Rheumatology: Current Research
Open Access
ISSN: 2161-1149 (Printed)
ISSN: 2161-1149 (Printed)
Research Article - (2023)Volume 13, Issue 5
Studies have shown that individuals with Knee Osteoarthritis (KOA) may benefit from cycling exercise. However, the supportive evidence remains unclear. This systematic review with meta-analysis aimed to investigate the effects of Cycling Rehabilitation Training (CRT) on the recovery of osteoarthritic knee joints. Five databases were searched with publishing date restrictions from 1 January 2000 to 1 June 2023. We included 21 studies with 1181 participants that 1) recruited participants with KOA, 2) used CRT in the intervention, 3) compared measurements before and after the intervention or between intervention group and control group, and 4) included the measurements of interest. The quality of the 21 included studies was moderate with a mean quality score of 18.76 which was assessed by the modified Downs and Black checklist. The random-effects meta-analysis of Western Ontario and McMaster Universities Arthritis scores, Lequesne index, and scores of Timed Up and Go test were conducted on eight studies which contain the numerical comparison results for the variables of interest. The changes in muscle strength, kinetics, and kinematics as a result of the intervention were summarized. CRT improved muscle strength and physical function (MD 8.39, 95% CI (5.56, 11.23)), and reduced pain (MD 3.02, 95% CI (2.21, 3.84)) and joint stiffness (MD 1.26, 95% CI (0.82, 1.70)) in KOA patients. Compared with healthy subjects, limited studies revealed that KOA patients showed increased extensor moments and abduction and adduction peak angles their knee joints, and decreased internal rotation moment and peak angles of knee flexion and extension. CRT was effective in relieving knee pain, restoring motor function, and improving lower limb muscle strength. Knee abduction moment may be an indicator of rehabilitation progress. This systematic review would give insights into the CRT intervention guideline for patients with KOA.
Cycling; Rehabilitation; Knee osteoarthritis; Muscle strength; Kinetics; Kinematics
Osteoarthritis (OA) is one of the most common musculoskeletal disorders in the elderly population. Knee Osteoarthritis (KOA) accounts for the majority of OA cases [1] and generates a major global medical burden [2]. More than half of patients over 65 with knee problems have KOA [3]. As a degenerative disease, KOA has few effective treatments other than Total Knee Arthroplasty (TKA), which is only recommended in the advanced stage. For most patients, non-invasive approaches, such as physiotherapy, drug therapy, and lifestyle adjustment, are recommended [4]. Exercise therapy is recommended by the Osteoarthritis Research Society International for the treatment of KOA, and this therapeutic method has attracted much attention for its non-invasiveness and high efficiency [5].
Cycling is a typical aerobic exercise. Studies have shown that moderate cycling exercise has positive effects on joint tissues [6]. Moreover, resistance training off the bike is useful for increasing lower body lean mass [7]. Even in older women, muscle power related to the joint force and movement velocity has been shown to improve significantly after an 8-week cycling training programme [8]. Hanna et al. found that regular cycling exercise increased the cartilage volume of the medial tibia, which protects the knee joints [9]. Due to the ease of adjusting training intensity and the availability of unlimited training sites, cycling exercise has been gradually introduced in rehabilitation programmes.
Some studies have confirmed the effects of Cycling Rehabilitation Training (CRT). Salacinski et al. [10] reported that KOA patients had reduced joint pain and significantly improved 6-minute walking ability compared with the daily-activities group (control group) after 12 weeks of community-based cycling. Alkatan et al. [11] showed that KOA patients had significantly increase in isokinetic knee peak torque at both 60°/s and 120°/s in comparison with baseline data. A 12-week cycling intervention in 140 KOA patients demonstrated reduced knee pain and this was associated with higher serum programmed cell death protein 1 levels [12]. A categorical comparison of these studies is necessary to discuss the biomechanics of CRT.
Studies of CRT have included developing training programmes and assessing the mobility of patients before and after the intervention. The interventions have typically been performed 2–6 weekly for 3–12 weeks. Long-term studies of more than 12 weeks are rare. Questionnaire scoring systems, such as the Knee injury and Osteoarthritis Outcome Score (KOOS) questionnaires, and physical motion tests such as the 6-Minute Walking Test (6 MWT) have mainly been used to evaluate the effects of training. Additionally, some studies have included biomechanical analysis, such as the numerical solution of dynamic equations [13], multi-body dynamic simulation [14], and Finite Element (FE) analysis. Joint angles, forces, and moments have also been determined. However, there is limited evidence to link these parameters directly to the recovery progress of KOA patients. Muscle activation measured by surface Electromyography (EMG) has also been widely used to determine the effects of CRT on muscle functions [15]. Compared with isokinetic muscle testing, EMG has the advantage of real-time monitoring during exercise. These biomechanical analyses have provided important information for assessing the effectiveness of CRT and preventing secondary injuries during exercise.
Although the biomechanics of CRT has been investigated using various methods, it remains challenging to develop clear guidance on the adjustment of bicycle configurations and rehabilitation programmes to provide the maximum benefit for patients. The integration and reanalysis of previous studies may help to reveal the underlying link between biomechanical variables and rehabilitation efficacy. Therefore, the purpose of this systematic review and meta-analysis was to review studies of CRT for KOA patients to provide more information to facilitate the development of CRT programmes and guide future studies.
Methods
This systematic review followed the guidelines described by the new Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [16]. Meta-analyses were performed for control group and cycling training group, pre and post-intervention assessment scale scores and test performance. To assess the kinematic and kinetic differences between patients and healthy subject groups, we conducted qualitative retrospective analyses of knee joint angles, knee joint moments, knee joint forces, and Pedal Reaction Forces (PRFs).
Search strategy
A systematic search of published articles was performed in five databases: PubMed, Scopus, Web of Science, EBSCO Essentials, and Cochrane Library. The search terms, such as cycling, bicycle, bike, ergometer, knee osteoarthritis, and knee arthritis, were predefined with Boolean operators, as shown in the Supplementary material. The publication date was restricted from 1 January 2000 to 1 June 2023, and the language was limited to English. The first literature search was performed between 10 February and 16 March 2022. The search results were retrieved again and supplemente from 1 June to 8 June 2023.
Study selection and data collection
Studies were included if 1) they were published in a peer-reviewed journal; 2) the participants with KOA were diagnosed clinically; 3) the main intervention was stationary cycling training; 4) measurement comparisons were performed before and after the intervention or between the control group and the experimental group; and 5) the measurements included at least one outcome from the KOOS, Western Ontario and McMaster Universities Arthritis Index (WOMAC), 6 MWT, or biomechanical variables including joint angle, joint force, or joint moment of the knee.
Study selection and data extraction were undertaken by a single reviewer (F.B.), with advice from a second reviewer (S.F.C.). The information extracted included demographic information and the research design, objectives, experimental protocol, outcomes of interest, and principal conclusions.
Risk of bias and quality of evidence assessment
The included studies were assessed independently by two assessors (F.B. and S.F.C.) using a modified Downs and Black checklist tool [17] for both randomised controlled and non-controlled trials. The checklist tool consists of five components and 27 questions, which examine the quality of the studies from with respect to reporting, external validity, internal validity (bias and confounding), and power, as shown in Supplementary Table S1 [18]. The possible answers to each question are Yes (a score of 2 for question 5 and a score of 1 for the other questions), Partially Yes (a score of 1 for question 5), No (a score of 0), or Unable to Determine (a score of 0). The possible total score is 28. The quality of the studies was categorised as excellent (scores of 26–28), good (20–25), fair (15–19), and poor (≤ 14) based on the previous reports [19]. The risk of bias was assessed based on random sequence generation, allocation concealment, the blinding of participants and outcome assessments, incomplete outcome data, selective reporting, and other biases. Two assessors (F.B. and S.F.C.) independently rated the results as low, high, or unclear risk of bias. When there were divergences in the assessments, a consensus was reached through discussion with a third professional assessor (M.Z.).
