Entomology, Ornithology & Herpetology: Current Research
Open Access
ISSN: 2161-0983
ISSN: 2161-0983
Research Article - (2014) Volume 3, Issue 1
Mold control is one of the most vital issues in insect rearing systems because mold outbreaks can alter the nutritional value of diets, harm insects, and even threaten the health of insectary workers. Because antifungal agents are widely used in insect diets their potential harmful effects on target insects’ quality is a major concern. This concern stems from the observations reported in several publications that high levels of antifungal agents in diets affect growth, development, survival, and fecundity. These observations and many unpublished observations on several insect species reared in our laboratory led us to study the mechanisms underlying those deleterious effects, using two representative lepidopterans, a butterfly and a moth, Vanessa cardui and Heliothis virescens larvae reared on different concentrations of three widely used antifungal agents: methyl paraben, potassium sorbate and sodium propionate. These antifungal agents were administered in concentrations of 1,000, 5,000, and 10,000 parts per million (ppm). The results show that the highest levels of antifungal agents suppress nutrient absorption (ECI) and increase metabolic costs (ECD). Relative consumption rates (RCR) and digestibility (AD) increased with increasing antifungal agent concentration, possibly to compensate for the declines in absorption and metabolism. Also, to determine the potential for antifungal agents’ effects on diet acceptance, we experimented with feeding responses to the three concentrations used in these experiments, showing a decreases of acceptance of diets with high concentration of methyl paraben and slight increases in acceptance of high concentrations of potassium sorbate. Finally, because pH of diet is intimately related to effectiveness of antifungal agents, we included experiments on pH and diet acceptance, but within the narrow range of diet pH values tested, there were no preferences displayed.
<Keywords: Heliothis virescens; Microbial colonization; Sorbates; Approximate digestibility
Insect rearing is important for research, mass production for programs in biological control, sterile insect techniques, host plant resistance, production of insects as food for other organism, and even for production of recombinant proteins (reviewed in 1). However, microbial contamination in artificial diet is one of the most serious problems in insect rearing systems. Aspergillus niger, Aspergillus flavus, Cladosporium spp., Fusarium spp., Rhizopusnigricans, Penicillium spp. and some yeasts and bacteria are among the most commonly found microorganisms in insect diets [1,2].
Microbial growth can adversely affect insects by altering the nutritional value of diet [2], preventing insect feeding [3], and generating toxins, such as ochratoxin A produced by Aspergillus and Penicillium [4], and even present health hazards to insectary workers [2]. Decreasing microbial colonization and increasing the time that insects can feed on uncontaminated diet have significant benefit for enhancing insect health, reducing the cost of diet ingredients and the labor involved with diet preparation [5].
Antifungal agents are commonly used to protect artificial diets from fungal contamination. For example, Funke [6] listed some commonly used antifungal agents, including methyl paraben, sorbic acid, formaldehyde, sodium benzoate, butyl paraben, and potassium sorbate, considering both the effectiveness on suppressing mold and their tolerance in target insects. A review of literature on diets by Cohen [1] drew a similar conclusion, that the predominant antifungal agents were sorbate compounds and methyl paraben. Both the acid form and salt form of some agents are used in diets; for example, benzoic acid is also used in the forms sodium benzoate and potassium benzoate, and sorbic acid is also used in the forms potassium sorbate and sodium sorbate [1]. Some antifungal agents are not only artificial diet additives, but can present in insect natural food.
Some chemicals, such as potassium sorbate and propionic acid are effective against both fungi and bacteria [5,7], and formalin is effective against these contaminants and nuclear-polyhedrosis virus in cabbage looper [8]. The efficiency of antifungal agents is influenced by several factors, especially concentration and pH. At pH 5.0, the effective concentrations of several antifungal agents vary dramatically: methyl paraben (1,000 ppm), propyl paraben (300 ppm), benzoic acid (2,000 ppm), sorbic acid (800 ppm), and propionic acid (800 ppm) [6]. Antifungal agents are usually more effective in microbial growth suppression when the pH is low, and the effective concentrations increase with higher pH values [1].
Antifungal agents with varied modes of action have been used in insect diets. Sorbates inhibit spore germination and catalase in molds, and they inhibit glucose and other nutrient transfer into fungal cells [9]. Propionates inhibit enzymes involved in glucose metabolism [10]. Antifungal agents with different functional mechanisms are often used together in diets to achieve better control [11]. Kishaba et al. [12] studied 7 antifungal agents and demonstrated that no agent was effective enough against mold when used singly, but the proper combination of antifungal agent suppressed mold far more effectively than individual ones—through a combination of different modes of action. Also, because the factors that inhibit mold growth may also affect insect metabolism, understanding the modes of action can help us select antifungal agents that will not adversely affect target insects.
It is well-documented that high levels of antimicrobial agents can be toxic to insects [13]. Methyl paraben and formalin significantly decreased fecundity of Lygushesperus females, while benzoic acid, propionic acid, and sorbic acid reduce biological fitness of Lygushesperus [14]. Antimicrobial agents can reduce size, the severity of size reduction correlating with concentrations [15]. Because effects of antimicrobial agents can differ from one species to another [1], the effects of antifungal agents on different insects should be studied on a per-case basis.
Nutritional ecology indices have been applied to understand many facets of insect feeding biology: nutritional quality, effects of toxins, microbial interactions, and plant secondary compounds [1]. These indices have been the subject of hundreds of studies of insects, with the works of Waldbauer [16] and Gordon [17] laying the foundation for numerous other studies. Slansky [18] demonstrated the utility of nutritional indices to study host plant resistance, leading to dozens of subsequent studies that focused on feeding ecology measurements through use of efficiency indices. Cohen and Patana [19] used nutritional indices for a comparison between artificial diet and natural food, and demonstrated that even a Heliothiszea lab colony with hundreds of generations reared on artificial diet can return to utilization of a natural food. Various nutritional indices have been used to treat quantitatively the nature and mechanisms of insects in response to their foods. Such studies include consumption index (CI), relative consumption rate (RCR), relative growth rate (RGR), approximate digestibility (AD), conversion of ingested food (ECI), and conversion of digested food into biomass (ECD) [16,20], with some nomenclature modifications made by Woodring et al. [21]. Some common nutritional indices are explained below.
