Enzyme Engineering
Open Access
ISSN: 2329-6674
ISSN: 2329-6674
Research Article - (2013) Volume 2, Issue 2
Keywords: Biodiesel, Enzymes, Transesterification, Solvent, Oil,Alcohol molar ratios, Reaction time, Reaction temperature
The high demand for fossil fuels and their limited supply have prompted the search for alternative renewable fuels such as biodiesel, bioethanol and biogas from biomass materials [1,2]. Biodiesel is a renewable fuel that can be used in compression-ignition engines instead of petroleum diesel [3,4]. It has several qualities over diesel including: being sulfur free, non-toxic, biodegradable and non-carcinogenic [5]. These characteristics make it more greener and eco-friendly than diesel [6-10].
There are many raw materials that can be used as a feedstock for the production of biodiesel including: plant oils, animal fats, microbial mass and waste materials [7]. The most popular plants used as a feedstock are jatropha, canola, coconut, cottonseed, groundnut, karanj, olive, palm, peanut, rapeseed, safflower, soybean and sunflower [3,7,10]. The most popular animal sources used as a feedstock are beef tallow, chicken fat, lamb fat, lard, yellow grease and hemp oil [3,11-13]. The waste materials include: waste cooking oil, greasy by-product from omega-3 fatty acid production and fish waste [7,10].
The main component of fats and oils are triacylglycerols (triglycerides) which are made of different types of fatty acids with one glycerol (glycerine) being the backbone. The types of fatty acids present in the triglycerides determine the fatty acids profile. Fatty acid profiles from plants and animal sources are different and each fatty acid has its own chemical and physical properties which can be a major factor influencing the properties of biodiesel [7,14].
Transesterification is a classic chemical process used to convert the vegetable oils and animal fats to biodiesel. Usually, a short chain alcohol is used with the feedstock to convert methyl esters and glycerin. The objective is to reduce the viscosity of oil by turning it into biodiesel [15]. Transesterification can proceed with one of three catalysts: acid, alkali and enzyme. With an acid catalyst, the proton is donated to the carbonyl group which makes it more reactive. A base catalyst is used to remove the proton from alcohol which makes the reactants more reactive. Both acid and alkali methods require more energy and a downstream processing step is required for removing the by-product (glycerin) [16]. An enzymatic catalyst cleaves the backbone of the glycerol which makes the reactants more reactive, giving the product without the need for a downstream processing step. The glycerol can be extracted easily and the energy required for the process is minimal.
The main aim of present study was to optimize the enzymatic transesterification process for the production of biodiesel from animal rendering waste. The specific objectives were: (a) to study the effectiveness of the experimental enzymecatalyst NS88001 with the short chain alcohol methanol and hexane as a solvent (b) to evaluate the effects ofoil : alcohol molar ratio (1:1, 1:2, 1:3, 1:4 and 1:5), reaction temperature (35, 40, 45 and 50°C) and reaction time (4, 8, 12 and 16 h) on the biodiesel yield and (c) to determine the enzyme reusability.
Animal tallow
The waste was obtained as beef tallow that had been rendered by the Company S.F Rendering, Centreville Nova Scotia. Samples (10 Kg) were collected and stored at -20°C in the Biotechnology Laboratory of Dalhousie University. The collected material was yellowish in colour.
Chemicals and enzymes
The immobilized Lipase was an experimental enzyme catalyst (NS88001) obtained from Novozyme (Franklinton, North Carolina, USA). The chemicals used in the study included: methanol, n-hexane, tertrahydrofuran, N, O - Bis (Trimethylsilyl) - trifluroacetamide (BSTFA) and hilditch reagent. They were purchased from Sigma Aldrich (St. Louis, Missouri, USA). The FAME standards, which included methyl myristate, methyl pentadecanote, methyl cis-11-eicosenoate, methyl all-cis-5,8,11,14,17- eicosapentaenoate (EPA), methyl erucate, methyl all-cis-7,10,13,16,19-docosapentaenoate (DPA) and methyl all-cis-4,7,10,13,16,19-docosahexenoate (DHA), were purchased from Sigma Aldrich (St. Louis, Missouri, USA). The other FAME standard, which included methyl palmitate, methyl palmitoleate, methyl stearate, methyl oleate, methyl linoleate and methyl linolenate,were purchased from Alltech Associates, Inc. (Deerfield, Illinois, USA). The FAME standard methyl-stearidonate was purchased from Cayman Chemical (Ann Arbor, Michigan, USA).
