VaD, and the heterogeneity test results were P=0.66 and I²=0%, indicating homogeneity among studies within the subgroup. Therefore, FEM was selected [17,21,23,25,26,28,31-33,37]. Meta-analysis showed an RR of 1.34 and corresponding 95% CI of 1.23, 1.46 (p<0.00001). The meta-analysis revealed a RR of 1.34, with a corresponding 95% CI of 1.23 to 1.46 (p<0.00001). Subgroup analyses demonstrated that the clinical effective rate of the modified DHYZ prescription was greater than that of Western medicine alone. However, the difference between the subgroups was not statistically significant (p=0.44, I²=0%), indicating consistent outcomes across the groups. The risk of bias for 17 studies was evaluated using a funnel plot, as illustrated in Figure 5. The distribution of scatter points appeared to be approximately symmetric, suggesting that there was no significant publication bias detected among the included studies.
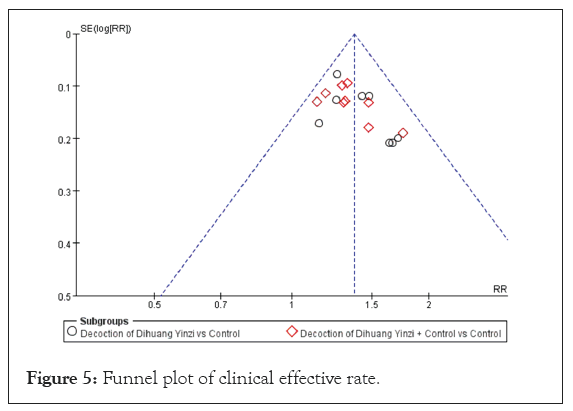
Figure 5: Funnel plot of clinical effective rate.
Activity of Daily Living (ADL) scores: Ten studies reported on ADL scores, encompassing a total sample size of 728 participants, with 386 in the experimental group and 342 in the control group. As the data consisted of continuous variables with consistent units, the Mean Difference (MD) was used for representation. The heterogeneity test revealed significant heterogeneity among the studies, with results of p<0.00001 and I²=98%, prompting the use of a Random Effects Model (REM) for analysis. The findings indicated that the modified DHYZ treatment for VaD did not result in a significant improvement in ADL scores compared to Western medicine alone. The analysis showed a notable difference in ADL scores between the two groups (MD=4.39, 95% CI [−0.47, 9.24], p=0.08) (Figure 6).
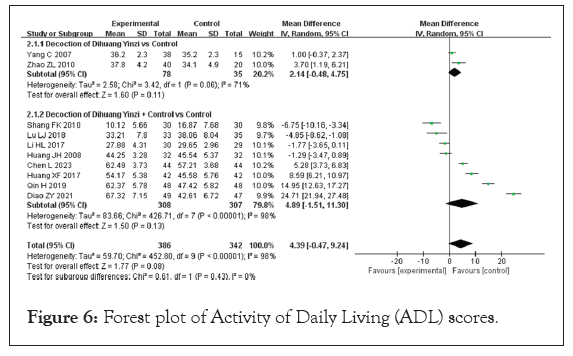
Figure 6: Forest plot of Activity of Daily Living (ADL) scores.
Subgroup analyses were performed to explore the impact of combining DHYZ decoction with conventional Western medicine, given the high heterogeneity observed. When DHYZ decoction was used alone, the improvement in ADL scores compared to the control group was not statistically significant (MD=2.14; 95% CI [−0.48, 4.75], p>0.05) [38,39]. The ADL score of the experimental group was not significantly enhanced compared with the control group after DHYZ decoction treatment combined with conventional Western medicine (MD=4.89; 95% CI [−1.51, 11.30], p>0.05) [17,19,20,23,24,30,32,37].
The findings from the subgroup analyses indicated that the inclusion or exclusion of rehmannia alone in the treatment of VaD did not lead to significant improvements in daily living abilities. Furthermore, the difference between the subgroups was not statistically significant (p=0.43, I²=0%), suggesting that the subgroups themselves were not responsible for the observed heterogeneity.
Given the significant heterogeneity among the studies, a sensitivity analysis was performed. The results indicated that while the ADL score demonstrated high sensitivity, it exhibited low robustness, warranting cautious interpretation. Additionally, funnel plots were created for the 10 studies to evaluate the risk of bias (Figure 7). The observed scatter asymmetry in the plots suggested the presence of publication bias.
