Maternal and Pediatric Nutrition
Open Access
ISSN: 2472-1182
ISSN: 2472-1182
Research Article - (2021)Volume 6, Issue 2
Objective: Post marketing study to determine the effect of 6 months consumption of nutritional supplement on growth and development of children aged 2-12 years.
Background: Young children in India suffer from some of the highest levels of stunting, underweight, and wasting observed in any country in the world. The levels of over-nutrition are also on a rise. Prevention of child malnutrition require diets providing adequate energy and essential nutrients to promote catch-up growth, strengthen resistance to infection, and support normal mental, physical and metabolic development.
Methods: This is an observational randomized controlled arm study, where anoral nutritional supplement is given to 776 children, aged 2-12 years, for 6 months along with normal diet. Anthropometric parameters (height, weight and BMI) are assessed at baseline, 3 and 6 months. The z score for height, weight and BMI is used to analyse the results using Khadilkar growth chart 2009.
Results: A total of 707 subjects are included in the analysis. Each child is grouped according to age bracket (2-3yrs; 4-6yrs; 7-9yrs; 10-12yrs). After consumption of nutritional supplement for 6 months, z-scores for height, weight and BMI shown improvement in almost all age groups, compared to baseline. The improvement is significant in weight and BMI z-score. The standard deviation scores from expected increase in mean of weight, height and BMI is well within the permissible range. No adverse event is observed.
Conclusion: This study showed that 6 months intake of nutritional supplement by children provided a significant improvement in anthropometric parameters, with no adverse event.
Nutritional supplements; Body mass index; Weight; Children; 2-12 years
Key message: A proper nutrient supplementation is required for growing children and various guidelines from American and European bodies support. This study adds the Indian perspective of food supplement to this group of children.
India, a developing country, faces several health challenges in terms of maternal and fetal growth, nutritional inequalities as well as disease management. Around 27% of Indian population (26% in urban areas and 28% in rural areas) has been determined to be below the poverty line, defined as the expenditure needed to obtain, on an average, 2400 Kcal per capita per day in the rural areas and 2100 Kcal in urban areas. Although the country has witnessed some great economic growth rate since few decades, poverty and under nutrition continue to top the chart of the issues that need immediate attention [1]. Poverty and nutritional problems often coexist and results in poor child growth, increased micronutrient deficiencies, increased susceptibility to diseases and hampered physical and mental development. Long term malnutrition in early age also leads to stunting, wasting, mortality and morbidity. India is home to 31% and 42% of the world's children who are stunted and underweight respectively, while many others are affected by micronutrient deficiencies [2].
The Indian Academy of Pediatrics (IAP) recommends use of IAP growth for monitoring height and weight and determining the need of intervention as appropriate [3]. The Academy of Nutrition and Dietetics (the Academy) and the American Society for Parenteral and Enteral Nutrition (ASPEN), recommend use of standardized set of diagnostic indicators to identify and document pediatric malnutrition/under nutrition in routine clinical practice. The recommended indicators include z scores for weight-for-height/ length, body mass index-for-age, or length/height-for-age or midupper arm circumference when a single data point is available [4]. Such a standardize approach would pave a way for establishing recommendations as well as determining the interventions needed for correction of nutritional deficiencies.
Nutritional supplements have been recognized as the most suitable method of improving growth and physical health of children in developing countries, they are also crucial for early development including cognition [5]. A meta-analysis including 29814 children from 20 developing countries suggested that nutritional supplementation could improve children’s cognitive development (d 0.08, 95% CI 0.03-0.13). Fortified food is also a suitable public health approach to increasing vitamin intakes [6]. Based on the available evidences, with an objective to provide adequate energy and essential nutrients and promote catch-up growth, strengthen resistance to infection, support normal mental, physical and metabolic development, we determined the effect of 6 months consumption of nutritional supplement on growth and development of children aged 2-12 years.
This was a post marketing study, to assess the effectiveness and safety of an oral nutritional supplement in children aged 2 to 12 years. The study was conducted according to the ethical guidelines. All approvals were obtained before start of the study.
All children were between 2-12 years and had regular eating habits. Data from the children were grouped based on their age group as 2-3 years, 4-6 years, 7-9 years and 10-12 years. Children with concomitant systemic infection or clinically significant diseases were excluded from the study. Children with stomach infection, infestations and suspected liver disorders were also not included in the study. In addition, children who were previously on a nutritional supplement, or have been taking multivitamins, calcium supplements in addition to the diet had to undergo a period of washout before enrolling in the study.