Approximate digestibility (AD) is a parameter which measures the efficiency of digestion of a given diet. Previous studies demonstrated that AD was reduced by feeding on a nutritionally poor diet. For example, younger instars often have a higher AD because they tend to ingest less indigestible crude fibers [22,23]. Low food digestibility can result from inhibition of essential hydrolytic enzymes [17]. Some environmental factors as well as gender and age are also correlated with AD.
Efficiency of conversion of ingested food to biomass (ECI) is a quantitative measurement of the utilization of food the insect ingested, and will rise and fall with AD and ECD. The ECI can reflect several important parameters in insects’ nutritional ecology, including antimetabolites and digestive organization (such as peritrophic complexes, extra-oral digestion, or digestive system structural peculiarities—all discussed by Cohen 1). The use of ECI can, for example, help to detect the presence of anti-metabolites which block nutrients and suppress absorption [17]. Peristalsis and the peritrophic complex also play important roles in digestive organization and nutrient absorption [1,24]. The ECI can be calculated without the weight of frass, thus the ECI provides a good parameter when the measurement of frass is not feasible, such as with subterranean insects [25] or liquid feeders [1].
Efficiency of conversion of digested food to biomass (ECD) is sometimes known as the metabolic efficiency; it measures how well the target insect has incorporated digested food into its own tissues. Metabolic rates, which include oxygen consumption, carbon dioxide production, and turnover of fuel molecules in general, are directly related to food intake; highest metabolic rates occur with maximal feeding and most rapid growth [21]. Thus, nutritional deficiency or the presence of antimetabolites can reduce metabolic efficiency [17]. Also, total metabolic efficiency can be lowered by increasing metabolic cost, for example, detoxification, secretion, enzyme production and other metabolically expensive metabolic processes.
Despite the clear advantage of using nutritional indices to understand the mechanisms and dynamics of insect/food interactions, relatively little attention has been paid to studies of the trophic effects of antifungal agents. Most work on antifungal agents has focused on developing and modifying diet formulas, their effects on growth and fitness [13-15], or their effectiveness against mold [26]. More research is needed to investigate the mechanism underlying the negative effects antifungal agents have on insect physiology and behavior. For example, if an antifungal agent is demonstrated to promote food intake, or have less negative effects on digestion, absorption and metabolism, then it could be considered a favorable choice for artificial diets. Several authors [1,16,17] discussed the utility of nutritional indices for comparing nutritional quality between diets; likewise, nutritional indices can also be used to compare effects of various antifungal agents on insect performance. Even if two antifungal additives yield insects of the same quality, the one with which insects have higher utilization of diet may be preferred in mass rearing. However, decisions need to be made on a case by case basis, and comparative research among different antifungal agents and insect species is necessary to facilitate this decision making.
Previous experience with Lepidoptera larvae in our rearing processes has resulted in anecdotal observations of lower growth and frass production in rearing trials where antifungal agents were at higher concentrations. In this study, we used nutritional indices to test the hypotheses that the effects of increasing levels of antifungal agents was 1) a decrease in feeding rate, 2) a decrease in digestive or absorptive efficiency, or 3) a decrease in metabolic efficiency of conversion of food to biomass. The two Lepidoptera used in this study (Vanessa cardui and Heliothisvirescens) were ideal subjects because they 1) are easily reared on artificial diets on artificial diets, 2) their relatively large size eases their handling (allowing accurate measurements of food consumption, frass production and growth rates, and 3) the two species represented two divergent taxa within the Lepidoptera thus allowing comparisons to be made of responses of disparate members of this order. The objective of this study was to determine if the effects of high levels of antifungal agents on V. cardui and H. virescens) are due to reduction in (1) feeding, (2) digestive efficiency, (3) amounts of absorption of nutrients, (4) metabolic capabilities or (5) a combination of effects. The answers to these questions and the testing of the ensuing hypotheses will provide better insights into the mechanisms of antifungal agent impact on insects at a physiological or metabolic level. To give us a perspective or context on how pH (within a limited range of choices) and the antifungal agents affect feeding behavior we supplemented the nutritional index studies with behavioral choice tests. Knowing the relationship between antifungal agents and insect nutritional ecology can provide us better insight into the interactions between antimicrobial agents and the insects that they are being used to protect from mold growth. Such understanding will help us develop artificial diets more efficiently. For example, by studying a certain behavioral or physical process (feeding, digestion, assimilation or metabolism), comparison among different antifungal agents can be made without rearing the insects for multiple generations. It would also help researchers to be aware of the potential influences by antifungal agents in their study, for example, to avoid diets that contain digestion-suppressing antifungal agents while studying the digestion of a certain species.
Insects
Vanessa cardui larvae used in this study were originally obtained from Carolina Biological Supply Company (Burlington, North Carolina), and were reared on our YC diet (Cohen modified Yamamoto Diet, unpublished observations) for three generations previous to the experiments. Heliothisvirescens used in the study were YDK strain [27] obtained from a colony kept at the insectary at Department of Entomology, North Carolina State University. This colony was originated from Yadkin County, North Carolina in 1988. The H. virescens are maintained on CSB (corn soy blend) diet [28]. Specimens from this colony were demonstrated to grow on YC diet, demonstrating the feasibility of using this diet for all our experiments. Because there was difficulty in preventing fungal growth in the rearing conditions used in the pilot studies unless the base mixture of 1,500 ppm of each antifungal, it was decided that all further diet trials would be done with a starting (base) YC Diet that contained 1,500 ppm of each of the three antifungal agents [29,30].