Purification of crude animal tallow
The animal tallow was first heated to 105-110°C with constant stirring at 50 rpm in a 500 ml round bottom flask for one hour as shown in Figure 1. During the process of melting the fats, the top layer (consisting of bubbles and impurities) was discarded regularly. Then, the extracted crude animal tallow oil was filtered four times using vacuum filtration with ultra filter paper (Whatman No.40, Fisher Scientific, Toronto, Ontario, Canada). The oil percentage was calculated as follows
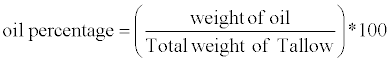 (1)
(1)
Experimental procedure
The enzymatic transesterification was carried out in order to extract fatty acid methyl esters from the animal tallow by the experimental enzyme catalyst NS88001 as shown in Figure 1. Five oil: alcohol molar ratios (1:1, 1:2, 1:3, 1:4 or 1:5), four reaction temperatures (35, 40, 45 or 50°C) and four reaction times (4, 8, 12 or 16 h) were investigated. The homogenized oil (2.3 ml corresponding to 2 g of fat) was placed into a 50 ml conical flask and heated on a hot plate (PC-620, Corning, New York, New York, USA). The immobilized experimental enzyme catalyst (NS88001) was added to the flask which corresponded to 25% of the oil weight of 0.5 g. The appropriate amount of alcohol (methanol) was added based on the selected oil:alcohol molar ratio (1:1, 1:2, 1:3, 1:4 or 1:5). The solution was mixed using a reciprocal shaking bath (2850 Series, Fisher Scientific, Toronto, Ontario, Canada) at 200 rpm. The desired temperature (35, 40, 45 or 50°C) was selected. After the desired reaction time was completed (4, 8, 12 or 16 hours), the enzyme was filtered by vacuum filtration as recommended by Nelson et al. [17]. Samples (100 μl) were taken from the mixture and analyzed using a gas chromatograph (Hewlett Packard 5890 series II, Agilent, Mississauga, Ontario, Canada).The same procedure was repeated with all oil:alcohol molar ratios, reaction temperatures and reaction times.
Determination of biodiesel conversion yield
The preparation procedure of biodiesel sample for gas chromatography analysis is shown in Figure 2. A 100 μL aliquot was taken from the transesterification process and flushed with nitrogen in a microprocessor-controlled water bath (280 series, Fisher Scientific, Toronto, Ontario, Canada) at 45°C in order to evaporate the hexane. A 10mg portion of the residue was dissolved in 100 μL of tetra hydrofuran and 200 μL of BSTFA. Then, the mixture was heated in the water bath at 90-95°C for 15 minutes. The sample was then cooled to room temperature for few minutes after which 5 mL of hexane was added. An aliquot of 1.5 ml mixture was transferred to the GC crimp vials and capped tightly for further analysis using Gas Chromatography.
A gas chromatograph, equipped with an AT-FAME capillary column 30 m in length, 0.32 mm of internal diameter and 0.25 μm film thicknesses (All tech Associates, Inc., Deerfield, Illinois, USA), was used for analyses. The column was a highly polar and stable bonded polyethylene glycol phase, coupled with flame ionization detector (FID) (HP5890 Series II, Agilent Technologies, Mississauga, Ontario, Canada). An aliquot of 10 μl of the mixture was injected directly into the column with the initial oven temperature of 60°C, followed by a flow rate of 20°C/min. A final temperature of 280°C was held for 10 minutes. The detection system was equipped with a flame ionization detector (FID) operating at 275°C with helium as a carrier gas at a flow rate of 0.6 mL/min. The total run time was 40 minutes. The yield was calculated as follows:
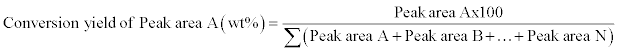 (2)
(2)
Where:
Peak area A=Methyl Oleate
Peak area B=Methyl Palmitate
Peak area N=no. of unknown peaks
Statistical analyses
Statistical analyses were performed on biodiesel results using Minitab Statistics Software (Ver 16.2.2, Minitab Inc., State College, Pennsylvania, USA). Both analyses of variance (ANOVA) and Tukey’s grouping were carried out.