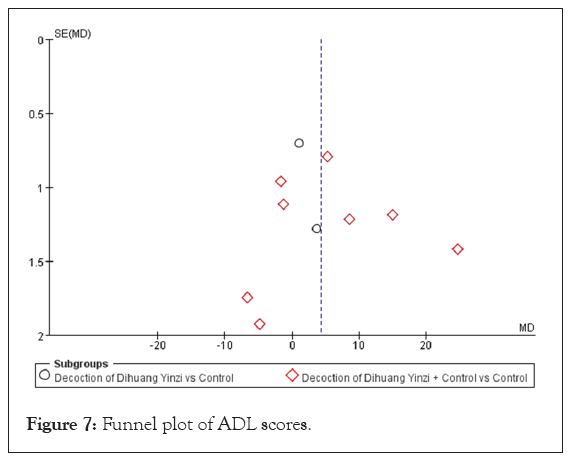
Figure 7: Funnel plot of ADL scores.
Mini-Mental State Examination (MMSE) scores: Ten studies reported MMSE scores for a total sample size of 794 cases, including 373 patients in the test group and 368 patients in the control group. Because the data of the included studies were continuous variables and the data units were consistent, the MD representation was applied. The heterogeneity test results were p<0.00001 and I²=96%, indicating large heterogeneity among studies, so the pooled summary of effect indicators was analyzed using REM. The meta-analysis showed that treatment of VaD significantly improved the MMSE score of patients (MD=4.09, 95% CI [2.22, 5.96], p<0.0001) (Figure 8).
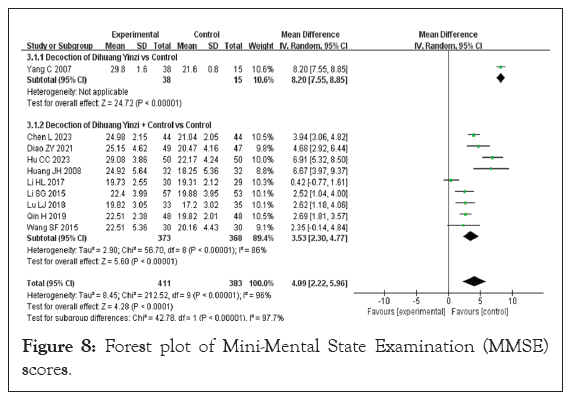
Figure 8: Forest plot of Mini-Mental State Examination (MMSE) scores.
With regard to the combination treatment test group, 7 included studies were subjected to subgroup analysis. Only one of these studies treated VaD with DHYZ decoction alone, and a meta-analysis results were MD=8.20 and 95% CI [7.55, 8.55] (p<0.00001) [38].
Nine studies used a combination of DHYZ decoction and conventional Western medicine to treat VaD. The heterogeneity test results were p<0.00001 and I²=86%, suggesting great heterogeneity among the subgroups; therefore, REM was used, and the results of the meta-analysis were MD of 3.53 and corresponding 95% CI of [2.30, 4.77] (p<0.00001) [17,20,21,23,27,30,32,33,37].
The results from the subgroup analyses demonstrated that both the administration of DHYZ alone and the integration of DHYZ decoction with conventional Western medicine led to a significant enhancement in patients' MMSE scores (p<0.00001, I²=98.4%). This finding indicates that the subgroup factor could be a contributor to the substantial heterogeneity noted among the included studies. Furthermore, the outcomes remained consistent even after conducting a sensitivity analysis, suggesting that the heterogeneity in the data had a minimal effect on the MMSE scores. To assess the risk of bias, a funnel plot was constructed for the ten studies (Figure 9), revealing scatter asymmetry that indicates potential publication bias.
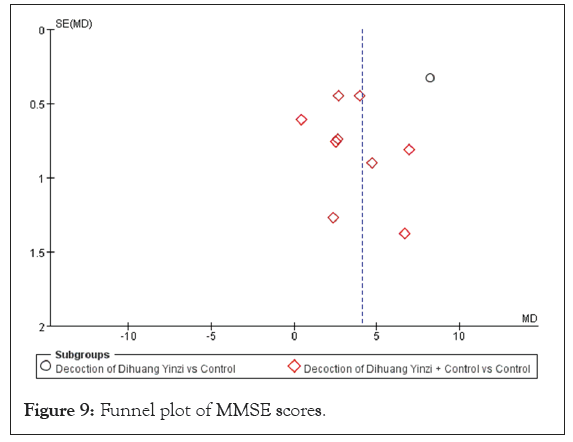
Figure 9: Funnel plot of MMSE scores.