Insect rearing
V. cardui were reared on YC Diet (Table 1) for three generations, with a colony size of 30 to 40 individuals. The YC Diet was developed by Dr. Allen Cohen as an offshoot of the Yamamoto Diet [31]. Butterflies were fed artificial nectar (water 65 g, sugar 30 g, honey 5 g, potassium sorbate 0.1 g). Leaves from potted mallow plants (Malva sp., Item # 144042 Carolina Biological Supply Company, Burlington, NC) were used as oviposition sites for the adults. Eggs were washed from oviposition substrates and sanitized with 5% bleach solution (5 ml sodium hypochlorite in 95 ml tap water), then hatched and reared communally in 8-cell trays (Product # SMRT8 Bio-Serv, Frenchtown, NJ) filled with YC diet (approximately 50 eggs and 70 g diet per cell). The trays were tipped 45° to allow frass to drop from diet, to keep the diet clean and minimize mold outbreaks on the substrate. Third or early fourth instar larvae were transferred to new trays with density being lowered to 2 to 3 individuals per cell, and fresh food was provided. Rearing and experiments for both of the two Lepidoptera species were conducted in a rearing room at 28 ± 1°C, 40-60% RH with a 14:10 L: D. Two HEPA (High-efficiency particulate air) filtration systems (Honeywell, Minneapolis, MN) were placed near to each heater, to remove air-borne mold spores, facilitate air circulation and disperse heat uniformly in the rearing room.
| Ingredient | Weight (g) |
|---|---|
| Wheat germ | 52 |
| Soy protein | 23.5 |
| Sucrose | 21 |
| Torula yeast | 16.8 |
| Rice (preparation) | 8.4 |
| Cannellini beans | 8.4 |
| Vanderzant Vitamins | 3.4 |
| Wesson salts | 3.4 |
| L-ascorbic acid | 3.4 |
| Cholesterol | 1.7 |
| Agar | 16.6 |
| Methyl paraben* | 1.6 (or 0.1, 0.5, 1.0) |
| Potassium sorbate* | 1.6 (or 0.1, 0.5, 1.0) |
| Sodium propionate* | 1.6 (or 0.1, 0.5, 1.0) |
| Water | 837.0 |
Table 1: The ingredients of YC diet. Dry mixture was triturated in a roller mill. and agar/antifungal agents solution were made separately and blended with a variable speed hand mixer.
Experimental design
Feeding experiment: A randomized complete block design with 9 treatments was used for V. cardui. Treatments were three antifungal agents tested at three concentrations each. Six replications were performed, and blocks consisted of replication in time. Caterpillars of V. cardui were fed with YC Diet with a mixture of 3 antifungal agents in it: methyl paraben, potassium sorbate and sodium propionate. The standard level of each antifungal agent was 1,500 ppm. The concentration of only one of the three anti-fungal agents was varied in each treatment, thus there were 3 groups: methyl paraben group (i.e. the concentration of methyl paraben was changed from 1,000 to 10,000 ppm while the other two agents were 1,500 ppm), potassium sorbate group, and sodium propionate group. Three levels of each chemical, 1,000, 5,000, and 10,000 ppm, were tested.
Each replication contained 3 groups with 3 treatments in each group, with 10 samples for each treatment. For both species one extra trial was done before the experiment to determine the interval to pupation. Experiments were run for one day shorter than the time required for pupation to maximize larval growth while avoiding pupation, to ensure that all the larvae were feeding for the same period. This measure was taken because of the observations of Waldbauer [16] and Cohen and Patana [19] that significant error was introduced if non-feeding periods were included in calculations of feeding, growth, and frass production. Because it took longer than 2 weeks for V. cardui to grow from neonate to wandering stage, V. cardui were tested from early 3rd instars, and reared individually in 2 ounce dessert cups (Item # SOLB200 Ace Mart Restaurant Supply, Texas). Each cup had ~8 g diet, and lids perforated with a #2 insect pin to enhance air exchange during larvae growth and freeze drying.
The diets and treatments for H. virescens were the same as for V. cardui. Heliothis virescens neonates were reared individually in 1 ounce dessert cups (Item # SOLP100 Ace Mart Restaurant Supply, Texas) fitted with plastic lids. Each cup was had 4-5 g of YC Diet. Holes were not perforated until freeze drying to escape of neonates. During the wandering stage, H. virescens larvae will dig and mix the diet with frass to make a pupal chamber (Zha, personal observation). It is not feasible to separate diet from frass in these samples, so the cups were set upside down to prevent larvae from making pupal chambers in diet. The 30 cups of each group were fitted into a 30-well (5×6 wells) cup tray (Product # 9040 Bio-Serv, Frenchtown, NJ) in the order of treatments (from 1,000 to 10,000 ppm), and then the trays were stacked on each other randomly and set upside down in the rearing room. The cups and their entire contents (uneaten diet, larvae, and frass) were frozen at -20°C after 11 days. The experiment was originally designed as a randomized complete block design as for V. cardui. Although trays were stacked randomly, cups on all trays were arranged in the same manner so that the 5,000 ppm treatment was in the middle of each tray, rendering these experiments as a stratified randomized block design. It should be noted that whatever non-random conditions might have been in effect, the greatest differences in all outcomes were between the 1,000 and 10,000 ppm treatments.
Cups contaminated with visible microbial growth were discarded. Dead individuals were discarded and mortality was not recorded. Frozen samples were freeze-dried, and the dry weights of larvae, uneaten diet and fecal materials were recorded. Original dry weights of the diets presented to larvae were estimated by multiplying wet weights by the percentage of dry material in the diet.
Choice test on different concentrations of antifungal agents
To determine whether neonates can make choices among different concentrations of antifungal agents five concentrations (0, 1,000, 1,500, 5,000, 10,000 ppm) of each antifungal agent (methyl paraben, potassium sorbate and sodium propionate) were tested in multiple choice tests. Diet was poured into 8 cell trays and diet columns in the same volume were made with a No. 7 cork borer. The 5 diet cylinders (1.3 cm diameter ×2 cm high) of the 5 antifungal agent concentrations were placed in an 8 cell tray with equal distance and angle. Twenty V. cardui or H. virescens neonates were introduced to the center of the cell right after hatching. Choice tests for each agent were replicated 20 times. Diet cylinders in each cell were rotated clockwise each time to standardize environmental factors. The trays were kept in rearing room conditions described above; and the number of neonates feeding on each diet was recorded the next day. Both V. cardui or H. virescens neonates seldom sat on the diet cylinders in preliminary experiments, so neonates which stayed near the diet at a distance less than their body length were recorded as “feeding on the diet”
pH choice test: To test the effects of pH on diet acceptability, diets at four different pHs were tested. Citric acid was used to alter the pH of the diet. To eliminate the possibility of the citrate anion affecting the diet’s palatability rather than pH, the diet’s pH was altered by adding the same weight but varying ratios of citric acid and potassium citrate mixture. This measure kept the concentration of the citrate ion relatively constant for all pH treatments.