Characterization of animal tallow
Table 1 shows the composition of the animal tallow used in this study. The filtration process removed about 7.5% of the total weight of tallow as impurities present in the animal fats. The homogenized oil was characterized by gas chromatography to identify and quantify the fatty acid composition of the tallow. Five fatty acids were identified in the animal tallow: oleic acids (44%), palmitic acids (28%), stearic acids (26%), linoleic acids (1%), and myristic acids (1%).
| Parameters | Value |
| Impurities (Kg) | 0.375 |
| Oil (%) | 92.5 |
| Impurities (%) | 7.5 |
| Fatty acids (wt%) | |
| Oleic acid | 44 |
| Palmitic acid | 28 |
| Stearic acid | 26 |
| Linoleic acid | 1 |
| Myristic acid | 1 |
| Tallow =5 kg |
Table 1: Composition of animal tallow.
Enzymatic Transesterification
Enzymatic transesterification using the experimental catalyst (NS88001) was first carried out to investigate the effects of reaction time (4, 8, 12 and 16 h), oil: alcohol molar ratios (1:1, 1:2, 1:3, 1:4 and 1:5) and reaction temperature (35, 40, 45 and 50°C) on biodiesel yield in solvent system (hexane). The results in Tables 2 and 3 shows the analysis of variance performed on the yield data. The effect of molar ratio, reaction time and reaction temperature were highly significant at the 0.001 level. All interactions between the parameters were also highly significant at the 0.001 level.
| Time (h) | Oil: Alcohol Molar Ratios | Reaction Temperature (°C) | ||
| 40 | 45 | 50 | ||
| 4 | 1:1 | 42.87 ± 0.86 | 58.90 ± 1.18 | 55.20 ± 1.10 |
| 1:2 | 46.51 ± 0.93 | 73.85 ± 1.48 | 63.10 ± 1.26 | |
| 1:3 | 58.39 ± 1.17 | 77.89 ± 1.56 | 69.28 ± 1.39 | |
| 1:4 | 78.07 ± 1.56 | 84.35 ± 1.69 | 73.24 ± 1.46 | |
| 1:5 | 51.31 ± 1.03 | 69.20 ± 1.38 | 46.73 ± 0.93 | |
| 8 | 1:1 | 47.29 ± 0.95 | 63.67 ± 1.27 | 60.90 ± 1.22 |
| 1:2 | 74.71 ± 1.49 | 79.85 ± 1.60 | 64.90 ± 1.30 | |
| 1:3 | 85.81 ± 1.72 | 88.94 ± 1.78 | 77.63 ± 1.55 | |
| 1:4 | 81.02 ± 1.62 | 85.62 ± 1.71 | 78.42 ± 1.57 | |
| 1:5 | 70.41 ± 1.41 | 79.56 ± 1.59 | 54.98 ± 1.10 | |
| 12 | 1:1 | 60.09 ± 1.20 | 78.72 ± 1.57 | 65.91 ± 1.32 |
| 1:2 | 74.98 ± 1.50 | 84.69 ± 1.69 | 71.92 ± 1.44 | |
| 1:3 | 85.93 ± 1.72 | 92.11 ± 1.84 | 82.25 ± 1.64 | |
| 1:4 | 82.82 ± 1.66 | 92.86 ± 1.86 | 79.87 ± 1.60 | |
| 1:5 | 72.94 ± 1.46 | 87.81 ± 1.76 | 69.13 ± 1.38 | |
| 16 | 1:1 | 66.82 ± 1.34 | 78.97 ± 1.58 | 70.03 ± 1.40 |
| 1:2 | 79.97 ± 1.60 | 92.56 ± 1.85 | 72.85 ± 1.46 | |
| 1:3 | 88.23 ± 1.76 | 94.43 ± 1.89 | 92.97 ± 1.86 | |
| 1:4 | 88.26 ± 1.77 | 95.75 ± 1.92 | 91.24 ± 1.82 | |
| 1:5 | 75.10 ± 1.50 | 88.04 ± 1.76 | 77.95 ± 1.56 | |
Table 2: Biodiesel yield (wt %) from animal tallow using 0.5 grams of experimental catalyst (NS88001) with methanol as alcohol and hexane as solvent at different reaction times, oil : alcohol molar ratios and reaction temperatures.