Outcomes
The most reported and quantifiable results were selected for the meta-analysis, including WOMAC (containing three subscales of pain, stiffness, and physical function), Lequesne index, and Timed Up and Go (TUG) test scores. Statistical analyses were performed separately between and within groups. Between-group analysis was the comparison of outcomes between the cycling group and the control group after the intervention. The within-group analysis compared the results before and after the intervention in the cycling group. The intervention periods lasted at least 4 weeks. If the study reported a non-standard score, the score conversion was performed first and the converted score was then compared with the standard scoring range. For example, the WOMAC score range in the study of Salacinski et al. was 0-100, with a lower score indicating more severe disease [10]. Each subscale was converted into standard scores with a possible range of 0–20 for pain, 0–8 for stiffness, and 0–68 for physical function [20], with higher scores indicating more severe OA conditions. In addition, a systematic review was performed to assess the associations of muscle strength, PRF, knee joint angle, and moment with the degree of KOA [21].
However, as there were few relevant studies and the experimental conditions significantly varied between studies, meta-analyse were not performed for these four variables.
Data analysis
All meta-analyses were conducted using RevMan 5.3 (The Cochrane Collaboration, London, UK). The Standardised Mean Differences (SMDs) with 95% Confidence Intervals (CIs) were determined for continuous data. If the Standard Deviation (SD) was not provided, it was calculated from the sample size and the Standard Error (SE). A random-effects model was used for calculations. The heterogeneity of the studies was assessed using Cochran chi-square (Cochran Q) and I2 tests. I2 describes the proportion of the total variation in study estimates that is due to heterogeneity [22]. When p<0.1 and I2<50%, none of the variation was assumed to be due to heterogeneity. If there was heterogeneity, a sensitivity test was performed by excluding each study and inspecting the effect sizes manually.
Study selection
A total of 547 articles were retrieved from the databases, and 21 studies met the inclusion criteria and were included in the systematic review. Eight of these studies were included in the meta-analysis. The detailed retrieval process and the reason for exclusion reasons are presented in Figure 1.
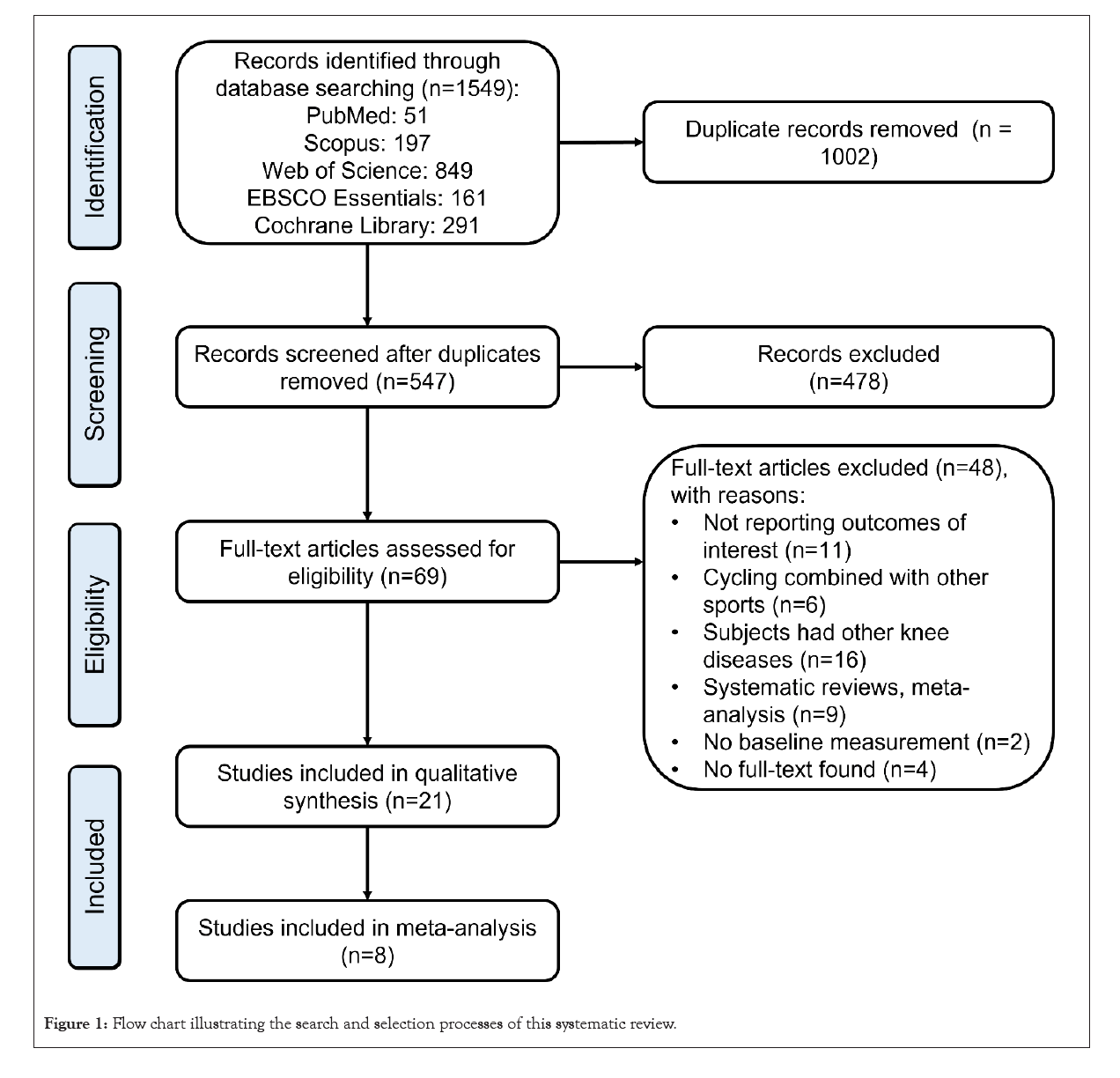
Figure 1: Flow chart illustrating the search and selection processes of this systematic review.
Study characteristics
The included studies comprised 1181 participants. There were 591 females and 282 males in the studies that reported gender information. The participants were mainly middle-aged or older (35–70 years old). There were 13 randomised controlled trials, accounting for 61.90% of the included studies. There were four case-control designed studies, two longitudinal studies, one descriptive research study, and one pretest-posttest designed study.
All included studies focused on KOA or included participants with KOA. Kellgren-Lawrence (K-L) grades and medical imaging diagnosis were used as the inclusion criteria for the participants in 13 studies. The minimum duration of pain was used in five studies instead of K-L grades. The basic demographic information is listed in Table 1. The characteristics of the studies, including the research protocol, outcomes of interest, and the main conclusions, are summarised in Table 2.