Comparisons of effects among the three antifungal agents
Nutritional indices of larvae reared in 5,000 ppm of each antifungal agent in the feeding experiment were used for comparison to determine the differences among the possible detrimental effects due to different antifungal compounds. Because the standard level of each antifungal agent was 1,500 ppm, the 5,000 ppm antifungal agent treatments can be described as basic diet with additional 3,430 ppm of one antifungal agent, with other environmental factors randomized. Thus, the comparisons were set up using complete randomized block design, and treatments were 5,000 ppm of three different antifungal agents. Six replications were performed, and blocks consisted of replication in time. The comparisons were done within each species separately.
Analysis
To obtain the dry weight of frass, eaten food, and net weight gain, the wet weight of initial diet in each cup was measured and converted to dry weight via dry diet to water ratios. The wet weight of each early 3rd V. cardui was recorded and converted to dry weight based on the water content of the larvae. The water content of the larvae was determined by dividing the weight of individual freeze-dried 3rd instar larvae by the fresh weight of the same larva, which had been frozen prior to measurement. The mean of 10 3rd instar larvae was used in these calculations. The weights of H. virescens neonates were considered negligible in the context of the total weight gain (based on the observation that neonates weighed less than 100 μg, and the final larval weights exceeded several hundred mg). Ten freeze dried diet samples from each species were treated by oven-drying at 110°C, 35 min to calculate the average water content of dried food.
For each sample, these calculations were made:
The amount of food ingested = initial dry diet – remaining dry weight of diet
The amount of frass produced = dry weight of fecal materials
Weight gain = dry weight of freeze dried larva- dry weight of 3rd instar larva (V. cardui)
Or weight gain = dry weight of freeze dried larva (H. virescens)
Once weight gain, amount of fecal materials produced, and diet consumed data were obtained, we calculated nutritional indices to evaluate the feeding, digestion and assimilation process of the insects. The nutritional indices can reflect how differences in insects’ foods affect the insects. The relative consumption rate (RCR), approximate digestibility (AD), the efficiency of conversion of ingested food to biomass (ECI) and the efficiency of conversion of digested food to biomass (ECD) are three commonly used nutritional indices [16].
The consumption rate (CR) is calculated as:
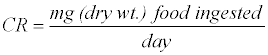
The relative consumption rate (RCR) is calculated as:
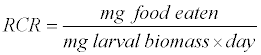
The approximate digestibility (AD) is calculated as:
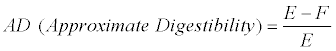
The efficiency of conversion of ingested food to biomass (ECI) is calculated as:
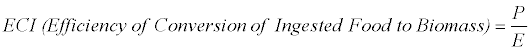
The efficiency of conversion of digested food to biomass (ECD) is calculated as:
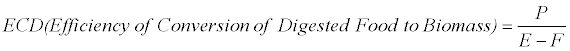
Where E=the amount of food ingested, F= the amount of frass produced, P=weight gain, all referring to dry weights.
The natural losses in fresh diet, for example include the degradation of diet components and water evaporation. These losses would increase with time, and would amplify the discrepancy between the dry weight calculated from percent dry matter in diet and the actual percent dry matter of the food. Waldbauer [16] pointed out that the error from failure to consider natural losses in diets can lead to significant deviation from actual consumption. Generally, magnification of introduced errors in nutritional indices is far greater when the larvae eat only a small fraction of the food [16,19].
Besides natural losses and diet aliquots causing error in measuring nutritional indices, several other factors can contribute errors that may be cryptic. For example, food consumption by insects can be affected by frequent disturbance by researchers while changing the diet and removing frass [16]. Thus, in our study more than enough food for larvae maximal growth was provided. The wet weight of diet was recorded immediately after the molten diet was distributed to individual cups, and then converted to dry mass. The average water content in freeze dried diet was also measured to minimize the errors.
All data were analyzed in JMP 10 (SAS Institute, Cary, North Carolina). For the variables in feeding experiment, i.e. frass weight, amount of diet eaten, weight gain, AD, ECI and ECD, analysis of variance (ANOVA) was performed to test for effect of antifungal agent concentrations on each variable. All significant ANOVA effects were followed by Tukey HSD test for mean comparisons. The number of neonates on each diet in multiple choice tests was recorded. The Friedman Test was used to model the ratings of 20 judges on treatment, i.e. different concentrations of one antifungal agent. For comparisons of effects of the three antifungal agents, the variables were subjected to analysis of variance (ANOVA) for possible effect of treatment on 5,000 ppm of different antifungal agents. All significant ANOVA effects were followed by Tukey HSD test for mean comparisons. An alpha level of 0.05 was used for all analyses.
Feeding experiment
Significant differences were detected among most of the treatments. Almost all parameters for V. cardui dropped significantly when the concentrations of antifungal agents increased except AD and RCR (Table 2). The amount of frass produced was reduced by diets containing 5,000 and 10,000 ppm methyl paraben (F=19.33; df=2,161; P<0.0001), and 10,000 ppm potassium sorbate/sodium propionate (F=26.85; df=2,173; P<0.0001. F=19.95; df=2, 171; P<0.0001). The weight gain of insects was reduced by diets containing 5,000 and 10,000 ppm methyl paraben (F=23.99; df=2,161; P<0.0001), and 10,000 ppm potassium sorbate/sodium propionate (F=37.93; df=2,173; P<0.0001. F=22.79; df=2,171; P<0.0001). CR was reduced by 5,000 and 10,000 ppm methyl paraben (F=35.64; df=2, 161; P<0.0001), 5,000 and 10,000 ppm potassium sorbate (F=35.03; df=2,173; P<0.0001), and 10,000 ppm sodium propionate (F=16.91; df=2,171; P<0.0001). RCR was increased by 5,000 and 10,000 ppm methyl paraben (F=5.43; df=2,161; P=0.0052), 10,000 ppm potassium sorbate (F=9.17; df=2,173; P=0.0002) and 10,000 ppm sodium propionate (F=7.85; df=2,171; P=0.0005). AD was increased by diet with 10,000 ppm methyl paraben (F=3.20; df=2,161; P=0.0432), 10,000 ppm potassium sorbate (F=3.59; df=2,173; P=0.0295), and 10,000 ppm sodium propionate (F=15.30; df=2,171; P<0.0001). ECI dropped with 10,000 ppm methyl paraben/potassium sorbate/sodium propionate (F=4.55, df=2,161; P=0.0120. F=11.46, df=2,173; P<0.0001. F=12.08; df=2,171; P<0.0001). ECD dropped with 10,000 ppm potassium sorbate/sodium propionate (F=9.04; df=2,173; P=0.0002. F=15.65; df=2,171; P<0.0001).