| Source | DF | SS | MS | F | P |
| Total | 179 | 31506.66 | |||
| Model | |||||
| MR | 4 | 11831.37 | 2957.84 | 1567.58 | 0.000 |
| RTI | 3 | 10275.81 | 3425.27 | 1815.30 | 0.000 |
| RTE | 2 | 5409.97 | 2704.98 | 1433.57 | 0.000 |
| MR*RTI | 12 | 895.27 | 74.61 | 39.54 | 0.000 |
| MR*RTE | 8 | 1083.51 | 135.44 | 71.78 | 0.000 |
| RTI*RTE | 6 | 616.79 | 102.80 | 54.48 | 0.000 |
| MR*RTI*RTE | 24 | 1167.52 | 48.65 | 25.78 | 0.000 |
| Error | 120 | 226.43 | 1.89 |
MR = Molar ratios; RTI = Reaction time; RTE = Reaction temperature; DF: Degree of freedom; SS: Sum of square; MS: Mean of square; R2 : 99.28%; CV : 8.65%
Table 3: ANOVA of biodiesel yield.
The results obtained from Tukey’s Grouping (Table 4) indicated that the five levels of oil: alcohol molar ratio (1:1, 1:2, 1:3, 1:4 and 1:5) were significantly different from one another at the 0.05 level. The highest mean yield of 84.29% was obtained with the 1:4, oil: alcohol molar ratio. The four reaction times (4, 8, 12 and 16 h) were significantly different from one another at the 0.05 level. The highest mean yield of 83.54% was achieved with 16 h reaction time. The reaction temperatures 40 and 50°C were not significantly different from each other but were significantly different from the reaction temperature 45°C at the 0.05 level. The highest mean yield of 82.39% was obtained at the reaction temperature 45°C.
| Factors | Level | N | MeanBiodiesel yield (%) | Tukey Grouping |
| Oil : Alcohol Molar Ratios | 1:1 | 36 | 62.49 | A |
| 1:2 | 36 | 73.32 | B | |
| 1:3 | 36 | 82.82 | C | |
| 1:4 | 36 | 84.29 | D | |
| 1:5 | 36 | 70.26 | E | |
| Reaction Time (hours) | 4 | 45 | 63.29 | A |
| 8 | 45 | 72.91 | B | |
| 12 | 45 | 78.80 | C | |
| 16 | 45 | 83.54 | D | |
| Reaction Temperature (°C) | 40 | 60 | 70.58 | A |
| 45 | 60 | 82.39 | B | |
| 50 | 60 | 70.95 | A |
Groups with the same letter are not significantly different from each other at the 0.05 level.
Table 4: Tukey's Grouping of the various parameters.
Effect of oil: alcohol molar ratio
Figure 3 shows the effect of oil: alcohol molar ratio on the biodiesel conversion yield using the experimental catalyst (NS88001) at different reaction temperatures and reaction times. Generally, there was an increase in the biodiesel conversion yield when the oil: alcohol molar ratio was increased from 1:1 to 1:4 which was followed by a decreases with a further increase in the oil: alcohol ratio from 1:4 to 1:5 for all reaction time (4, 8, 12 and 16 h) and reaction temperatures (40, 45 and 50°C).
The biodiesel conversion yield in the solvent system at the 4 h increased from 42.87 to 78.07% (82.13%), from 58.9 to 84.35% (43.20%) and from 55.20 to 73.24% (31.49%) when the oil : alcohol molar ratio was increased from 1:1 to 1:4 for the reaction temperatures of 40, 45 and 50°C, respectively. A further increase in the oil: alcohol molar ratio from 1:4 to 1:5 decreased the biodiesel conversion yield from 78.07 to 51.31% (34.27%), from 84.35 to 69.2% (17.96%) and from 73.24 to 46.73% (36.19%) for the reaction temperatures of 40, 45 and 50°C, respectively. Similar trends were observed with the 8, 12 and 16 h reaction times at all reaction temperatures (40, 45 and 50°).
Effect of reaction time
Figure 4 shows the effect of reaction time on the biodiesel conversion yield using the experimental catalyst (NS88001) at different reaction temperatures and oil: alcohol molar ratios. Generally, there was an initial rapid increase in the biodiesel conversion yield with increases in reaction time during the first 4 hours which was then followed by a gradual increase till the end of the experiment (16 h) for all reaction temperatures (40, 45 and 50°C) and oil : alcohol molar ratios (1:1, 1:2, 1:3, 1:4 and 1:5).