| Study | Group | Sample size | Age (y) | BMI/Height/Weight | Healthy state |
|---|---|---|---|---|---|
| Breugem et al. [18] | KOA group | 52 females, 41 males | 60.4 | - | - |
| TKA group | 75 females, 43 males | 71.9 | |||
| Meniscal or ligamentous injury group | 51 females, 36 males | 42.3 | |||
| Oliveira et al. [23] | Exercise group | 45 females, 3 males | 61.5 ± 6.94 | 29.72 ± 4.11 kg/m2 | K-L grades: 91.18% II |
| 5.88% III | |||||
| 2.94% IV | |||||
| Instruction group | 47 females, | 58.78 ± 9.6 | 30.00 ± 5.05 kg/m2 | K-L grades: 92.68% II | |
| 3 males | 4.88% III | ||||
| 2.44% IV | |||||
| Salacinski et al. [10] | Cycling group | 15 females, 4 males | 55.1 ± 10.5 | 22.4 ± 3.3 kg/m2 | K-L grades of I to III |
| Control group | 12 females, 6 males | 60.6 ± 8.4 | 25.7 ± 6.3 kg/m2 | ||
| Sheth et al. [24] | Cycling group | 7 females, 8 males | 50.86 ± 5.39 | - | Pain duration: 7.6 ± 1.6 months |
| Treadmill group | 8 females, 7 males | 50.46 ± 4.69 | Pain duration: 8.0 ± 2.0 months | ||
| Gardner et al. [25,26] | KOA group | 13 | 56.8 ± 5.2 | 26.6 ± 3.6 kg/m2 | K-L grades of I to IV |
| 1.80 ± 0.14 m | |||||
| 83.2 ± 22.3 kg | |||||
| Control group | 11 | 50.0 ± 9.7 | 25.9 ± 5.4 kg/m2 | Healthy | |
| 1.75 ± 0.12 m | |||||
| 80.17 ± 23.13 kg | |||||
| Alkatan et al. [11] | Cycling group | 24 | Middle-aged and older adults | 31.6 ± 1.7 kg/m2 | K-L grades of I to III |
| 84.5 ± 3.8 kg | |||||
| Swimming group | 24 | 34.6 ± 2.1 kg/m2 | |||
| 92.0 ± 4.7 kg | |||||
| Silvis et al. [27] | Upright cycle | 9 females, 11 males | 57.6 | 30.8 kg/m2 | K-L grades of I to IV (average score: 2.17) |
| Land treadmill | 13 females, 8 males | 58.1 | 31.5 kg/m2 | ||
| Water treadmill | 16 females, 4 males | 59 | 32.7 kg/m2 | ||
| RAJ et al. [28] | Group A: supervised exercise with cycle ergometry | 15 | - | - | Knee pain age ≥ 50 years |
| Group B: supervised exercise | 15 | ||||
| Control group | 6 females, 6 males | 66.1 ± 7.3 | 165.8 ± 6.6 cm | ||
| 67.7 ± 10.6 kg | |||||
| Buddhadev et al. [29] | Control group | 6 females, 6 males | 66.1 ± 7.3 | 165.8 ± 6.6 cm | K-L grades of I to IV |
| 67.7 ± 10.6 kg | |||||
| KOA group | 6 females, 6 males | 67.3 ± 7.6 | 171 ± 9.7 cm | ||
| 74.5 ± 14.9 kg | |||||
| Kabiri et al. [30] | Control group | 6 females, 6 males | 66.1 ± 7.3 | 165.8 ± 6.6 cm | K-L grades of II or III |
| 67.7 ± 10.6 kg | |||||
| Arm ergometer group | 19 females, 4 males | 60.72 ± 2.37 | 26.24 ± 0.47 kg/m2 | ||
| Treadmill group | 19 females, 5 males | 56.92 ± 1.37 | 25.89 ± 0.45 kg/m2 | ||
| Keogh et al. [31] | MICT cycling group | 7 females, 1 male | 66.1 ± 8.8 | 28.2 ± 6.9 kg/m2 | Duration of diagnosis: 4.9 ± 3.2 years |
| 165.5 ± 6.3 cm | |||||
| 77.8 ± 23.0 kg | |||||
| HIIT cycling group | 6 females, 3 males | 59.1 ± 6.7 | 27.0 ± 4.0 kg/m2 | Duration of diagnosis: 4.6 ± 5.8 years | |
| 170.0 ± 6.2 cm | |||||
| 78.5 ± 13.5 kg | |||||
| Liu et al. [32] | Cycling group | 23 females, 4 males | 40-70 | 23.4 ± 2.6 kg/m2 | K-L grades of II or III |
| Tai Chi group | 22 females, 6 males | 40-70 | 22.8 ± 2.2 kg/m2 | ||
| Baduanjin group | 24 females, 5 males | 40-68 | 23.1 ± 2.7 kg/m2 | ||
| Control group | 14 females, 10 males | 40-70 | 23.4 ± 3.3 kg/m2 | ||
| Bhattacharya et al. [33] | Group A: conventional treatment and forward cycling group | 8 females, 4 males | 56.5 | - | K-L grades of I to III |
| Group B: conventional treatment and backward cycling | 6 females, 6 males | 51.2 | |||
| Group C: conventional treatment | 10 females, 2 males | 58 | |||
| Rezasoltani et al. [34] | Aquatic cycling group | 15 | 50.8 ± 3.5 | 25.9 ± 2.1 kg/m2 | Pain duration: |
| 177.1 ± 6.3 cm | ≥ 3 months | ||||
| 79.5 ± 5.5 kg | |||||
| Control group | 15 | 51.2 ± 2.2 | 25.6 ± 3.0 kg/m2 | ||
| 176.4 ± 4.2 cm | |||||
| 81.0 ± 2.8 kg | |||||
| Rewald et al. [35] | Aquatic cycling group | 39 females, 16 males | 59 ± 9.5 | 29 ± 5.6 kg/m2 | K-L grades of I to III |
| Usual care group | 24 females, 23 males | 61 ± 7.4 | 29 ± 5.4 kg/m2 | ||
| Thompson et al. [36] | KOA group | 13 | 56.8 ± 5.2 | 26.6 ± 3.6 kg/m2 | K-L grades of I to IV |
| 1.80 ± 0.14 m | |||||
| 83.2 ± 22.3 kg | |||||
| Control group | 11 | 50.0 ± 9.7 | 25.9 ± 5.4 kg/m2 | Healthy | |
| 1.75 ± 0.12 m | |||||
| 80.17 ± 23.13 kg | |||||
| Golightly et al. [37] | One group | 19 females, 10 males | 63 ± 7 | 18.5-50 kg/m2 | Pain subscale of the WOMAC ≥ 6/20 |
| Smith-Ryan et al. [38] | One group | 16 | 59.9 ± 8.3 | 29.0 ± 4.3 kg/m2 | K-L grades of II to IV, and pain score of WOMAC ≥ 6 |
| 1.69 ± 0.086 m | |||||
| 86.0 ± 14.7 kg | |||||
| Oiestad et al. [39] | Stationary cycling group | 56 | 35-70 | - | Mild to moderate radiographic osteoarthritis |
| Strength training group | 55 | - | |||
| Usual care group | 57 | - | |||
| Rosadi et al. [40] | Group 1: strengthening and stationary cycling intervention | 4 females, 6 males | 63.3 ± 8.3 | 25.2 ± 3.6 | KOA duration: 72.3 ± 8.9 months |
| Group 2: walking and physiotherapy modalities | 6 females, 4 males | 63.0 ± 6.9 | 28.5 ± 4.6 | KOA duration: 71.8 ± 7.4 months | |
| Group 3: physiotherapy modalities | 9 females, 1 males | 64.0 ± 7.4 | 25.4 ± 3.1 | KOA duration: 72.6 ± 4.3 months |
Note: ACL: Anterior Cruciate Ligament; BMI: Body Mass Index; KOA: Knee Osteoarthritis; K-L: Kellgren-Lawrence; MICT: Moderate-Intensity Continuous Training; HIIT: High-Intensity Interval Training; TKA: Total Knee Arthroplasty; WOMAC: Western Ontario and McMaster Universities Arthritis Index.
Table 1: Demographic information in the included studies.