| Antifungal agent | Concentration, ppm | Frass (mg) | Weight gain (mg) | CR (mg/day) | RCR (day-1) | AD (%) | ECI (%) | ECD (%) |
|---|---|---|---|---|---|---|---|---|
| Methyl paraben | 1000 | 231.5 ± 10.57a | 141.0 ± 5.81a | 80.1 ± 2.66a | 0.6 ± 0.02b | 60.0 ± 0.91a | 24.6 ± 0.57a | 42.0 ± 1.34a |
| 5000 | 151.7 ± 10.09b | 92.9 ± 5.93b | 56.8 ± 2.07b | 0.8 ± 0.05a | 64.0 ± 1.56a | 21.8 ± 0.98ab | 37.3 ± 2.41a | |
| 10000 | 142.0 ± 13.80b | 83.4 ± 7.57b | 51.8 ± 2.96b | 0.8 ± 0.07a | 64.9 ± 1.91a | 20.9 ± 1.11b | 35.5 ± 2.64a | |
| Potassium sorbate | 1000 | 223.0 ± 11.16a | 137.7 ± 6.26a | 78.7 ± 3.07a | 0.6 ± 0.02b | 60.8 ± 0.71b | 24.4 ± 0.45a | 40.9 ± 1.03a |
| 5000 | 184.3 ± 8.31b | 116.7 ± 4.26b | 66.8 ± 2.31b | 0.6 ± 0.01b | 61.3 ± 0.71ab | 24.9 ± 0.53a | 41.4 ± 1.22a | |
| 10000 | 132.8 ± 6.00c | 78.9 ± 3.70c | 50.6 ± 1.54c | 0.7 ± 0.03a | 63.5 ± 0.89a | 21.6 ± 0.63b | 34.9 ± 1.32b | |
| Sodium propionate | 1000 | 218.8 ± 11.90a | 132.2 ± 6.88a | 77.9 ± 3.20a | 0.7 ± 0.05b | 61.5 ± 1.10b | 23.2 ± 0.78a | 39.5 ± 1.77a |
| 5000 | 186.3 ± 10.58a | 112.5 ± 5.93a | 69.1 ± 2.93a | 0.7 ± 0.03b | 62.8 ± 0.85b | 22.7 ± 0.58a | 37.0 ± 1.26a | |
| 10000 | 126.0 ± 8.91b | 75.3 ± 5.23b | 54.4 ± 2.47 b | 1.0 ± 0.10a | 68.9 ± 1.06a | 18.6 ± 0.84b | 28.2 ± 1.46b |
Table 2: Effects of antifungal agents on V. cardui feeding and growth.
Most parameters for H. virescens dropped significantly when the concentrations of antifungal agents increased except AD (Table 3). The amount of frass produced was reduced by the diets with 10,000 ppm methyl paraben/potassium sorbate/sodium propionate (F=7.81; df=2,151; P=0.0006. F=18.04, df=2,116; P<0.0001. F=30.15; df=2,137; P<0.0001). The weight gain of insects was also reduced in the diets with 10,000 ppm methyl paraben/potassium sorbate/sodium propionate (F=10.48; df=2,151; P<0.0001. F=21.06; df=2,116; P<0.0001; F=29.77; df=2,137; P<0.0001). The CR was reduced by the diets with 10,000 ppm methyl paraben/potassium sorbate/sodium propionate (F=7.65; df=2,151; P=0.0007. F=17.42; df=2,116; P<0.0001. F=28.91, df=2,137; P<0.0001). The RCR was increased in insects fed diets with 10,000 ppm methyl paraben/potassium sorbate/sodium propionate (F=7.56, df=2,151; P=0.0007; F=8.64, df=2,116; P=0.0003. F=9.84; df=2,137; P=0.0001). The AD was increased in insects fed diets with 10,000 ppm methyl paraben/potassium sorbate/sodium propionate (F=4.70; df=2,151; P=0.0105. F=14.42; df=2,116; P<0.0001. F=26.19; df=2,137; P<0.0001). The ECI dropped with diets containing 10,000 ppm methyl paraben/potassium sorbate/sodium propionate (F=10.30; df=2,151; P<0.0001. F=16.03; df=2,116; P<0.0001. F=14.90; df=2,137; P<0.0001). The ECD also dropped with diets containing 10,000 ppm methyl paraben/potassium sorbate/sodium propionate (F=9.95; df=2,151; P<0.0001. F=18.70; df=2,116; P<0.0001. F=21.83; df=2,137; P<0.0001).