The biodiesel conversion yield in a solvent system at the 40°C reached 42.87%, 46.51%, 58.3%, 78.07% and 51.31% for the oil: alcohol molar ratios of 1:1, 1:2, 1:3, 1:4 and 1:5, respectively. Further increases in the reaction time from 4 h to 16 h, increased the biodiesel conversion yield from 42.87 to 66.82% (55.86%), from 46.51 to 79.97% (71.94%), from 58.39 to 88.23% (51.10%), from 78.07 to 88.26% (13.05%) and from 51.31 to 75.10% (46.36%) for the oil : alcohol molar ratios of 1:1, 1:2, 1:3, 1:4 and 1:5, respectively. Similar trends were observed at the 45 and 50°C reaction temperature and with the oil: alcohol molar ratios (1:1, 1:2, 1:3, 1:4 and 1:5).
Effect of reaction temperature
Figure 5 shows the effect of reaction temperature on the biodiesel conversion yield using the experimental catalyst (NS88001) at different reaction time sand oil: alcohol molar ratios. There was an increase in biodiesel conversion yield when the reaction temperature was increased from 40 to 45°C which was then followed by a decrease when the reaction temperature was further increase from 45 to 50°C for all reaction times (4, 8, 12 and 16 h) and oil:alcohol molar ratios (1:1, 1:2, 1:3, 1:4 and 1:5).
The conversion yield of biodiesel at the 4 h reaction time increased from 42.87 to 58.90% (37.39%), from 46.51 to 73.85% (58.78%), from 58.39 to 77.89% (33.39%), from 78.07 to 84.35% (8.04%), from 51.31 to 69.20% (34.86%) when the reaction temperature was increased from 40 to 45°C for the oil:alcohol molar ratios of 1:1, 1:2, 1:3, 1:4 and 1:5, respectively. A further increase in reaction temperature from 45 to 50°C decreased the biodiesel conversion yield from 58.90 to 55.20% (5.43%), from 73.85 to 63.10% (14.55%), from 77.89 to 69.28% (11.05%), from 84.35 to 73.24% (13.17%), from 69.20 to 46.73% (32.47%) for the oil:alcohol molar ratios of 1:1, 1:2, 1:3, 1:4 and 1:5, respectively. Similar trends were observed with the 8, 12 and 16 h reaction times at all oil: alcohol molar ratios (1:1, 1:2, 1:3, 1:4 and 1:5).
Enzyme reusability
The effect of the number of enzyme cycles on the biodiesel conversion yield using NS88001with methanol and hexane at the optimum conditions (45°C reaction temperature, 1:3 oil:alcohol molar ratio and 8 h reaction time) is shown in Figure 6. There was a gradual decrease in conversion yield by the enzyme catalyst NS88001 with increases in the number of cycles beyond 10 cycles. When the number of cycles was increased to 10, the biodiesel conversion yield slightly decreased from 86.12 to 85.6% (0.6%). A further increase in the number of cycles from 10 to 50 decreased the biodiesel conversion yield gradually from 85.6 to 15.2% (82.24%).
Extraction profiles of the raw material
After melting and homogenizing the animal tallow, the impurities (7.5%) were removed by filtration. The fatty acids analysis by Hilditch procedure indicated that the homogenized oil contained high percentages of oleic acid (44%), palmitic acid (28%) and stearic acid (26%) as well as lower percentages of myristic acid (1%) and linoleic acid (1%). A high concentration of oleic acid improves the characteristics of biodiesel resulting in a high cetane index and combustion temperature [9]. Biodiesel produced from feedstocks containing a high level of oleic acid showed similar characteristics to these of conventional diesel [4,9]. Therefore, the biodiesel produced for oil extracted from animal tallow is expected to have good characteristics as a biofuel.
The extracted oil can be transformed to biodiesel by chemical or enzymatic transesterification. Watanabe et al. [18], Dorado et al. [19] and Kulkarni and Dalai [20] reported that oxidized oil can inhibit the chemical transesterification process and increase the oxidation of methyl esters. Kulkarni and Dalai [20] stated that an increase in the oxidation of methyl esters might increase the cetane number which tends to delay the ignition time in the engine. Nelson et al. [17] and Watanabe et al. [18] reported that oxidation in crude tallow or oil containing high free fatty acids is a common problem and no negative effects of the oxidized oil substrate on the transesterification process by enzyme was observed. Watanabe et al. [18] stated that in the enzymatic process, the oxidized substrate becomes a non recognition site for the enzyme to bind and the process continues with the substrates which are not oxidized. However, the authors stated that using oxidized oil might reduce the biodiesel stability. Nelson et al. [17] reported that the stability of biodiesel can be increased by blending the biodiesel with conventional diesel especially in cold environment.