| Study | Experiment Protocol | Outcomes | Main conclusions | ||||
|---|---|---|---|---|---|---|---|
| Score | Test | Kinematics | Kinetics | Muscle | |||
| Breugem et al. [18] | Patients were evaluated at the clinic and completed 4 questionnaires | SF-36, Oxford 12-item, IKDC, Cycling questionnaire | - | - | - | - |
|
| Oliveira et al. [23] | Exercise group did 8-week exercise including cycling, hamstrings stretching, and quadriceps strengthening. Instruction group did 8-week instructions about KOA. | WOMAC, KOOS, Lequesne Index | TUG test | - | - | - |
|
| Salacinski et al. [24] | Cycling group participated in a community-based 12-week cycling program. Control group did daily activities and routine exercise. | WOMAC, KOOS, KOS-ADL, VAPS, IPAQ | 6MWT, 1RM test | Gait velocity | - | Lower-body muscle strength |
|
| Sheth et al. [25] | Group A did cycling exercise program and group B did supervised walking exercise. Both performed 30 min/day and five days/week for 3 weeks. | VAS, Lequesne Index, SF-36 | - | - | - | - |
|
| Gardner et al. [25] | All subjects pedalled in 3 cycling conditions (neutral, 5° toe-in, 10° toe-in) with 80 W at 60 rpm. | KOOS, VAS | - | Flexion, adduction and external rotation angles | Three PRFs, Extensor, abduction, internal rotation moments | - |
|
| Alkatan et al. [11] | Subjects performed cycling or swimming exercise training for 45 min/day, 3 days/week for 12 weeks. | WOMAC, SF-36, Godin physical activity score | 6MWT | Walk speed | - | Grip strength, Isokinetic knee extensor and flexor peak torque |
|
| Gardner et al. [26] | All subjects pedalled in 3 cycling conditions (neutral, 5° wedge, 10° wedge) with 80 W at 60 rpm | KOOS, VAPS | - | Peak flexion, adduction and external rotation angles | Three PRFs, Peak extensor, abduction and internal rotation moments | - |
|
| Silvis et al. [27] | Exercise sessions (including cycling/land treadmill/water treadmill) were completed three times weekly for 8 weeks. | WOMAC, KOOS, SF-12 | - | - | - | - |
|
| Raj et al. [28] | Group A and B subjects performed supervised exercise program with and without low-intensity cycling for a period of 4 weeks, respectively. | WOMAC | 6MWT | - | - | - |
|
| Buddhadev et al. [29] | All subjects performed 2-min bouts cycling at 4 workload-cadence conditions (75 W at 60 rpm, 75 W at 90 rpm, 100 W at 60 rpm, and 100 W at 90 rpm). | KOOS | - | Power outputs, Cadence, Asymmetry index | Anterior-posterior and normal PRFs | - |
|
| Kabiri et al. [30] | All patients participated in 12 supervised exercise sessions (treadmill/cycle ergometer/arm ergometer), 3 times/week for 8 weeks. | KOOS, VAS | 6MWT, TUG test, 30s chair stand test | - | - | - |
|
| Keogh et al. [31] | Two groups involved four unsupervised home-based MICT and HIIT cycling sessions (~25 min per session) each week for 8 weeks, respectively. | WOMAC, Lequesne index | TUG test, STS test, | Gait speed | - | Muscle mass, |
|
| Liu et al. [32] | The 3 exercise groups performed cycling, Tai Chi, and Baduanjin, respectively. The control group received basic health education. The intervention lasted 12 weeks with 5 days a week. | KOOS | - | - | - | - |
|
| Bhattacharya et al. [33] | Group A, B, and C performed forward cycling, backward cycling, and conventional exercise respectively for 4 weeks with 4 days a week. | WOMAC, NPRS | - | ROMs | - | - |
|
| Rezasoltani et al. [34] | The intervention group performed aqua-cycling and the control group followed lifestyle recommendations for weeks. | KOOS | - | - | - | Quadriceps and hamstrings muscle strength |
|
| Rewald et al. [35] | AC group performed cycling exercises of 45 min each 2 times per week for 12 weeks. The Control group received the usual care for 12 weeks. | KOOS, LEFS, PGA, NPRS | TUG test, 6MWT | - | - | Quadriceps muscle strength |
|
| Thompson et al. [36] | Subjects cycled with 4 pedalling conditions (neutral, 5° wedge, 10° wedge, 5° toe-in, 10° toe-in). 600 muscle-actuated inverse-dynamic simulations were performed. | - | - | - | Joint reaction forces and moments | EMG of BF, GM, RF, SM, TA, VM |
|
| Golightly et al. [37] | 12-weeks HIIT including cycling/treadmill was completed two times a week. | WOMAC | TUG test, 20m fast-paced walk test, 30s chair-stand test, Stair-climb test | - | - | Body composition, Isokinetic knee extensor and flexor peak torque |
|
| Smith-Ryan et al. [38] | Participants trained twice a week for 6 weeks for 12 training sessions on an electronically braked cycle ergometer. | WOMAC | Fasting blood draw, OGTT, VO2 peak test | - | Peak watts | Body composition, Lean and fat mass |
|
| Oiestad et al. [39] | The trial randomized participants to strength training, stationary cycling, and usual care groups for 12- weeks of structured training with 4-month and 1-year follow-ups. | KOOS, NPRS, Arthritis Self-Efficacy Scale, QoL index | VO2 peak test | - | - | Quadriceps strength |
|
| Rosadi et al. [40] | Groups 1 and 2 completed 12-week sessions of combined strengthening and stationary cycling intervention, walking, and physiotherapy modalities, respectively. Group 3 underwent 6-week physiotherapy modalities. | WOMAC, Quality of life of SF-36, VAS | TUG test, 6MWT, STS test | - | - | - |
|
Note: AC: Aquatic Cycling; BF: Biceps Femoris; EMG: Electromyography; GM: Gluteus Maximus; IKDC: International Knee Documentation Committee; IPAQ: International Physical Activity Questionnaire; KOOS: Knee Injury and Osteoarthritis Outcome Score; KOS-ADL: Knee Outcome Survey Activities of Daily Living subscale; LEFS: Lower Extremity Function Scale; NPRS: Numeric Pain Rating Scale; PGA: Patient Global Assessment; Prfs: Pedal Reaction Forces; RF: Rectus Femoris; ROM: Range Of Motion; SF-36: Short-Form 36; SM: Soleus Muscle; ST: Semitendinosis; STS: Sit To Stand; TA: Tibialis Anterior; TUG: Timed Up And Go; VAPS: Visual Analog Pain Scale; VAS: Visual Analog Scale; VL: Vastus Lateralis; VM: Vastus Medialis; 1RM: 1-Repetition-Maximum; 6MWT: 6-Minute Walk Test; OGTT: Oral Glucose Tolerance Test; QoL: EuroQoL 5D-5L.
Table 2: Characteristics of included studies.
Assessment of the included studies
The inter-rater reliability of the study assessments was 0.54, calculated using Cohen’s kappa statistic, which indicated a moderate level of agreement between the two assessors. All scores were checked by the third assessor (M.Z.). The final modified Downs and Black checklist scores, which ranged from 13 to 26 (out of 28), with a mean score of 18.76, are shown in Table 3. The assessment results of each assessor are presented in Supplementary Tables S2 and S3. One study was rated as ‘excellent’ quality, eight (38.10%) were rated as ‘good’ quality, nine (42.86%) as ‘fair’ quality, and three (14.29%) as ‘poor’ level. Inadequate justification of the sample size, control of confounders, and blinding of evaluators and participants resulted in low quality scores. The risk of bias in the reviewed studies is summarised in Figures 2 and 3. Green, red, and yellow indicate low, high, and unclear risks of bias, respectively. Most studies had a high risk of performance bias due to the non-blinding of participants. Estimation of sample size was performed in six studies. An inappropriate sample size increases the risk of violating the normal distribution. Nine studies reported effect sizes using Cohen’s d-statistic [23,26,29,31,32,34,41] or the Eta-squared statistic [10,30]. Most studies reported complete demographic information, but one studies did not report the age of the participants, and five studies did not report the Body Mass Index (BMI) or weight and height. Double-blinding was difficult to achieve in most of the studies, but the blinding of assessors was achievable to protect against detection bias. Blindness was indicated in eight studies. The number of participants who dropped out and the associated reasons were described in 13 studies.