| Antifungal agent | Concentration, ppm | Frass (mg) | Weight gain (mg) | CR (mg/day) | RCR (day-1) | AD (%) | ECI (%) | ECD (%) |
|---|---|---|---|---|---|---|---|---|
| Methyl paraben | 1000 | 93.5 ± 6.87a | 56.1 ± 4.17a | 24.3 ± 1.11a | 0.5 ± 0.03b | 67.4 ± 1.30b | 19.7 ± 0.85a | 30.5 ± 1.67a |
| 5000 | 91.5 ± 6.41a | 53.5 ± 3.65a | 24.6 ± 1.08a | 0.6 ± 0.03b | 68.2 ± 1.24b | 18.5 ± 0.69a | 28.3 ± 1.38a | |
| 10000 | 62.5 ± 5.12b | 34.8 ± 2.80b | 19.5 ± 0.89b | 0.7 ± 0.05a | 72.6 ± 1.32a | 15.0 ± 0.71b | 21.6 ± 1.29b | |
| Potassium sorbate | 1000 | 99.7 ± 8.67a | 58.7 ± 4.61a | 25.0 ± 1.42a | 0.5 ± 0.04b | 66.0 ± 1.47b | 20.2 ± 0.88a | 31.8 ± 1.74a |
| 5000 | 98.8 ± 9.17a | 52.3 ± 4.51a | 24.1 ± 1.48a | 0.5 ± 0.03b | 65.2 ± 1.51b | 18.6 ± 0.82a | 29.7 ± 1.72a | |
| 10000 | 51.1 ± 3.87b | 28.6 ± 2.29b | 16.8 ± 0.67b | 0.7 ± 0.05a | 73.9 ± 1.12a | 14.4 ± 0.69b | 20.2 ± 1.18b | |
| Sodium propionate | 1000 | 100.5 ± 8.13a | 57.8 ± 3.86a | 25.8 ± 1.41a | 0.5 ± 0.04b | 66.4 ± 1.50b | 19.8 ± 0.72a | 30.9 ± 1.41a |
| 5000 | 95.7 ± 6.79a | 56.7 ± 3.58a | 23.5 ± 1.10a | 0.5 ± 0.03b | 65.6 ± 1.22b | 20.8 ± 0.75a | 32.8 ± 1.44a | |
| 10000 | 41.0 ± 3.26b | 27.4 ± 2.20b | 15.2 ± 0.63b | 0.8 ± 0.07a | 76.9 ± 1.15a | 15.3 ± 0.84b | 20.8 ± 1.38b |
Table 3: Effects of antifungal agents on H. virescens feeding and growth.
Choice test
Numbers of neonates feeding on different diets were significantly different among treatments. Feeding of V. carduineonates was reduced by high levels of methyl paraben (F=8.08; df=4, 19, 76; P<0.0001). Comparatively, potassium sorbate showed positive effect on diet acceptance by neonates (F=5.41; df=4, 19, 76; P=0.0007). Sodium propionate did not affect choices (Table 4). Analysis of H. virescens feeding showed that neonates consumed less diet at high levels of both methyl paraben and potassium sorbate (F=4.31; df=4, 19, 76; P<0.0034. F=3.70; df=4, 19, 76; P=0.0083). Sodium propionate did not affect feeding choice (Table 5). The pH (5.5, 5.1, 4.8 and 4.5) had no significant effect for feeding choices of either V. cardui and H. virescens (Table 6).
| Concentration of antifungal agent (ppm) | 0 | 1000 | 1570 | 5000 | 10000 |
| Larvae choice on methyl paraben | 4.5 ± 0.51a | 3.4 ± 0.48ab | 4.5 ± 0.52a | 1.3 ± 0.23b | 0.9 ± 0.20b |
| Larvae choice on potassium sorbate | 1.9 ± 0.36b | 2.7 ± 0.48ab | 3.6 ± 0.38a | 3.8 ± 0.47a | 3.2 ± 0.36a |
| Larvae choice on sodium propionate | 3.4 ± 0.47a | 3.5 ± 0.29a | 3.4 ± 0.29a | 3.5 ± 0.30a | 3.1 ± 0.36a |
Table 4: Number of V. cardui larvae fed on diet cylinders containing different concentrations of antifungal agents.
| Concentration of antifungal agent (ppm) | 0 | 1000 | 1570 | 5000 | 10000 |
| Larvae choice on methyl paraben | 3.8 ± 0.43a | 3.3 ± 0.45a | 3.0 ± 0.32a | 2.7 ± 0.50ab | 1.2 ± 0.31b |
| Larvae choice on potassium sorbate | 3.2 ± 0.51ab | 3.8 ± 0.38a | 3.9 ± 0.39a | 2.8 ± 0.35ab | 1.6 ± 0.29b |
| Larvae choice on sodium propionate | 3.2 ± 0.40a | 2.8 ± 0.30a | 2.2 ± 0.37a | 2.5 ± 0.37a | 2.2 ± 0.37a |
Table 5: Number of H. virescens larvae fed on diet cylinders containing different concentrations of antifungal agents.
| pH | 5.5 | 5.1 | 4.8 | 4.5 |
| V. cardui | 4.4 ± 0.6a | 3.6 ± 0.3a | 4.3 ± 0.5a | 3.5 ± 0.6a |
| H. virescens | 4.2 ± 0.4a | 3.6 ± 0.3a | 3.9 ± 0.2a | 3.6 ± 0.4a |
Table 6: Number of larvae fed on different diet cylinders with different pH.
Comparison of effects among the three antifungal agents
Vanessa cardui performed differently with the addition of different kinds of antifungal agents to the diet. V. cardui reared on a high level of methyl paraben had lower amount of frass, less weight gain, reduced CR, elevated RCR and reduced ECI (F=4.02; df=2, 176; P=0.0196. F=5.51; df=2, 176; P=0.0048. F=7.10; df=2, 176; P=0.0011. F=7.69; df=2, 176; P=0.0006. F=4.93; df=2, 176; P=0.0082). H. virescens reared on the three diets did not show any significant differences; i.e. the performance of H. virescens did not differ from each other when there was an extra high level of methyl paraben, potassium sorbate, or sodium propionate, indicating a relative insensitivity to these agents by H. virescens.
We found that high levels of antifungal agents decrease frass production and weight gain by reducing (i) consumption rate, (ii) absorption rate and (iii) metabolic capabilities. However, relative consumption rate (RCR) and approximate digestibility (AD) were unchanged or, more often, elevated with increased level of antifungal agents in diet. This raises and important point: nutritional indices are not independent from each other, and they are also influenced by insect age, gender, and various environmental factors [16]. Therefore interpretation of the mechanics of nutritional index-related responses with antimicrobial concentrations must be interpreted in the context of insect size, gender, and our experiments’ environmental factors.
Reduced growth under high antifungal agent concentrations
Both V. cardui and H. virescens gained significantly less body weight and produced less frass at highest concentrations of all three antifungal agents. Similar results were found in previous studies [14,15]. The consumption rate (CR) was also reduced as in previous studies, which indicated that daily food consumption is positively related to daily growth, and later instars ingested more food than did earlier instars [22]. Thus, consumption rate is directly related to the size of the larvae; since the individuals reared on diets with the highest concentration of antifungal agents are much smaller, their consumption rate is also lower than it is for larger individuals. Larvae may feed less in the presence of large amount of antifungal agents, acting as they might with distasteful allelochemicals. Reduction in feeding in response to common antifungal agents has not been well-studied despite the longstanding observations (such as 15) that antifungal agents are toxic in high concentration. The relationship between size of larvae and the gape of their mouthparts is discussed by Cohen [1].