In this study, enzymatic transesterification was carried out and no oxidation stability test was performed on crude tallow oil nor was antioxidants used. A high biodiesel conversion yield of 95.75% was achieved using the experimental enzyme catalyst NS88001 at 25% enzyme concentration. Nelson et al. [17] reported biodiesel conversion yield of (94.5%) using immobilized Mucor miehei with tallow, ethanol and 25% of enzyme concentration. Kumari et al. [21] reported biodiesel conversion yield of (96%) using immobilized Pseudomonas cepacia with mahua oil, ethanol and 25% of enzyme concentration.
Effect of oil: alcohol molar ratios
Increasing in the oil: alcohol molar ratio from 1:1 to 1:4 at the 4 h reaction time increased the biodiesel conversion yield by 82.13, 43.20 and 31.49% and when the oil: alcohol molar ratio was further increased from 1:4 to 1:5 at the 4 h reaction time, the biodiesel conversion yield decreased by 34.27, 17.96 and 36.19% at the reaction temperatures of 40, 45 and 50°C, respectively.
Kumari et al. [22] reported that the biodiesel conversion yield increased when the oil: alcohol molar ratio was increased upto 1:4 and then decreased when the oil:alcohol molar ratio was further increased to 1:5. Chen et al. [23] reported that increasing the oil : alcohol molar ratio from 1:1 to 1:4 promoted the methanolysis reaction with waste cooking oil, but the formation of methyl esters decreased when the oil : alcohol molar ratios was increased from 1:4 to 1:5 due to an excess of methanol in the system.
The decrease in conversion yield of methyl esters from oil substrate at higher oil: alcohol molar ratios might be due to the presence of insoluble methanol in the reaction system. Tamalampudi et al. [24] suggested that this would cause the active site on the surface of the lipase to be locked resulting in less access of Novozyme 435 to the surface of oil substrate. Dizge and Keskinler [25] also reported that the use of excessive amount of methanol (short-chain alcohol) might deactivate the lipase in the reaction. Chen et al. [23] suggested that the excess methanol distort the essential water layer needed to stabilize the structure of the enzyme.
In this study, the increase of the oil:alcohol molar ratio from 1:4 to 1:5 deactivated the lipase catalyst and resulted in low conversion yield. It is likely that once the maximum level of esters was formed, a further increase in number of moles of alcohol decreased the formation of methyl esters in the reaction due to enzyme inactivation [17,23-27].
Short chain alcohols such as methanol are responsible for deactivation and inhibition of immobilized lipase [28,29]. Deactivation of enzyme likely occurred by the insoluble alcohol present in the reaction due to its tendency to be absorbed by the surface support matrix [30,31]. Several studies suggested that the theoretical 1:3 stoichiometric oil : alcohol molar ratio is needed to complete the reaction in the following continuous steps (a) the conversion of triglycerides to diglycerides, (b) the conversion of diglycerides to monoglycerides and (c) the conversion of monoglycerides to methyl esters and glycerol [11,32,33].
However, in this study the optimum level was achieved at 1:4, oil:alcohol molar ratio in a solvent system using NS88001. From the results obtained from this study, increases in the oil: alcohol molar ratio from 1:3 to 1:4 at 4 h with solvent increased the conversion yield of biodiesel for NS88001 by 82.13, 43.20 and 31.49% at the reaction temperatures of 40, 45 and 50°C, respectively. The lipase catalyst (NS88001) used in this study, showed different activity due to their mass transfer, use of alcohol and solvent system. Based on the stoichiometeric reaction, the use of an amount of alcohol equal to the number of fatty acids residues was sufficient to complete the conversion reactions.
Effect of reaction time
In this study, at the initial phase of the reaction, the enzymes, oil and alcohol appeared to be static and the reaction started when the stirring speed reached 200 rpm which promoted the initial mixing and increased the mass transfer between substrate and enzyme catalyst. Formation of esters increased with increases in reaction time from 1 to 4h.When the reaction time was increased from 4 to 16 h at the 40°C reaction temperature, the biodiesel conversion yield was increased by 55.86, 71.94, 51.10, 13.05 and 46.36% for the oil: alcohol molar ratios of 1:1, 1:2, 1:3, 1:4 and 1:5, respectively. The maximum conversion yield of 95.75% was obtained by NS88001 with hexane and methanol at the 16 h. [18,23,34] observed similar trends from crude tallow, waste cooking oil and vegetable oil. Freedman et al. [32], Ma et al. [35], Leung and Guo [36], Peter et al. [37] and Eevera et al. [38] reported that the rate of conversion of fatty acid esters increased with increases in reaction time as the reaction proceeds rapidly due to the initial mixing and dispersion of alcohol into the oil substrate and the activation of enzyme. Chen et al. [23] reported that after alcohol was dispersed, it rapidly interacted with fatty acids giving a maximum conversion yield. However, a further increase in the reaction time decreased the conversion yield due to the backward reaction of transesterification.