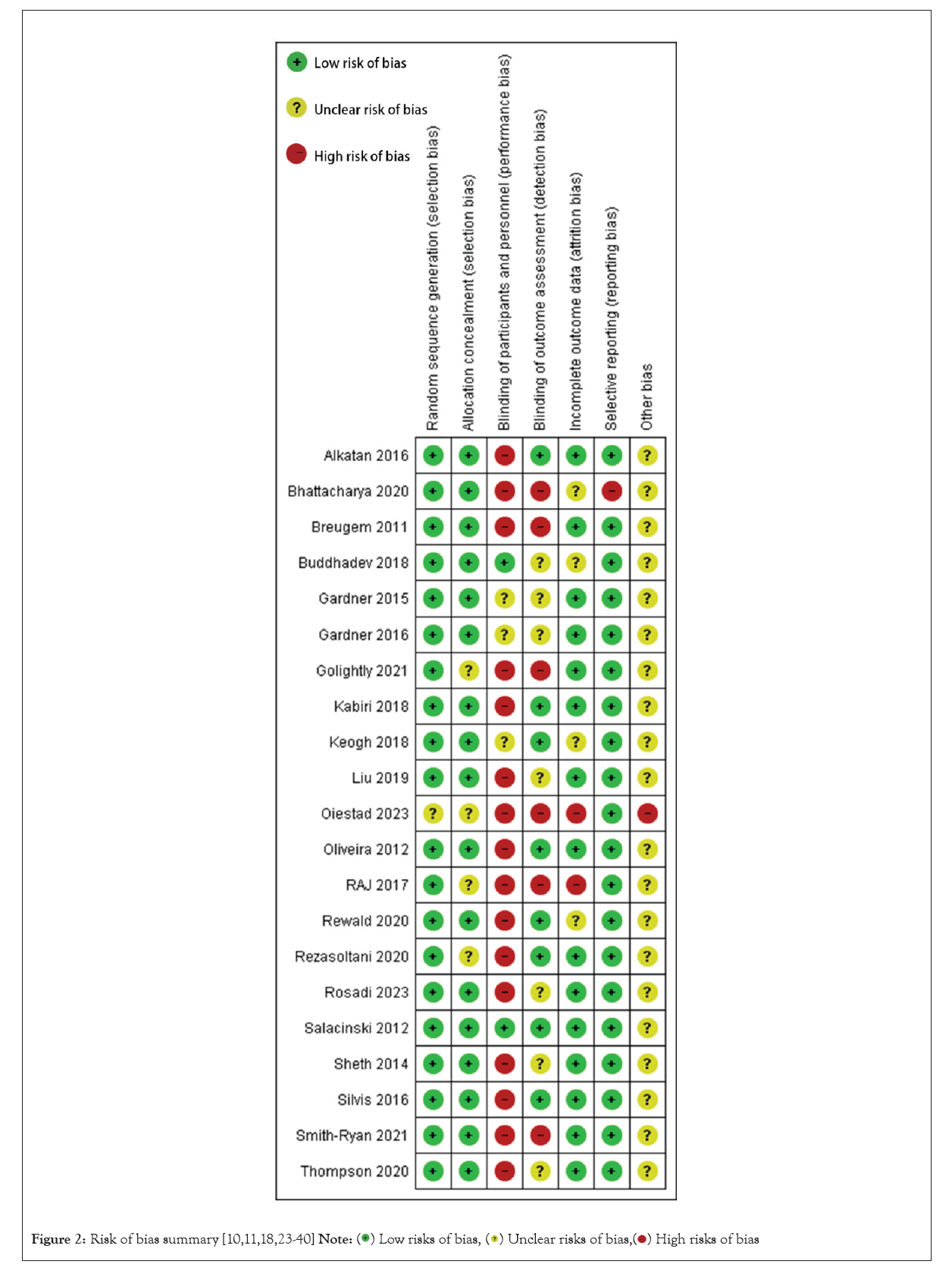
Figure 2: Risk of bias summary [10,11,18,23-40] 
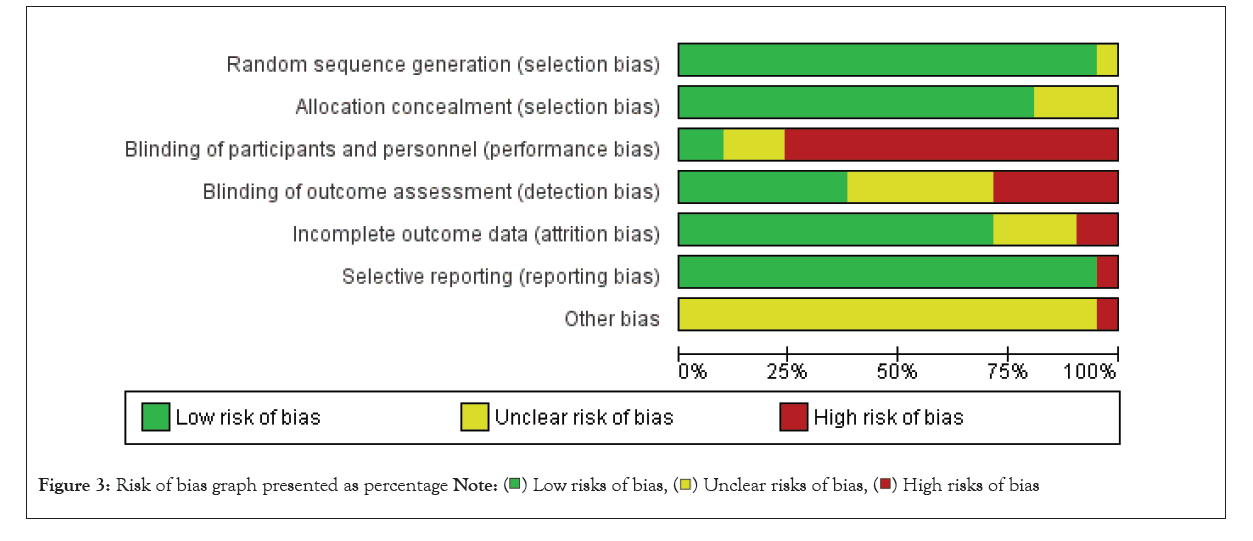
Figure 3: Risk of bias graph presented as percentage 
| Study | Research design | Assessment score | Quality level |
|---|---|---|---|
| Breugem et al. [18] | Descriptive research study | 17 | Fair |
| Oliveira et al. [23] | Randomized controlled trial | 21 | Good |
| Salacinski et al. [10] | Randomized controlled trial | 26 | Excellent |
| Sheth et al. [24] | Randomized controlled trial | 17 | Fair |
| Gardner et al. [25] | Case-control design | 20 | Good |
| Alkatan et al. [11] | Randomized controlled trial | 20 | Good |
| Gardner et al. [26] | Case-control design | 18 | Fair |
| Silvis et al. [27] | Randomized controlled trial | 18 | Fair |
| RAJ et al. [28] | Pretest-Posttest design | 14 | Poor |
| Buddhadev et al. [29] | Case-control design | 18 | Fair |
| Kabiri et al. [30] | Randomized controlled trial | 24 | Good |
| Keogh et al. [31] | Randomized controlled trial | 23 | Good |
| Liu et al. [32] | Randomized controlled trial | 23 | Good |
| Bhattacharya et al. [33] | Randomized controlled trial | 13 | Poor |
| Rezasoltani et al. [34] | Randomized controlled trial | 18 | Fair |
| Rewald et al. [35] | Randomized controlled trial | 20 | Good |
| Thompson et al. [36] | Case-control design | 18 | Fair |
| Golightly et al. [37] | Longitudinal study | 17 | Fair |
| Smith-Ryan et al. [38] | Longitudinal study | 20 | Good |
| Oiestad et al. [39] | Randomized controlled trial | 13 | Poor |
| Rosadi et al. [40] | Randomized controlled trial | 16 | Fair |
Table 3: Assessment scores for the included studies. Quality levels were excellent (scoring 26-28), good (scoring 20-25), fair (scoring 15-19), and poor (scoring ≤ 14).
Research methods
The duration of the long-term intervention training was usually 4–12 weeks. Assessment scales and functional motor tests were used to evaluate the rehabilitation effects before and after the training. The KOOS was used in 11 studies, the WOMAC in 10 studies, the Visual Analog Scale in six studies, the 36/12-Item Short Form Survey in five studies, the Lequesne index in 3 studies, and the 6 MWT and TUG test in six studies. To capture kinematic and kinetics data, a motion capture system (Vicon Motion Capture Inc., Oxford, UK) was used in three studies [26,25,36] and two video cameras were used to record the positions of reflective markers in one study [42]. Two studies used Visual 3D (C-Motion Inc., Germantown, PA, USA) to obtain joint moments [25,26], and Thompson et al. [36] performed inverse dynamics calculations using OpenSim (Stanford University, Stanford, CA, USA). In addition, muscle activation function was determined by EMG or dynamic simulation [36]. Methods, which are easier to execute but less informative, such as isokinetic muscle strength tests and functional physical tests, were used in six studies [10,11,34,35,37].