Hasegawa Dementia Scale (HDS) score: Seven studies in the included literature reported HDS scores, with a total sample size of 570 cases, including 314 patients in the test group and 256 cases in the control group [19,26,28,29,34,36,39]. The heterogeneity test results were p=0.0001 and I²=78%, indicating large inter-study heterogeneity. Therefore, a random-effects model was used to analyze the pooled effects. Meta-analysis results demonstrated a significant difference in the HDS score between the groups (MD=3.26, 95% CI [1.87, 4.65], p<0.00001), indicating a significant effect of DHYZ decoction on HDS score (Figure 10). Four studies used DHYZ decoction alone to treat VaD [29,34,36,39]. The heterogeneity test results were p=0.002 and I²=79%, indicating large inter-study heterogeneity within this subgroup. However, there was no significant difference in the results before and after the sensitivity analysis, with an MD of 2.68 and corresponding 95% CI of [1.05, 4.30] (p=0.001). Three studies used the combination of DHYZ decoction and conventional Western medicine to treat VaD [19,26,28]. The heterogeneity test results were p=0.27 and I²=25%, indicating no inter-study heterogeneity between subgroups. In summary, the treatment of VaD with DHYZ decoction alone or DHYZ decoction combined with Western medicine significantly improved the HDS score of the patients. A funnel plot was constructed across the 7 studies to assess the risk of bias (Figure 11). The results showed scatter asymmetry, indicating publication bias.
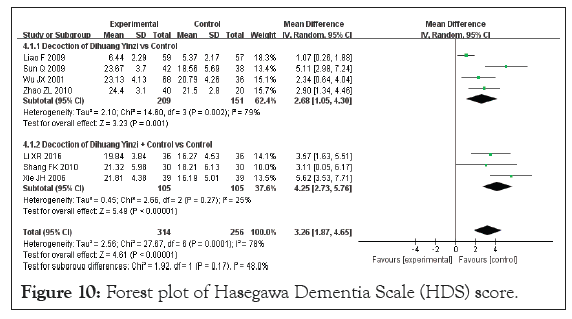
Figure 10: Forest plot of Hasegawa Dementia Scale (HDS) score.
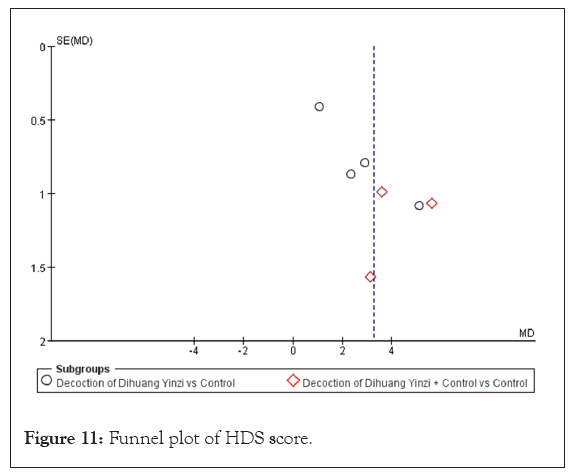
Figure 11: Funnel plot of HDS score.
TCM syndrome score: Five studies evaluated the efficacy of Traditional Chinese Medicine (TCM) syndrome, encompassing a total sample size of 381 participants, with 194 in the experimental group and 187 in the control group [20,23,27,30,31]. Given that the data from these studies were dichotomous, RR was used for analysis. The heterogeneity test results indicated p=0.02 and I²=64%, which suggests significant heterogeneity among the studies. Consequently, a REM was utilized to analyze the variations between the studies. Meta-analysis showed that treatment of VaD with DHYZ decoction was significantly better than treatment with Western medicine alone (RR=1.51, 95% CI [1.20, 1.89], p=0.0004) (Figure 12). When excluding the study by Lu, no heterogeneity between studies was observed, so FEM was used. The combined test of heterogeneity results were p=0.88, I²=0%. The results of the meta-analysis indicated an RR of 1.37 and 95% CI of [1.19, 1.56] (p<0.00001). There was no significant change in the results after the sensitivity analysis. The sensitivity analysis indicated that the results remained consistent, showing no significant changes. To evaluate the risk of bias, a funnel plot was created for the five studies. The findings revealed scatter asymmetry, suggesting the presence of publication bias (Figure 13).
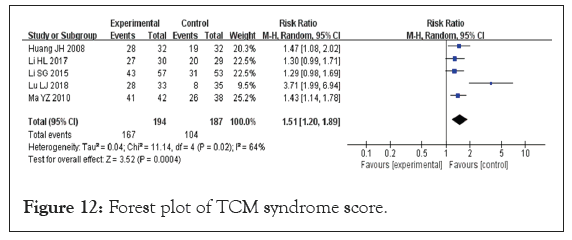
Figure 12: Forest plot of TCM syndrome score.
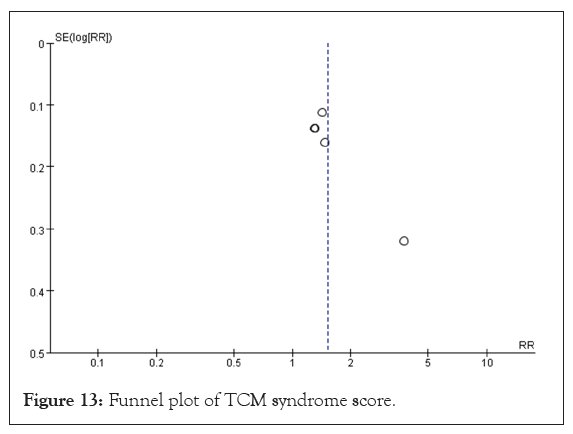
Figure 13: Funnel plot of TCM syndrome score.
Safety evaluation: Eight studies provided safety evaluations, encompassing a total of 430 participants, 230 in the experimental group and 200 in the control group [17,20,27,30,31,33,37,38]. Notably, three of these studies reported no adverse reactions in either group. However, one study highlighted a significant adverse reaction rate, with 61.2% in the experimental group and 61.7% in the control group. All studies indicated that there were no dropouts due to adverse reactions. The data from the included studies were dichotomous and presented as Relative Risk (RR). The heterogeneity test showed p=0.60 and I²=0%, indicating no heterogeneity among the studies, which allowed for the application of a FEM to summarize the effect indicators. The results revealed that treatment with DHYZ decoction did not significantly decrease the incidence of adverse reactions compared to Western medicine alone, with no statistically significant difference found (RR=0.94, 95% CI [0.68, 1.30], p=0.60) (Figure 14). A funnel plot for the eight studies (Figure 15) demonstrated that the scatter was roughly symmetrical, indicating no significant bias in the included studies [39].
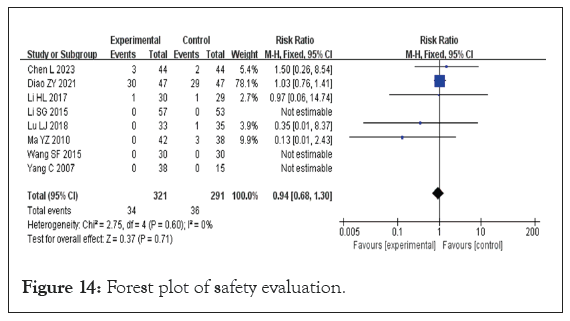
Figure 14: Forest plot of safety evaluation.
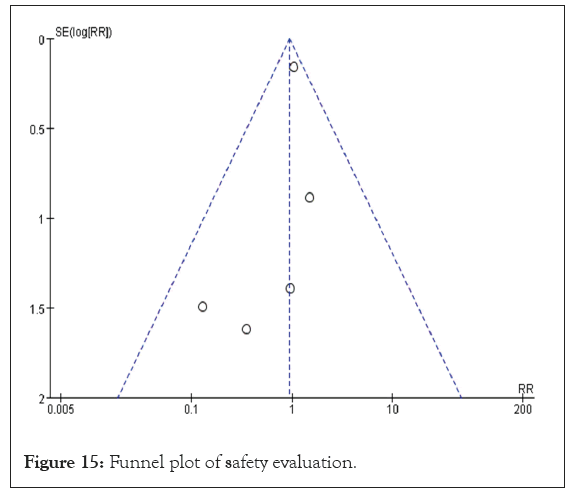
Figure 15: Funnel plot of safety evaluation.
GRADE quality of evidence assessment: We utilized GRADE Profiler 3.6 software to evaluate the quality of evidence related to the outcome indicators. All 23 articles included in this analysis were clinical RCTs conducted in China, and they were initially rated as providing the highest level of evidence for the total effective rate. Following this, the evidence underwent a thorough evaluation based on five key factors that could warrant downgrading: Risk of bias, inconsistency, indirectness, precision, and publication bias. A comprehensive assessment of the evidence quality is detailed in Supplementary Table 1.