The Oral Nutritional Supplement (ONS) was given to children for 6 months along with normal diet and they were asked to follow-up at regular intervals.ONS in this study (Groviva®) is a signature child nutrition supplement with 38 key nutrients- including Certi5TMdual protein, dietary fiber, DHA, probiotics and calcium which has been formulated for Indian children as per ICMR guidelines. Anthropometric parameters (height, weight and Body Mass Index [BMI]) were assessed at baseline, 3 and 6 months. The z-score for height, weight and BMI was used to analyze the results using Khadilkar growth chart 2009. Safety was assessed by monitoring of adverse events.The p value <0.05 was considered statistically significant.
Patient and public involvement
• The research question was designed by the set of KOLs, and it was decided to collect the data retrospectively from their clinics/Institutions, etc.
• We collected the already existed patient data for analysis
• No patients were recruited for study, data was collected from the database
• Data was analysed retrospectively
• Patients advisors are acknowledged
The data obtained from studies conducted at 145clinics across 116 cities in India from 1stMarch 2018 to 30th October 2018. A total of 776 children were enrolled and received the nutritional supplement (Figure 1). The compliance observed was 100% with zero dropouts. The demographics and baseline characteristics are presented in Table 1. The median age was 5.2±0.4 years (range 02- 12) and the gender distribution include451 males and 256females. None of the children enrolled had wasting (z score ≥ -2), however the children in the 10-12 years age group showed stunted growth (z score ≥-2); however most of them were underweight (z score ≤ -1.88).
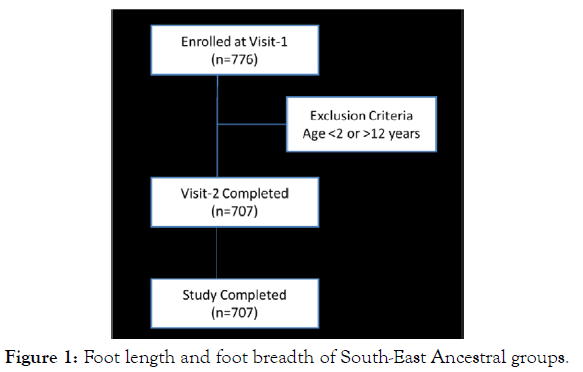
Figure 1: Foot length and foot breadth of South-East Ancestral groups.
| Variables | All Children (N=707) |
|---|---|
| Male | 451 |
| Female | 256 |
| Height (cm) | 100.3 |
| Weight (kg) | 14.9 |
| BMI* | 14.5 |
Table 1: Baseline demographics.
After consumption of nutritional supplement, changes were observed as early as 3 months. The changes in height, weight and BMI at both 3 and 6 months are presented in Table 2. A substantial increase in 3 parameters, among all age groups was observed at both 3 months and 6 months due to consumption of ONS. In the children aged 2-3 years, a statistically significant (p<0.05) weight increase from 11.6 to 12.9 and 13.8 kg was observed from baseline to 3 months and 6 months respectively, which also led to increase in corresponding BMI values. In age group of 8-10 years at both 3 months and 6 months significant increase (p<0.05) in weight with corresponding BMI was observed. Similar increase in age group 11- 12 years was seen at 6 months consumption.
| AGE | Parameter | Baseline | 3 months | 6 months |
|---|---|---|---|---|
| 2-3 yrs | Height (cm) | 89.8 | 91.2 | 92 |
| Weight (kg) | 11.6 | 12.9 | 13.8 | |
| BMI | 14.4 | 16.1 | 18.1 | |
| 4-6 yrs | Height (cm) | 104.4 | 105.7 | 107.6 |
| Weight (kg) | 160 | 16.6 | 17.9 | |
| BMI | 14.6 | 14.9 | 15.5 | |
| 7-9 yrs | Height (cm) | 119.7 | 121.4 | 121.8 |
| Weight (kg) | 20.8 | 22 | 23.4 | |
| BMI | 14.5 | 14.9 | 28.9 | |
| 10-12 yrs | Height (cm) | 131.9 | 133 | 135.6 |
| Weight (kg) | 27.9 | 29.1 | 30.6 | |
| BMI | 15.9 | 16.3 | 16.4 |
Table 2: Change in height, weight & BMI from baseline to month 3 and 6 months.
The data was also presented in form of Z-score. In the children aged 2-3 years, the z scores for height, weight and BMI improved from -0.89, -1.01 and -0.42 respectively at baseline to -0.46, -0.33 and -0.06 at Month 3. Similar results were noted for age group 4-6 (Baseline: height, -1.67, weight, -1.32, BMI, -0.32 to Month 3: height, -1.73, weight, -0.87, BMI, -0.05); age 7-9 years (Baseline: height, -1.67, weight, -1.43, BMI, -0.65 to Month 3: height, -1.46, weight, -1.11, BMI, -0.43) and age 10-12 years (Baseline: height, -2.16, weight, -1.87, BMI, -0.99 to Month 3: height, -1.91, weight, -1.6, BMI, -0.83).After consumption of nutritional supplement for 6 months, substantial improvements were observed in z-scores for height, weight and BMI compared to baseline in almost all age groups (Figure 2). The improvements were significant in weight, height, and BMI z-score. The standard deviation scores from expected increase in mean of weight, height and BMI were well within the permissible range. No adverse events were observed.
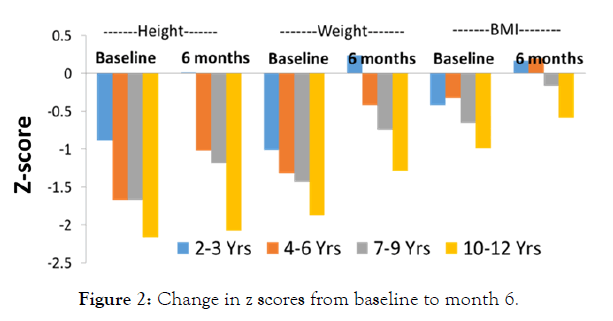
Figure 2: Change in z scores from baseline to month 6.
Long-term malnutrition is a concern not only to the individual but also the nation as it leads diet related disorders, improper growth, increased morbidity, reduced work capacity as well as untimely death in few cases. This results in increased healthcare loss, great economic burden and is detrimental to the development of a country like India. The National Family Health Survey 4 (NFHS-4) showed that at birth 31.9%children present with signs of wasting, and 17.7% under five years of age still continue to have the problem. Moreover around <1% children in almost all the States suffer from both severe wasting and severe stunting, increasing the risk of mortality in them [7]. Micronutrient deficiencies are also common in India, and high prevalence of anemia (14–88%) and low dietary iron intakes (30–50%) of the Recommended Dietary Allowance (RDA) has been observed in school children. Furthermore, around 44–66% of children even from affluent backgrounds suffer from vitamin A, B2, B6, B12, and C deficiencies [8]. To combat this challenge, proper nutrition is a mandate.
In the present study keeping in view the nutritional needs of growing children, the nutritional supplement provided was scientifically formulated with 38 key nutrients to support cognitive function, natural immunity, normal growth and development, gut health, bone health and decrease fatigue in children between 2-12 years. The changes in height, weight and BMI were visible as early as 3 months and substantial after 6 months of use. This is in line with the reports of the European Food Safety Authority (EFSA) which noted that young child formulae and supplement consumption was the shortest way to cover the EFSA nutrient requirements of U.K. children [9]. Previous studies reported positive association between specific nutrient intake such as protein, DHA, dietary fibers or calcium with linear growth and development, suggesting that intake of certain nutrients may be specifically important to promote growth [10]. Nutritional supplements not only aid in improving the health status of normal children but are also useful for children with Attention-Deficit Hyperactivity Disorder (ADHD) [11], autism [12] etc.
The results of our trial support the results of the study which determined the 1-year effectiveness and safety of nutritional supplementation with the study formula on linear growth and weight gain in short and lean prepubertal children. Throughout the entire year height continued to improve, with a total gain of 0.19 ±0.14 SD. The study concluded that 1 year of a nutritional supplement was effective in promoting the linear growth of short and lean prepubertal children, with no change in body mass index status [13].
Some limitations of this current 6 month study include absence of a comparator, restricting the enrolled children to healthy individuals and limited assessment parameters. The present study however paves a way for further recognizing the nutritional needs of children in India and also identifying suitable interventions that can be recommended to correct malnutrition/under nutrition. The Government should also play a crucial role in eradicating nutrition related problems by providing easy access to the supplements. Further studies are required to assess the clinical effectively and safety of such nutritional supplements in a larger group of population with or without disease conditions.
In6 months of observational study, young children of age 2-12 year supplemented with ONS as nutritional supplement to their diet, led to significant increase in height, weight and BMI. No adverse effects were observed across the study.
No conflicts of interest to disclose.
This study was funded by Signutra Inc, New Delhi.
Citation: Chetan M, Bhasin JS, Khadilkar V, Kochar IPS, Pai U, Mittal G, et al. (2021) Effectiveness of Nutritional Supplement in Growth and Development of Children aged 2-12 years. Matern Pediatr Nutr 6:126.
Received: 10-Dec-2020 Accepted: 15-Apr-2021 Published: 22-Apr-2021 , DOI: 10.35248/2472-1182.21.6.126
Copyright: © 2021 Chetan M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : Signutra Inc, New Delhi.