Outcomes related to knee joint
The results from 16 studies involving KOA assessment scales, 10 studies involving motor functional tests, seven studies assessing muscle function, four studies assessing joint moments, and four studies assessing PRFs were compiled for evaluation.
Assessment scales and tests
Six studies that examined WOMAC scores before and after CRT were included in the meta-analysis [10,11,22,25,37,38]. The analysis of WOMAC scores, as shown in Figures 4a-4f, revealed statistically significant results for pain (MD=3.02, 95% CI (2.21, 3.84), I² = 0%, p<0.001, Figure 4a), stiffness (MD=1.26, 95% CI (0.82, 1.70), I²=0%, p<0.001, Figure 4b), physical function (MD=8.39, 95% CI (5.56, 11.23), I²=0%, p < 0.001, Figure 4c), and total score (MD=12.03, 95% CI (7.31, 16.75), I²=0%, p<0.001, Figure 4d). Only two of the studies using the KOOS met the inclusion criteria [10,30], and therefore, a meta-analysis was not performed for KOOS. However, the subscales scores of KOOS for pain, symptoms, daily living function, sports function, and quality of life improved after CRT.
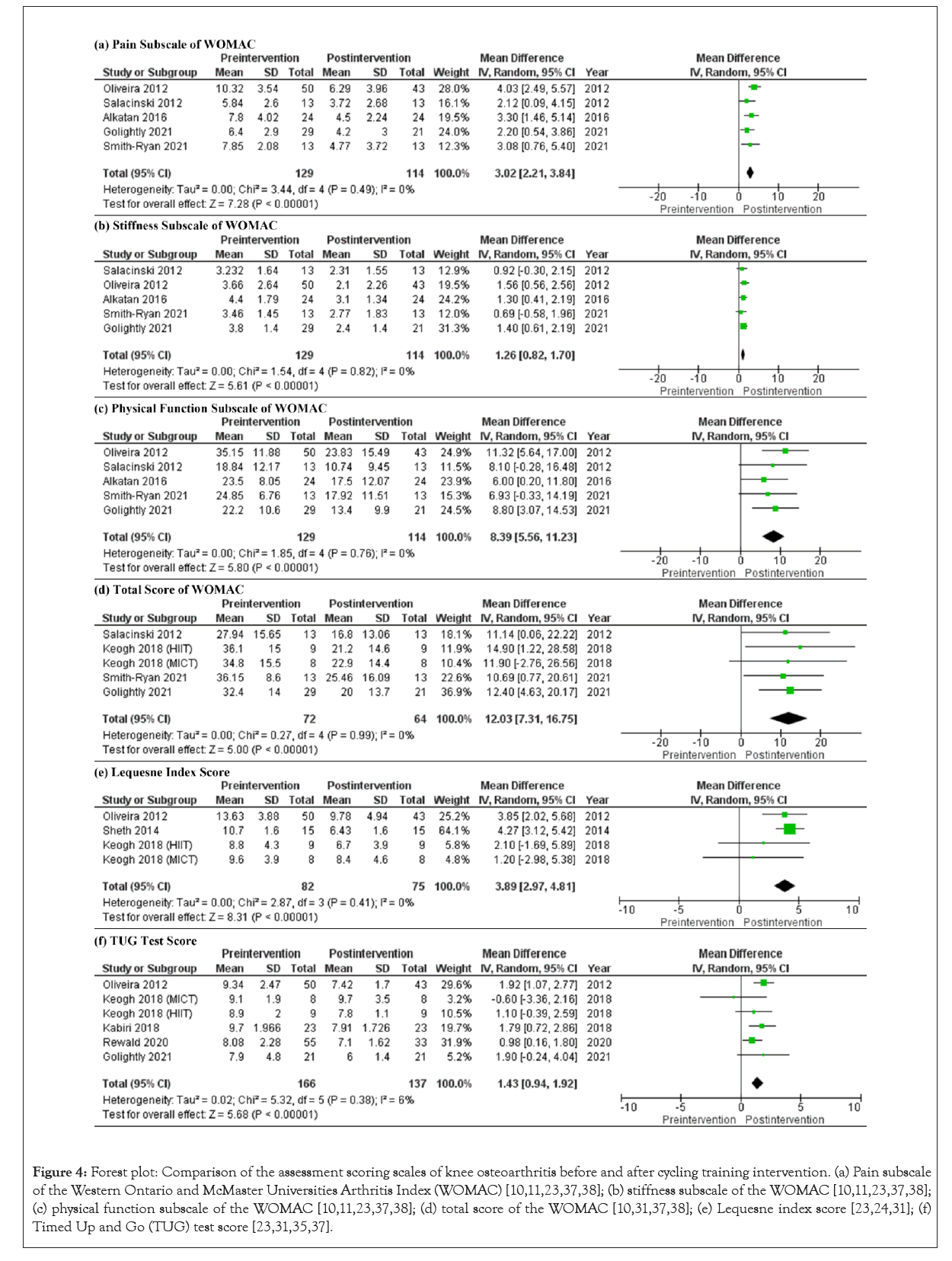
Figure 4: Forest plot: Comparison of the assessment scoring scales of knee osteoarthritis before and after cycling training intervention. (a) Pain subscale of the Western Ontario and McMaster Universities Arthritis Index (WOMAC) [10,11,23,37,38]; (b) stiffness subscale of the WOMAC [10,11,23,37,38]; (c) physical function subscale of the WOMAC [10,11,23,37,38]; (d) total score of the WOMAC [10,31,37,38]; (e) Lequesne index score [23,24,31]; (f) Timed Up and Go (TUG) test score [23,31,35,37].
For WOMAC outcomes, only two studies reported results for the CRT and control groups that met the requirements for meta-analysis. Figures 5a-5c show the results between group for pain (MD=1.85, 95% CI (-0.46, 4.16), I2=61%, p=0.12), stiffness (MD=1.07, 95% CI (0.32, 1.83), I²=0%, p=0.005), and physical function (MD=7.00, 95% CI (1.87, 12.13), I²=0%, p=0.007). These indicated that after experiencing the same amount of rehabilitation time, the CRT group had more significant WOMAC improvement than the control group.

Figure 5: Forest plot: Comparison of the assessment scoring scales of knee osteoarthritis between control and cycling training groups. (a) Pain subscale of the Western Ontario and McMaster Universities Arthritis Index (WOMAC) [10,23]; (b) stiffness subscale of the WOMAC [10,23]; (c) physical function subscale of the WOMAC [10,23].
The Lequesne index of OA severity was often used to evaluate the physical function of the joint [43]. The meta-analysis of this index included four groups of comparative data before and after cycling training from three studies [23,31,36]. High-Intensity Interval Training (HIIT) and Moderate-Intensity Continuous Training (MICT) groups were included as two separate outcomes, according to the data reported by Keogh et al. [31]. As shown in Figure 4e, cycling training had positive effects on Lequesne index scores (MD=3.89, 95% CI (2.97, 4.81), I²=77%, p<0.001). The sensitivity test results suggested that the high I² was caused by the high mean difference in the study of Sheth et al. [24]. The SD of the Lequesne index was much smaller in the study of Sheth et al. than in the other studies, due to differences in the contents and training frequencies of the CRT programmes. Additionally, Sheth et al. only implemented the CRT intervention for 3 weeks, whereas the other studies applied it for at least 8 weeks, which may have increased the SD of the results in the other studies.
The 6 MWT and TUG test were used as physical motion tests. However, as the measurement variables, such as the number of steps and walking distance, used in the 6 MWT in each study were not uniform, a meta-analysis was not possible. Figure 4f shows six groups of comparative data for the TUG test (MD=1.43, 95% CI [0.94, 1.92], I² = 6%, p<0.001) from five studies [23,30,31,35,37]. The HIIT and MICT results from the study of Keogh et al.'s [31] were analysed as two separate sets of data. The TUG test scores decreased significantly after CRT. The non-zero I2 was caused by the conflicting results between the MICT and HIIT groups in the study of Keogh et al. [31]. The conclusion that HIIT was better than MICT for KOA patients suggested the MICT programme may provide insufficient exercise.
Muscular strength
The isokinetic dynamometry [11,37] and physical motion tests [31], such as the 1-repetition maximum test [10] and the 6 MWT [11], are common methods for evaluating muscular strength. The strength of the isokinetic knee flexor and extensor, and the power of the muscle groups around the knee were evaluated using an isokinetic dynamometer. Alkatan et al. [11] found that a 12-week CRT programmes significantly increased the maximal isokinetic knee extensor and flexor strength, and this increased more significantly in the flexional direction. The increased maximal isokinetic strength ratio of the joint flexor and extensor suggested better muscular balance around the joint. Golightly et al. [37] evaluated changes in the average and maximum isokinetic strength during 12 weeks of HIIT. They demonstrated that CRT significantly enhanced isokinetic knee extensor strength and balance, with the greatest enhancement occurring in the first 6 weeks. Rezasoltani et al. [34] reported that 12 weeks of an aqua-cycling intervention significantly improved quadriceps and hamstring muscle strength. Similar changes in quadriceps strength were documented in another investigation [35]. EMG allows real-time recording of muscle activity signals during exercise [36,42]. Studies have shown that less activation of the Rectus Femoris (RF) and Vastus Lateralis (VL) muscles in affected limbs leads to a reduction in the peak net knee joint extensor moment [42]. Moreover, the study participants showed decreased fat mass, slightly increased muscle mass, and thus slightly decreased BMI after CRT [31,37].
Knee joint angles
The peak knee joint motion angles were reported with extension, flexion, adduction, abduction, and external rotation directions in two included studies. The peak angles of knee flexion, extension, and external rotation were reduced, and the peak angles of knee adduction and abduction were increased, in the KOA groups compared with the healthy groups [25,26]. The results showed that the frontal plane knee angles remained adducted for the entire crank cycle in most KOA participants. However, there was no significant statistical difference between the neutral, 5° toe-in, 10° toe-in, 5° wedge, and 10° wedge cycling conditions due to a limited amount of data. The authors also cautioned that this trend may not be truly meaningful.
Knee joint moments
The peak knee joint moments with extensor, flexor, abduction, and internal rotation directions were reported in five studies. Under the same cycling conditions, the internal rotation moments and abduction moments of KOA patients decreased compared with healthy participants [25,26]. The extensor moments of KOA patients increased under neutral cycling conditions, but these changes were not consistent under other cycling conditions. Gardner et al. [25] reported that the position of the foot relative to the pedal affected this moment. At neutral and 5° toe-in angle positions, KOA patients had a larger extensor moment than healthy subjects, but the opposite effect was observed by increasing the toe-in angle to 10°. These differences showed a trend but were not statistically significant. The joint torque in the experiment may have been affected by the variable cadence and power, which is a possible explanation for these findings [42].
Pedal reaction forces
PRF is a critical mechanical index in CRT. However, only a few studies measured PRF, and even fewer reported data that were eligible for the meta-analysis. Although Buddhadev et al. [29] measured anterior–posterior and normal PRFs, they only calculated the effective force and the effective crank torque. The post-TKA lower limbs of KOA patients produced lower PRFs than the other limb without TKA [41]. They found that all of the PRFs of limbs treated with TKA were lower except for the medial PRF under the cycling condition of 100 W. The studies by Gardner et al. [25,26] were more comparable because they used the same participants and cycling equipment. They examined the differences in PRFs between a KOA group and a healthy group under five cycling conditions. The vertical and medial PRFs were lower, while the posterior PRF was slightly higher, in the KOA group than in the healthy group.
Assessment scales and tests
The meta-analysis showed statistically significant differences in WOMAC, Lequesne index, and TUG test scores before and after CRT, and between the control and CRT group. Improvements in these assessment results and 6WMT scores for KOA patients were observed after CRT. These assessments are widely used for KOA patients in clinical practice [44]. Although there was variability in the meta-analysis results of the WOMAC pain scores between groups, this may be due to the small number of included studies. Additionally, subjective bias may exist. For example, the differences in individual pain tolerance may lead to inconsistent ratings of the pain subscale in the WOMAC and KOOS assessments [45].
Physical tests are relatively objective, and both scales and physical tests only evaluate the progress of knee function rehabilitation from a macro perspective. In comparison, medical imaging technology is a more accurate method to observe joint tissues at the microscopic level [46]. Moreover, motion capture systems can provide kinematic information. However, there is still a gap between the use of biomechanical indices in scientific research and clinical rehabilitation applications.
Muscular strength
Lower-limb muscle strength was measured in four studies using different methods, with all studies showing an improvement in the muscle strength of KOA patients after CRT [10,11,34,35,37]. However, only two studies reported a statistically significant difference in isokinetic knee peak torques before and after CRT [11,34]. This may be related to the different CRT programmes used and individual cycling modes, such as the degree of knee extensor engagement during the pushing period. However, even if the results were not statistically significant, muscle strength increased in all of the studies, demonstrating that CRT might strengthen the lower-limb muscles which deserve further investigation. Moreover, the muscle strength around the knee joint was associated with pain of knee joint [47]. The increased muscle strength may explain the changes in the pain subscale scores in the WOMAC, to some extent. However, these physical motion tests could only be performed before and after training.
EMG enables the real-time recording of muscle activity [48]. The onset and offset timing of the lower limb muscles of KOA patients were statistically different from those of healthy subjects [49]. The changes in the strength of the quadriceps were related to knee pain [50]. By comparing the sEMG ratio of the Vastus Medialis Oblique (VMO) and Vastus Lateralis (VL) muscles, Wills et al. stated that the Open Stance Cycling Protocol (OSCP) activated the VMO muscle more preferentially than the Traditional Cycling Foot Position (TCFP) [51]. This ratio is related to the range of motion angle, the angular velocity of the knee joint, and contraction type of the VMO and VL muscles [52]. Changes in this ratio reflect general improvements in muscle strength and the motor function of the knee joint. Therefore, OSCP may be a better choice for patients. Larger amplitudes of the root mean squared and integrated EMG signals were discovered with increasing power at the same cadence. However, these amplitudes were not always linearly correlated with cadence at the same power [53].
In addition, EMG signals may reflect both muscle strength and symmetry between limbs. The shape symmetry index and area symmetry index are calculated from the linear envelopes of EMG signals [54]. These variables have been used in real-time feedback training systems to help patients improve their cycling smoothness. The improved cycling performance including smoother cycling, more power output and symmetry are likely to result from better control of the rectus femoris muscle activation with visual feedback [54]. Tim et al. successfully alleviated the knee pain of a cyclist using the ComputrainerTM feedback system which can display force outputs of the right and left lower limbs throughout the stroke cycle [55]. Adjusting muscle asymmetry subjectively by real-time feedback would be better for patients than changing external conditions.
Kinematics of the knee joint
Cross-sectional studies have shown that an increased knee abduction angle is common in patients with knee diseases compared with healthy individuals [56]. As legs mainly move in the sagittal plane when cyclin, the increased motion angle of the knee joint in the coronal plane indicates decreased stability of the knee and an increased risk of injury [57]. Moreover, people with KOA may have reduced peak flexional and external rotation angles of their knee joints, but increased peak adduction knee angles compared with healthy people [25,26,41]. These results may be due to the reduced motion range of the ankle joint in the dorsiflexion direction, which limits the flexion of the knee joint leading to an increase in knee abduction angle and a decrease in knee flexion angle [58]. Therefore, these joint motion angles may be related to the progression of KOA. As the extension and flexion angles of the knee joint were significantly affected by bicycle configurations such as the saddle height, the angles of motion in the abduction direction were less affected [59]. Furthermore, KOA is often accompanied by pain on the medial side of the knee joint [60]. To relieve this pain, patients tend to avoid movement of the medial joint and increase the abduction motion angle of the knee joint [61]. Therefore, a CRT programme may be designed to reduce the knee angle during the abduction motion.
Fatigue is also a cause of increased joint motion angle [62]. As fatigue sets in, muscle stiffness increases, and the muscle force produced by each contraction decreases. The cyclist may increase the motion angles of their knee joint, especially in the adduction and the external rotation directions, to maintain the original joint moments and cycling intensity [62]. Another study confirmed that the changes in motion angles of the ankle and knee joints were positively and synchronously correlated with changes in median EMG frequencies [63]. Therefore, analyses of changes in kinematics should account for the contribution of fatigue in cycling experiments lasting more than 30 minutes.