There is no clear term for "vascular dementia" in TCM, and VaD is therefore classified into the category of "dementia" according to its clinical manifestations [40,41]. DHYZ is a TCM decoction that nourishes kidney yin, tonifies kidney yang, and resolves phlegm, aligning with the treatment principle of tonifying the kidney and resolving phlegm for dementia management. Research has indicated that DHYZ can reverse the decreased expression of extracellular signal-regulated protein kinase following ischemic brain injury, inhibit synapse loss, and promote synapse regeneration after such injuries. This action helps prevent learning and memory impairments associated with ischemic brain injury [42]. In VaD rats, DHYZ can also promote PI3K and Akt expression in the hippocampus, reduce caspase-3 expression, and inhibit apoptosis [42,43]. Despite the growing number of RCTs exploring the treatment of VaD with DHYZ, no meta-analyses have yet evaluated its clinical efficacy and safety. This study aims to assess the effectiveness and safety of DHYZ in treating VaD by analyzing the available clinical evidence.
Several studies have evaluated the efficacy of TCM in treating dementia [44,45] . TCM has a long-standing history of use in treating dementia, making the safety of these treatments a critical concern [46,47]. While several RCTs have investigated the use of DHYZ for treating VaD, no meta-analyses evaluating its clinical efficacy and safety have been published. This paper aims to assess the efficacy and safety of DHYZ for VaD treatment based on current clinical evidence.
The authors performed a systematic review and meta-analysis of 23 RCTs examining the efficacy of DHYZ decoction in treating VaD. The results revealed that DHYZ significantly outperformed conventional treatments, resulting in notable enhancements in the clinical symptoms associated with VaD. The statistically significant differences observed between the treatment groups highlight the unique benefits of DHYZ in the management of this condition.
This systematic review demonstrates that DHYZ decoction, whether administered alone or alongside conventional Western medicine, significantly improves cognitive function and daily self-care capabilities in patients with VaD. However, there are some limitations to the study. Notably, DHYZ decoction both as a standalone treatment and in conjunction with conventional therapies led to significant enhancements in overall clinical response rates, Traditional Chinese Medicine (TCM) syndrome efficacy, Hasegawa Dementia Scale (HDS), and Mini-Mental State Examination (MMSE) scores, as well as a reduction in dementia severity.
However, when comparing DHYZ decoction, whether used alone or in combination with conventional Western medicine, to the exclusive use of Western medicine, no significant differences were found in the improvement of ADL scores. Furthermore, the incidence of adverse reactions showed no significant differences between the two treatment approaches. Due to the considerable heterogeneity, high sensitivity, and low robustness of the results, further research is necessary. Thus, the findings presented in this evaluation should be interpreted with caution.
RCTs assessed the long-term efficacy and safety of total treatment for VaD, most focused on evaluating clinical symptoms and scales, which increases the risk of subjective bias. Similar to many trials involving Chinese medicine interventions, there are concerns regarding selective reporting that could affect the interpretation of the conclusions. Although all trials reported a positive effect of DHYZ, no unpublished negative reports were identified. Given that we only examined published studies, the potential for selective reporting bias may exist. To fully evaluate the safety and efficacy of DHYZ decoction, multi-center RCTs with larger samples, more-rigorous experimental designs, implementation of blind and hidden allocation schemes, and unified clinical efficacy judgment criteria will be needed to improve the methodological quality of the studies and obtain more reliable evidence on which to base conclusions.
This study has several limitations. First, all 23 selected articles were from China, introducing geographical limitations. Second, the methodological quality of the RCTs was generally low. Few studies provided detailed descriptions of their randomization procedures using random number tables, and there was limited information on the concealment and blinding of assignments. Consequently, we cannot rule out the possibility that some studies may not have been true RCTs.
This work was financially supported by Traditional Chinese Medicine Inheritance and Innovation “Hundreds and Tens of Thousands” Talent Project (Qihuang Project) (No. (2022) No. 6). Open fund of Key Laboratory of Ministry of Education for TCM Viscera-State Theory and Applications, Liaoning University of Traditional Chinese Medicine (zyzx2307).
Data are available from the corresponding author upon reasonable request.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Li TT, Lu T, Lv XD, Zhana LB (2024). Effectiveness and Safety of Dihuang Yinzi in Treatment of Vascular Dementia: A Systematic Review and Meta-Analysis. Angiol Open Access. 12:528.
Received: 11-Nov-2024, Manuscript No. AOA-24-35068; Editor assigned: 14-Nov-2024, Pre QC No. AOA-24-35068 (PQ); Reviewed: 28-Nov-2024, QC No. AOA-24-35068; Revised: 05-Dec-2024, Manuscript No. AOA-24-35068 (R); Published: 12-Dec-2024 , DOI: 10.35841/2329-9495.24.12.528
Copyright: © 2024 Li TT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.