Nelson et al. [17] reported a maximum biodiesel conversion yield of 83.8% at 16 h reaction time with 1:3 oil: alcohol molar ratio using 25% concentration of the enzyme Candida antarctica (SP 435) with hexane and 2-butanol alcohol in the system. Chen [23] achieved a maximum biodiesel conversion yield of 85.12% after 30 h with 1:4 oil:alcohol molar ratio using 30% concentration of the immobilized enzyme Rhizopus oryzae and waste cooking oil as substrate. Modi et al. [34] reported a maximum biodiesel conversion yield of 93.4% after 8 h with 1:4 oil:alcohol molar ratio using the enzyme Candida antarctica (Novozyme 435) with vegetable oil. The biodiesel conversion yield obtained in this was slightly higher than those reported in the literature and were achieved in a shorter time.
Effect of reaction temperature
In this study, when the reaction temperature was increased from 40 to 45°C with 4 h reaction time, the biodiesel conversion yield increased by 37.39, 58.78, 33.39, 8.04 and 34.86% and when the reaction temperature was further increased from 45 to 50°C with the 4 h reaction time, the biodiesel conversion yield decreased by 5.43, 14.55, 11.05, 13.17 and 32.47% for the 1:1, 1:2, 1:3, 1:4 and 1:5 oil : alcohol molar ratios, respectively. Chen et al. [23], Dizge and Keskinler [25], Rodrigues et al. [39] and Nie et al. [40] observed similar trends from waste cooking oil, canola oil, vegetable oil and salad oil.
Chen et al. [23] reported that the biodiesel conversion yield increased (reaching a maximum of 87%) when the reaction temperature was increased from 30 to 40°C and then decreased when the reaction temperature was further increased from 40 to 70°C during conversion of waste cooking oil to methyl esters using Lipozyme RM IM. Dizge and Keskinler [25] reported that the biodiesel conversion yield increased (reaching a maximum of 85.8%) when the reaction temperature was increased from 30 to 40°C and then decreased when the reaction temperature was further increased from 40 to 70°C when converting canola oil to methyl esters using Lipozyme TL. Rodrigues et al. [39] reported a maximum biodiesel conversion yield of 53% at 35°C which then decreased with increases in reaction temperature above 35°C during the conversion of soybean oil to methyl esters using Novozyme 435. Nie et al. [40] reported a maximum biodiesel conversion yield of 90% at 40°C which then decreased when increasing the reaction temperature above 40°C. In this study, higher conversion yield were obtained at 45°C which was higher than those reported in the literature.
However, increasing the reaction temperature reduces the viscosity of the oil and enhances the mass transfer between the substrate and enzyme catalyst which results in higher conversion yield of biodiesel can be obtained. Kumari et al. [22] and Antczak et al. [13] reported that the interactions between enzyme polymer surface and substrate appears to be dependent on reaction temperature due to hydrogen bonding and ionic interactions which play important roles in maintaining the thermostability of lipase in the system. However, a higher temperature may denature the specific structure of enzymes which results in a decrease in the methyl esters formation. Denaturation of enzyme support matrix may also promote the enzyme to leak from the outer layer of the support matrix [40]. However, the optimum reaction temperature is dependent on other parameters such as oil: alcohol molar ratio, enzyme activity, stability and type of system used.
Use of solvent
In this study, a maximum biodiesel conversion yield of 95.75% was obtained by the NS88001 in the solvent system using hexane at 45°C and 1:4, oil: alcohol molar ratio, respectively. Mittelbach [41], Soumanou and Bornscheuer [42] and Kumari et al. [21] reported similar biodiesel conversion yields with solvent system.