Kinetics of the knee joint
The Musculoskeletal (MSK) multibody dynamic model is commonly used to obtain joint forces and moments. Knee pain is mainly due to abnormal external and internal moments of the knee joints which can be represented as the net efforts of the surrounding muscle. The Knee Abduction Moment (KABM), a surrogate variable for loading in the medial compartment of the knee [64], is an important indicator associated with KOA [65]. A positive correlation was found between the K-L grade and the peak of the abduction moment [25]. In addition, the KABM did not change as saddle height increased within a peak knee extension angle ranging from 20° to 40°, but the knee extension moment decreased [66]. Bini's analysis of three-dimensional knee loads also illustrated that changes in saddle height have a minimal influence on knee abduction/adduction moments and forces, but a considerable influence on knee extension moment and temporal patterns of knee flexion [59]. KOA patients tended to increase the abductive rather than the adductive motion angles of the knee joint. A change in KABM would be more representative of this phenomenon. Although KABM may be affected by the joint motion angles of ankle-dorsiflexion and muscle activation of the gluteus maximus [67], both of these factors are internal factors of the patients. KABM may be a more stable variable than other moments when different external cycling conditions are configured. Therefore, KABM may be a feasible indicator of the recovery of KOA patients. Recently, some attempts have been made to rectify the abnormal KABM in KOA patients by modifying bicycle configurations and cycling postures. A 22% decrease in the peak KABM can be achieved using a 10° lateral shoe wedge [26]. Cycling under the 5° and 10° toe-in angle conditions resulted in a 61% and a 73% decrease in peak KAM, respectively, compared to the neutral condition in participants with KOA [25].
Joint power, which is calculated from the product of the joint moment and the angular velocity, represents the contribution of each joint to the crank power output. The knee and hip joints contribute up to 80% of total joint power [68]. As KOA patients produce abnormal joint moments during cycling, their joint power would also differ from the joint power of healthy people at the same cadence. Consequently, joint power may be an alternative variable for joint moments and an indicator of KOA progression. The joint power is divided by the crank power to obtain the relative contribution of each joint to cycling [63], which can be used to assess joint motion coordination, which cannot be reflected by joint moment. However, few studies have examined the joint power of KOA patients, and this needs further exploration.
The joint force of the knee joint is very important for KOA recovery. Kutzner et al. [69] directly measured the tibiofemoral contact forces of TKA patients in-vivo during cycling using instrumented knee implants. The lowest forces were observed when cycling at a low power level, a high cadence, and a high saddle height [69]. This conclusion was consistent with the finding of Fang et al. [70] that the increased cadence at a constant workload resulted in increased peak medial and vertical PRFs. As PRFs are transmitted indirectly through the ankle to the tibia, they change synchronously with tibiofemoral contact forces to some degree. However, in-vivo measurements are invasive and their use greatly limits the sample size. FE analysis can be used to calculate the stress distribution of the knee joint. Many FE knee models have been used to study patients with TKA [71] or ACL [72]. However, few such studies have been conducted on KOA patients, especially under cycling conditions. The development of an FE model would facilitate an exploration of the biomechanics of cycling and relevant rehabilitation indicators for KOA patients.
Pedal reaction forces
A positive correlation was revealed between knee joint forces and PRFs [73]. PRFs may reflect joint loading, and they are much easier to measure than joint forces. Many studies have focused on the vertical PRF followed by the tangential force perpendicular to the frontal plane and the shear force perpendicular to the sagittal plane. The symmetry and efficiency of cycling may be indicated by PRFs. Because of pain, KOA patients tend to use their healthy side as the preferred limb. This asymmetry during cycling may affect the results of CRT [74]. Interlimb asymmetry was found to decrease as the pedalling rate increased [75]. However, cycling training intensity for KOA patients should be moderate. The relationship between cycling cadence and symmetry is worth considering, especially for KOA patients.
The effective force is the force perpendicular to the pedal surface. Cycling efficiency is determined by the proportion of the effective force to the total force. Patients tend to cycle less efficiently than healthy people. They may waste more force in acting towards the axis of the crank and in the opposite direction of pedalling [76]. Consequently, real-time feedback on PRFs has been recommended as it may help patients improve their cycling efficiency [77]. Although PRF data from previous studies could not be used to explicitly reflect the recovery of patients’ lower limbs, providing patients with real-time information on PRFs may help to promote rehabilitation and assess the effectiveness of CRT.
Study limitations and future research directions
A review of previous studies and the results of a meta-analysis support the opinion that cycling benefits recovery from KOA. However, this study also has some limitations. First, the number of studies of CRT for KOA patients is limited. Further, the criteria used to recruit subjects varied widely among the studies included in this systematic review. Most studies used the K–L grading scale as a diagnostic criterion [78], but the selection range of grades was not uniform. Recruiting subjects with the same score, rather than within a score range, would reduce the bias of the results. Second, insufficient control of confounders resulted in low quality scores for the reviewed studies. Most KOA patients are middle-aged or older and women have more severe OA in the knee joint than men [79]. Similar age and gender distributions between the patient and healthy control groups were confirmed in most studies. However, many studies did not fully report height and weight, which significantly affect bike fitting and the kinematics of cycling. Third, the included studies were limited to macroscopic evaluations of motion ability, and the changes in kinetic indices are inconclusive.
In future studies, more KOA patients should be recruited for long-term CRT experiments, to increase the generalisability of the findings. Patient recruitment criteria and demographic information need to be elaborated in more detail. The interactive effects of the cycling conditions for KOA patients, such as work rate and saddle height, should also be considered [80]. The development of FE models for the knee joint and MSK multibody dynamics models for cycling would provide more comprehensive biomechanical information. Additionally, as static bike fitting cannot distinguish differences between patients and healthy people, it is necessary to improve and apply the dynamic bike-fitting method which has higher precision [81].
We performed a systematic review and meta-analysis of the WOMAC, Lequesne index, and TUG test scores. CRT was found to reduce joint pain and stiffness and improve the mobility of KOA patients, with a significant improvement in pain. Therefore, cycling may be used for the rehabilitation of KOA patients. Compared with healthy people, KOA patients tended to show a decreased flexion angle and an increased abduction angle and moment under the same cycling conditions. The angles and moments of the knee joint of KOA patients in other directions were also different compared with healthy individuals, but these changes were inconsistent across studies. KABM may be used as a kinetic indicator of the progression of KOA due to its correlation with K–L grades and stability when external cycling conditions change. The results of both EMG and physical tests indicated that CRT contributed to the recovery of lower-limb muscle strength. However, as few studies met the quantitative analysis requirements, these results may not be generalizable. Furthermore, the development of dynamic simulation and calculation models may provide theoretical guidance for formulating effective personalised training programmes in future studies.
This study was supported by the National Natural Science Foundation of China (11732015, 11972315). There are no other funding sources.
F.B. performed the literature search, completed data extraction, and wrote the first draft of the manuscript. F.B. and S.F.C. contributed to the assessment of the study quality and risk of bias, and the interpretation of the results. Y.W., S.F.C., and G.Z. provided critical feedback and commented on the manuscript. Y.W. and M.Z. helped with the supervision of the project and provided critical comments. All authors reviewed the manuscript.
The authors declare no competing interests
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Bing F, Wang Y, Chen SF, Zhang G, Zhang M (2023) Effects of Cycling Rehabilitation Training on Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Rheumatology (Sunnyvale). 13: 362
Received: 16-Jun-2023, Manuscript No. RCR-23-25146; Editor assigned: 19-Jun-2023, Pre QC No. RCR-23-25146 (PQ); Reviewed: 03-Jul-2023, QC No. RCR-23-25146; Revised: 10-Sep-2023, Manuscript No. RCR-23-25146 (R); Published: 17-Sep-2023 , DOI: 10.35841/2161-1149.23.13.362
Copyright: © 2023 Bing F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.