It appears that feeding was not suppressed by the existence of antifungal agents. Instead, the antifungal agents showed direct detrimental effects on absorption or metabolism as they decreased digestion and/or absorption and subsequently, ECI and ECD. Thus, the slow growth rate would affect CR indirectly by holding insects in earlier instars and smaller sizes.
Few studies targeted palatability of antifungal agents on insects. Benedict et al. [29] found that methyl paraben in diet was avoided by Anopheles gambiae but not A. arabiensis. Our choice tests showed that different antifungal agents have effects on insect feeding behavior, and the effects may vary among species. Methyl paraben is a feeding deterrent for both V. cardui and H. virescens. Potassium sorbate reduced feeding response in H. virescens, but appeared to be reducing diet palatability in V. cardui. Sodium propionate showed neutral effect on both species. Another factor that must be considered is that the counterions in sorbate and propionate compounds (potassium and sodium) in antifungal agents can affect insect feeding behavior negatively or positively. Another factor that could modify palatability of diet antifungal additives could include pH changes influenced by acid forms of compounds such as sorbic acid/sorbate salt, propionic acid/propionate salt, and benzoic acid/benzoate salt (Cohen 2003). The influences of widely-used antifungal agents on feeding behavior are worth further study on case–by-case basis.
Increasing feeding and digestion under high antifungal agent concentrations
Interestingly, although CR dropped as antifungal agents concentrations increased, the relative consumption rate (RCR) was actually increased. While gross consumption necessarily increases as larvae get larger, RCR often declines with increasing insect age and the accompanying increase in body mass. Previous studies showed that RCR is highest in early instars, then declines in later instars [30]. Since larvae in our high antifungal agent trials were usually smaller and stunted at earlier instars, it is not surprising that they had higher RCR values. Besides the age factor, a high RCR can compensate for a low ECI; the larvae may feed relatively more when they are not absorbing the food efficiently enough. Blake et al. [30] found that feeding insects with foliage resulted in higher RCR, while artificial diet resulted in higher ECI. Since high concentrations of antifungal agents reduced both ECI and ECD, the insects may offset that negative effect by feeding more intensively. In other words, the rate of feeding and amounts consumed by insects reared on high levels of antifungal agents is probably enhanced instead of suppressed.
Similar to RCR, AD of V. cardui and H. virescens is also increased with higher levels of antifungal agents. Approximate digestibility is another nutritional index that will decrease as the insect grows and develops to later instars. Waldbauer [16] reviewed and calculated data from some previous studies and concluded that AD declined from first to last instars. Some later studies further proved the negative relationship between AD and age [22,23]. These authors [22,23] stated that the decreased AD was due to selective feeding in younger larvae; older larvae will ingest not only tender part of the leaves but also indigestible crude fibers, which decrease the overall digestibility. Insects in our study reared on artificial diet also showed declined AD with well-developed individuals reared on low antifungal agents, so it was very likely affected directly by the presence of antifungal agents. However, Gordon [17] also suggested that when an animal doubled its weight and volume, the surface area of its digestive tract increased by only a factor of 1.8, so the digestibility would decrease as the animal grows. Therefore, the difference among insect ages in our study is again confounding.
Reduced ECI & ECD under high antifungal agents concentrations
As discussed above, an increasing CR and decreasing RCR and AD are related to larval ages and body mass. However, the relationship between ECI, ECD and age are complex. Waldbauer [16] stated that there is an obvious tendency for the ECI to decrease with age, and suggested that the decline of AD with age was responsible for the decline of ECI. Conversely, many other studies showed an increasing of ECI with age [22,23,30]. The increasing of ECI through age is possibly related to the factors that also lead to decline of RCR. Scriber [20] also showed that RCR was negatively correlated to ECI due to a “power and efficiency” trade-off, i.e. insects eat less when the assimilation is more efficient and vice versa. From those previous studies, we can suggest that ECI is closely correlated to both AD and RCR. Insects will absorb better if they digest well, so a low AD will decrease ECI; and a decreased RCR will make a higher ECI value. Since both AD and ECI decrease with age, their effects on ECI would be more complex. Thus, later instars could have an increased, decreased or possibly fluctuating ECI values.
In this study, the ECI is significantly reduced in high antifungal agent trials. The increased AD is not responsible for the decline of ECI, since AD is positively correlated with ECI [18]. Instead, the increased RCR in high antifungal agent trials is possibly triggered by declining ECI. The absorption of digested food is negatively influenced by a high level of antifungal agent, although the food may get thoroughly digested in the gut. Cohen [1] discussed the factors that influence rates and efficiencies of digestion and absorption. Factors that influence absorption are i) exposure of the digested food to sites of absorption, especially receptors in the gut lining ii) possible interference at absorption sites by competing molecules iii) presence and concentration of diet components that facilitate absorption, and iv) the organization and characteristics of the digestive system, especially the peritrophic matrix (PM). At the onset of the experiments discussed here, the hypothesis that antifungal agents—especially ones at high concentrations—might impact nutritional indices at one of the above levels of digestive system organization. It was hypothesized that a factor such as modification of the rate of peristalsis by an antifungal agent or influence by the agent on the characteristics of the PM (such as thickness, number of layers, or possible damage to the PM’s integrity) could all impart a downstream effect on the digestion and absorption efficiency with respect to diet components. This issue was discussed by Pechan et al. [24] regarding PM characteristics and influence on nutritional indices and by Cohen [1] regarding the comprehensive characters mentioned above.
If the rate of peristalsis in the midgut was decreased by the high concentrations of antifungal agents, this could account for the increase in AD. Thus, food stayed in the gut for a longer time and was digested more thoroughly due to the increase in gut residence period. At the same time, there would be a decrease in ECI because the reduction in gut movements, which, according to Cohen [1] involves a churning action whose reduction would lead to a reduced amount of absorption. Another possible reason is that the activity of nutrient receptors in the gut is suppressed by antifungal agents; and since AD is enhanced, the activity of digestive enzymes is less likely suppressed by antifungal agents. This study points to the possible mechanism of direct effects of antifungal agents on gut receptors/absorption sites and would merit further study in future research.
Few studies have focused on the change of ECD with age. ECD is usually inversely related with AD and increases with advancing age and developmental progress [23]. As more food is assimilated, a smaller proportion of food is converted into biomass. ECD reflects metabolic efficiency, and can be reduced by lowered efficiency of conversion of digested food or enhanced metabolic cost. Metabolic efficiency can be reduced by antimetabolites, which may lower the efficiency of biochemical reactions by binding with the active sites of enzymes. Phytophagous insects are evolutionarily adapted to feeding on allelochemical-rich host plants, and several phytophagous species have been shown to develop effective detoxification enzyme systems [32]. High concentrations of antifungal agents may trigger detoxifying processes in the gut and bring extra metabolic cost, thus decreasing the net metabolic efficiency.
Another kind of metabolic cost can be caused by a damaged peritrophic complex. The peritrophic complex is a very important membrane structure in the insect midgut, which holds the ingested food, protects gut cells from physical abrasion, and prevents the invasion of pathogens and parasites. Chang et al. [33] and Pechan et al. [24] showed that ECI and ECD of fall armyworm were reduced by resistant corn plant tissue containing a 33-kDa cysteine protease, which damages the peritrophic membrane. In our study, if a high level of antifungal agents has deleterious effect on the peritrophic membrane, then the metabolic cost in enzyme production and secretion will also reduce ECD.
In summary, high levels of all three antifungal agents impacted feeding, digestion, absorption and metabolism. Although nutritional index values are correlated with insect size, the lag in development of larvae reared under highest antifungal agent level do not explain the observed changes in nutritional indices. Clearly, this affected the insects’ trophic physiology. Antifungal agents may slow down gut peristalsis and increase the extent of digestion. It also seems plausible that ECI is reduced possibly by slowed peristalsis and interference with nutrient receptors. ECD is also reduced, possibly by increases in metabolic cost of the detoxification of antifungal agents and possibly toxin-induced increases in metabolic rate. As a response to decreased assimilation, more food is ingested, which increases RCR. Also although insects are feeding more and digesting more thoroughly, thus compensating for the loss in absorption and metabolism, their growth and development are still negatively influenced by high levels of antifungal agents overall.
Because we understand the tremendous importance of the relationships between insects and microbes, a brief discussion of insect/ microbe relationships, especially those pertaining to gut microbes is in order. Dillon & Dillon [34] reviewed the large body of information about the roles of gut microbes, including improving the ability of some insects to live on suboptimal diets, improving digestive efficiency, and providing of essential nutrients. Many widely used antifungal agents, including the three agents in our study, have relatively general modes of action, and can inhibit a wide spectrum of fungi and bacteria [35,36]. We do not know the exact roles gut microbes in V. cardui and H. virescens play when insects feed on artificial diet. Since many insects can obtain their gut microbes from food [35], the gut microbes in lab colonies and wild populations could be dramatically different by feeding on food with different microbe communities. Many insect colonies have been kept in lab for generations and decades, for example, the H. virescens colony used in this study has been kept in lab conditions for 25 years, so it is possible that the colony has developed a unique gut microbe flora, which may be far different from original field populations.
Comparisons among the effects of different antifungal agents
Previous studies on detrimental effects of antifungal agents dealt with biological parameters such as biomass, mortality, and fecundity. Here, we evaluated the potential toxicity of antifungal agents via nutritional indices. Since the concentration of only one antifungal agent was varied each time and the other agents maintained at basic level of 1,500 ppm, both 5,000 and 10,000 ppm trials can be considered as having an extra amount of a given antifungal additive in basic diet and used in comparison. We chose the 5,000 ppm to compare the antifungal agents, because 10,000 ppm methyl paraben treatments caused solubility problems. Although the 10,000 ppm methyl paraben treatment showed significant changes in nutritional indices, the results vary dramatically among individuals. Many larvae remained very small, while grew normally. This was not a problem with potassium sorbate or sodium propionate, but to keep our comparisons consistent, we used the three concentrations of all three antifungal agents. Our study showed that in addition to the conventionally-measured factors (weight, fecundity, and survival); nutritional indices can be used in measuring the deleterious effects of agents. Thus methyl paraben is a harsher chemical than the other two antifungal agents for V. cardui. Similar results were reported by Singh and House [13,15] and Alverson and Cohen [14], who found methyl paraben to be more toxic than several other antimicrobial agents. However, the value of methyl paraben is still considerable in terms of the stability of this antifungal agent and the relatively small amounts of this preservative required controlling mold.
In contrast to the findings on V. cardui, the three antifungal agents have similar effects to one-another in the tests with H. virescens. This may be caused by differences among species or strains. Since H. virescens were assigned to each cup right after hatching, no holes were made on the lids to limit larval escape; thus the increased moisture inside the rearing cups facilitated mold growth. This point, about the strong relationship between high water activity and mold is discussed extensively by Cohen [1].
The effect of anti-fungal agents varies dramatically with pH. Many anti-fungal chemicals are most effective at lower pH, and the effective concentrations must increase when pH is elevated. On the other hand, most insects do not do well with diets whose pH is below a speciesspecific threshold. The pH of YC diet used in the experiment is about 5.7~5.9. The anti-fungal agents used in YC diet are mostly the salt forms instead of acid forms, since salt forms are easier to dissolve in water. Consequently, it would be advantageous in terms of feeding behavior and microbial management to reduce the pH of the YC Diet for both V. cardui and H. virescens neonates, so the use of citric acid in YC diet to enhance mold suppression could be considered in future usage.
In summary, our analysis of nutritional indices and choice tests showed that effects of different antifungal agents vary between the two insect species included in this study. Matching antifungal agents to species in a wide variety of insects, especially in terms of effects of these agents on feeding behavior and growth can lead to substantial improvements in artificial diet-based rearing.
We thank Drs. Yasmin J.Cardoza.David Orr and Clyde Sorenson for their constructive comments on this study,including many helpful suggestions about experimental design. We thank Dr. Consuelo Arellano for her advice on statistical analysis. We thank Dr. Fred Gould and Ms. Sandra Paa for providing the Heliothis virescens for these experiments.