Xu et al. [43] and Shimada et al. [44] reported that the decreases in biodiesel conversion yield in solvent-free system using immobilized Lipozyme TL at oil: alcohol molar ratio of 1:1 was due to the inactivation of lipase in the presence of insoluble methanol in reaction. Antczak et al. [13] reported that the use of organic non polar solvents for transesterification process might help to reduce the viscosity of the oil substrate and increase the mass transfer between the enzyme and the substrate. The authors suggested that by using n-hexane in the reaction might help to stabilize the enzyme in the reaction in spite of toxicity of alcohol.
Nie et al. [40] reported that a maximum conversion yield of biodiesel of 96% was observed when with using Candida sp 99-125 with salad oil and n-hexane (non-polar solvent) as solvent in the system and when acetone (polar solvent) was used the yield decreased to (40%). They indicated that the organic non-polar solvents with a log P value (value obtained by octanol/ water experiments to determine the non polarity of a solvent) greater than 2 are considered to be suitable in the transesterification reaction due to their hydrophobic property so that water cannot be stripped from the enzyme and the spatial conformation of the active site of the enzyme is maintained. The authors suggested that n-hexane (log P=3.5) can preserve the catalytic reaction, thus increasing the biodiesel conversion yield. Lu et al. [45] suggested that the n-hexane increased the biodiesel conversion yield with less water residue in the reaction and the non-polar solvent which promotes the usage of short chained alcohols like methanol (a polar alcohol).
Enzyme reusability
In this study, the activity of experimental enzyme catalyst NS88001 in the presence of methanol and hexane at the optimum conditions (a reaction temperature of 45°C, an oil: alcohol molar ratio of 1:3 and a reaction time of 8 h) remind relatively constant for 10 cycles and the decreased gradually reaching zero after 50 cycles. Several researchers [42,46,47] stated that repeated use of enzyme in the reaction without removing glycerol from the system might inhibit the interaction between the substrate and lipase. Ghamgui et al. [47], Xu et al. [48] and Bernardes et al. [26] obtained similar results from immobilized Lipozyme Thermomyces lanuginosus, immobilized Lipozyme Rhizomucor miehei and immobilized Rhizopus oryzae. Xu et al. [43] reported that while using methyl acetate as acyl acceptor, no glycerol was produced in the reaction with no loss of enzyme activity for 10 cycles in the reaction. The byproduct from the reaction was triacetylglycerol instead of glycerol which did not affect the product quality.
The effectiveness of enzymatic transesterification of animal fat using the experimental enzyme catalyst NS88001 was studied. The effects of oil: alcohol molar ratio (1:1, 1:2, 1:3, 1:4 and 1:5), reaction temperature (35, 40, 45 and 50°C) and reaction time (4, 8, 12 and 16 h) on the biodiesel conversion yield were evaluated. n-Hexane was used as a solvent. The highest conversion yield of biodiesel was obtained at the 1:4 oil:alcohol molar ratio, 16 h reaction time and 45°C reaction temperature. The rate of conversion of fatty acid esters increased with increases in reaction time. The reaction proceeds slowly at the beginning due to the initial mixing and dispersion of alcohol into the oil and activation of enzyme. After dispersion of alcohol, the enzyme rapidly interacted with fatty acids esters. Increasing the reaction time from 4 to 16 h increased the biodiesel conversion yield by 13.05-71.94%, depending on the reaction temperature and oil: alcohol molar ratio. Increasing the oil: alcohol molar ratio from 1:1 to 1:4 increased the biodiesel conversion yield by 32.68-82.11% while increasing the oil: alcohol molar ratio from 1:4 to 1:5 decreased the biodiesel conversion yield by 5.43-34.27%, depending on the reaction time and reaction temperature. The increase in the oil: alcohol molar ratio promotes the reaction between the enzyme and substrate. The interactions between enzyme polymer surface and substrate appears to be dependent on reaction temperature due to hydrogen bonding and ionic interactions which play a important roles in maintaining the thermostability of lipase in the system. Increasing the reaction temperature from 40 to 45°C increased the biodiesel conversion yield by 3.64-58.78%. Higher temperature from 45 to 50°C denatured the specific structure of enzymes and resulted in a decrease in biodiesel conversion yield by 4.3-32.47%. Using n-hexane in the reaction helped to stabilize the enzyme and minimize the toxicity of alcohol. The activity of experimental enzyme catalyst (NS88001) in the presence n-hexane was slightly reduced after 10 cycles. However, the enzyme activity decreased rapidly when the number of cycles was increased above 10 and stopped completely after 50 cycles.
The research was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada.