
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2023)Volume 13, Issue 2
Di-(2-Ethylhexyl) Phthalate (DEHP), used as a popular plasticizer to enhance the flexibility of plastics, is a major pollutant in aquatic environments. DEHP poses severe risks to aquatic organisms since it is an endocrine-disrupting compound. To comprehensively evaluate the toxicity of DEHP on the growth and livers of male Xenopus tropicalis (X. tropicalis), sexually mature male X. tropicalis were exposed to environmentally relevant concentrations of DEHP, 0.2, 0.6, 1.8, 5.4 mg/L, for 49 days. The results showed that DEHP had a severe toxic effect on the livers of male X. tropicalis. Histopathological analysis of livers in all the DEHP-exposed groups showed changes in terms of vacuolization, loose cell cords, and an increasing amount of melanin. Large lipid droplets were markedly formed, and there were changes in the mitochondrial morphology upon DEHP exposure. In addition, oxidative stress (excessive ROS and inhibition of antioxidants) was induced through the suppression of biochemical indicators and the down regulation in the mRNA expression of genes (nrf2, CAT, SOD, GST, and GPX) related to oxidative stress. A reduction in the expression of the fatty acid metabolism-related gene (PPARα) was seen post-DEHP exposure. Thus, our study suggests that the hepatotoxicity induced by DEHP could be attributed to oxidative stress and disordered fatty acid metabolism. In conclusion, long-term exposure to DEHP at environmentally relevant concentrations poses ecological risks to aquatic organisms, which serves as a reminder that the application of DEHP and other plasticizers should be limited.
Xenopus tropicalis; Di-(2-ethylhexyl) phthalate; Oxidative stress; Lipid metabolism; Environmentally relevant concentrations; Environmental toxicology
Di-(2-Ethylhexyl) Phthalate (DEHP) is a plasticizer that is widely used in the manufacture of flexible plastics and plastisol [1]. In addition to its close correlation to the plastic production industry, DEHP is also used as an additive in cosmetics, pharmaceuticals, and other fields [2-4]. Plastic production, with DEHP applications, owns a worldwide annual production of plastics as high as 394,000 tons [5,6]. As the non-covalent bonding with polymer materials, DEHP is unstable and easily releases out into the environment upon alteration of environmental conditions [7]. It is worth noting that a variety of foods contains DEHP for emulsifiers. As a result, humans are exposed to DEHP by diet, inhalation and skin absorption, which causes a hazard to humans [8-11]. EHP is listed as a priority pollutant by The Chinese General Environmental Monitoring Station and The US Environmental Protection Agency [12]. Since it is closely associated with high risks to both humans and the natural environment, the ecotoxicity of DEHP deserves further investigation.
Surface water is the main environmental media, which the DEHP has been detected [13]. Our study presented the environmental concentration distribution of DEHP in surface water around the world (Supplementary Table S1), which includes data from some freshwater regions (rivers, lakes, source water, ponds, and estuaries). The lowest concentration in China was detected in the Changjiang River Estuary, at 0.26 ng/L [4,14]. The Yellow River basin, one of the drainage basins that suffers from the most severe DEHP contamination in China, has been reported to have a DEHP concentration of up to 6.35 mg/L [15]. The detection frequency of DEHP reached up to 95% in most of the aquatic mediums and the highest concentration in China was detected in the Liao River, at as high as 13.05 mg/L [1,16]. In other parts of the world, the occurrence of DEHP in surface water has been monitored. A concentration of DEHP exceeding 2 mg/L has been reported in South Africa, while the lower level (0.33-97.8 μg/L) was detected in the surface water of the USA and Canada. Xenopus tropicalis, a species living in surface water, possibly suffered the threat and ecological risk posed by DEHP.
In the past, plenty of reports have been determined the toxic effects of DEHP on animals, such as damages on embryo development, reproduction, immune system and liver function [15-18]. However, the studies about hepatotoxicity mostly focused on the toxicity of DEHP to mice, birds and fish. After long-term exposure to DEHP in Coturnix japonica, there was an occurrence of hepatotoxicity mediated by alterations in mitochondrial function and redox homeostasis [18]. A recent study found that the inhibition of PPARα or PPARγ caused by DEHP was related to oxidation in the liver of mice [19]. There are some relevant studies on amphibians. At 3.9 mg/L DEHP, up to 21.2% of Western clawed frog embryos appeared tail abnormalities. The consequence of DEHP on Rana chensinensis tadpoles was weight loss and the reduction of the abundance of butyrate-producing bacteria. The study rarely pays attention to hepatotoxicity, and the potential mechanisms of hepatotoxicity on amphibians deserved more exploration. Thus, our study investigated the hepatotoxicity of DEHP on amphibians in terms of pathological and molecular biology assessments.Compared to other animal models, X. tropicalis is an ideal model animal for toxic experiments, because its genomic library has been constructed completely and it is similar to humans in many biological processes [3,20]. Thus, in this study, male X. tropicalis were exposed to DEHP (0.2, 0.6, 1.8, and 5.4 mg/L) for 49 days, to identify the ensuing toxic effects. The main aims of the current study were to: (1) Identify the organs targeted by the toxic effects of DEHP through the developmental index (total body weight and hepatosomatic index) and pathological assessment; (2) Determine the toxic effects of DEHP on the target organs by means of biochemical assessment; and (3) Clarify the mechanism of the toxic effects using real-time quantitative reverse transcription PCR (RT- qPCR).
Maintenance of X. tropicalis
Throughout the whole experiment, adult X. tropicalis were selected in aquariums with circulating system that was kept running continuously in order to ensure adequate dissolved oxygen (6.0 mg/ L<concentration<7.5 mg/L). The aquariums were installed in our laboratory, maintained the temperature in the range of 26.0°C to 26.5°C, and provided a light supply with 12 h light and 12 h dark. In addition, exposure solution was monitored in these conditions: pH 7.4 ± 0.5; hardness 128.5 ± 15.2 mg/L, in terms of CaCO3.
Analysis of DEHP in solution
The practical concentrations of DEHP were measured in T0 (at the time of replacement) and T48 (48 hours after replacement). The exposure solution for each treatment was used for a gas chromatography tandem mass spectrometer (7890B-7000C, Aglient, California, USA). The analytical conditions were provided in supplementary information.
Chronic exposure and tissue pre-processing
The male frogs were randomly selected and exposed to the following treatments for 49 days: Control group (0 mg/L, contained 0.01% dimethyl sulfoxide), 0.2 mg/L, 0.6 mg/L, 1.8 mg/L, and 5.4 mg/L of DEHP. A series of DEHP treatments were produced by diluting the DEHP stock solution (containing 0.01% Dimethyl Sulfoxide, DMSO) in dechlorinated tap water, while the control group contained DMSO (water with 0.01% DMSO). Each group contained 6 individuals, and the experiment was conducted in an aquarium (density: 1 frog/5 L). It is necessary to highlight that the lowest exposure concentration of 0.2 mg/L was chosen based on the DEHP level reported in the Haihe river [21]. During the exposure period, the frogs were fed every two days, and the exposure solution of each treatment was replaced every 48 h, to maintain an accurate concentration of DEHP. The total body weight of the frogs was recorded every seven days. After 49 days of exposure, the frogs were frozen on ice for dissection, and livers were rinsed and immediately weighed on an electronic scale, following which they were divided into 4 pieces for further assessment. Two hundred milligram samples were fixed in 4% paraformaldehyde for histological analysis and one hundred milligram samples were fixed in 1% osmic acid solution for ultrastructural analysis. One thousand milligram samples were snap-frozen in liquid nitrogen and stored at 80°C for biochemical analysis. Five hundred milligram samples were placed in tubes with Trizol Reagents (Ambion™, ThermoFisher, USA) and stored at -4°C for 24 h, used for RT-qPCR. The Hepatosomatic Index (HSI) was expressed as the ratio of the weight of livers to the total weight of frogs. All animal protocols and procedures were approved by the Institutional Animal Care and Use Committee of the Guangdong University of Technology (Ethics number: GDUTXS002).
Histological analysis
Livers tissues were fixed with 4% paraformaldehyde for 24 h. Afterward, dehydration of livers was carried out in a gradient concentration of alcohol. Following that, tissues were embedded in paraffin wax, sectioned into 4 μm-thick thickness slices, and stained with Hematoxylin-Eosin (HE). Finally, the processed slices were observed under an optical microscope (CX43, Olympus, Japan) for histological analysis.
Based on the hepatosomatic index and histopathological changes, the liver is speculated to be one of the targets of DEHP in male X. tropicalis. After fixation, tissues were placed in a 15% sucrose solution at 4°C and then transferred into a 30% sucrose solution for dehydration. The processed tissues were embedded with optimal cutting temperature compound (Solarbio Bioengineering Institute, Shanghai, China), sectioned at 8-10 μm, and stained with Oil Red O. Oil Red O was used to identify the lipid droplets and measure the degree of lipid accumulation. Slices were observed and photographed under an optical microscope (CX43, Olympus, Japan). The area of the lipid droplets (stained by Oil Red O) was analysed using Image J software (NIH, Windows version, USA), according to a previous study [22].
Ultrastructural analysis
Fresh liver tissue was cut into 1 mm3 piece, fixed in 1% osmic acid solution (pH=7.4), and then rinsed three times in phosphoric acid buffer (pH=7.4). After dehydration in a gradient concentration of alcohol, the tissues were penetratively embedded in an embedding agent. The resin block in the ultrathin slicer was cut into 60-80 nm ultrathin slices (15 UL, Daitome, Swiss Confederation), stained with 2% uranium acetate-saturated alcohol solution, and observed under a transmission electron microscope (HT7800/HT7700, Hitachi, Japan), and analysed.
Biochemical analysis
The Reactive Oxygen Species (ROS) content was measured using the Dihydroethidium (DHE) method [23]. Fresh livers sample from 6 individuals per treatment were used to conduct the assay. Frozen slices were incubated in DHE solution for 30 min at 37°C, and then washed three times with phosphate-buffered saline (pH=7.4). The residual liquid was cleaned, and the sections were buried in an antifade mounting medium. The treated sections were scanned using fluorescence microscopy (Eclipse C1, Nikon, Japan) at an excitation wavelength of 510-560 nm. The fluorescence pictures were captured, and the intensity of fluorescence was used to determine the ROS content using Image J software (NIH, Windows version).
Determination of antioxidant capacity included assessments of the activities of total superoxide dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), and Glutathione-S-Transferase (GST), as well as, Malondialdehyde (MDA) and Glutathione (GSH) content. Fresh livers samples from 6 individuals per treatment were ground with saline solution at 4°C. Centrifuged at 3500 × rpm for 10 min, the supernatants were used to determine the antioxidant capacity. The antioxidant index was measured using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the specifications of the manufacturer. SOD activity was determined based on the degree to which hydroxylamine can be oxidized by superoxide anion radicals [24]. CAT activity was determined based on the consumption of hydrogen peroxide [25]. GPX activity was evaluated in terms of the reduced glutathione consumed in the body [26]. The content of GSH was evaluated in terms of the combination of sulfhydryl with 5, 5-dithiobis (2-nitrobenzoic acid), which can reflect the activity of GST [27]. MDA can combine with Thiobarbituric Acid (TBA) resulting in the generation of red products at 532 nm [28]. The activity unit (U per mg protein; U per g protein) was performed to reflect the antioxidant capacity. The analysis of the total protein content of individuals from treatments was according to the reaction of anion and -NH3+.
qRT-PCR
Total RNA was extracted using Trizol reagent (Ambion™, ThermoFisher, USA) and rinsed with 75% alcohol to remove the impurity and residual liquids. An ultra-micro spectrophotometer (NanoDrop One™, ThermoFisher) was used to measure the concentration and quality of the obtained total RNA. cDNA synthesis was performed using the qPCR reverse transcription kit (FSQ-101, Toyobo, Japan) with efficient Oligo dT primers in a PCR amplifier (Mastercycler®, Eppendorf, Germany). The amplification of cDNA was performed using SYBR™ Green qPCR Mix (QPS-201, Toyobo) and a real-time PCR detection system with a two-step qRT-PCR procedure and a CFX Connect Module (Bio- Rad, Berkeley, USA). The reaction volume was 20 μL under the following conditions: Denaturation at 95°C for 1 min, followed by 40 cycles at 95°C for 15 s and 60°C for 45 s. Detailed information about the primer sequences used is presented in supplementary information, Supplementary Table S2. The relative expression (fold change of control group) of the target genes was calculated using the 2−ΔΔCt method and the gene ornithine decarboxylase was used as the reference gene [28]. Triplicate replicates were set for each RNA sample from different treatments.
Statistical analysis
Data have been expressed as mean ± Standard Deviation (SD), and the data were tested for normality. Total body weight, the relative weight of organs, fluorescence levels, the content of antioxidants, and related expression of genes were statistically analyzed by oneway ANOVA. The Least Significant Difference (LSD) test was used to determine significant differences between the control and DEHP treatment (version 26.0; SPSS, USA), and the differences were considered statistically significant at *P<0.05, and **P<0.01.
Exposure concentration
Measurement of the concentrations of Di-(2-Ethylhexyl) Phthalate (DEHP) was displayed in Supplementary Table S3. The deviation between the nominal concentrations and experimental concentrations of DEHP was less than 20%. Thus, the nominal concentrations of DEHP can represent the experimental concentrations of DEHP used in the study.
Effects of DEHP on the hepatosomatic index of male Xenopus tropicalis
The hepatosomatic index was measured at the end of the exposure, to assess the chronic toxicity of DEHP in male X. tropicalis. The liver is an important organ involved in the metabolic process and detoxification process of vertebrates [30]. In the present study, HSI was significantly increased (Figure 1, P<0.05). The increase in HSI of the liver may be due to disturbed lipid metabolism and lipid accumulation [31,32]. In a relevant study, Zhang Q, et al. [18], illustrated that DEHP could cause inhibition in HSI by restraining the metabolism of toxicants and amassing the toxic effect in the organisms. The results indicated that the liver may be one of the targets for DEHP toxicity to male X. tropicalis. Moreover, the liver is an organ that is directly attacked by xenobiotics, making it a suitable organ for assessing the toxicity of external pollutants. Thus, further studies need to be conducted on the hepatotoxicity caused by DEHP in male frogs.
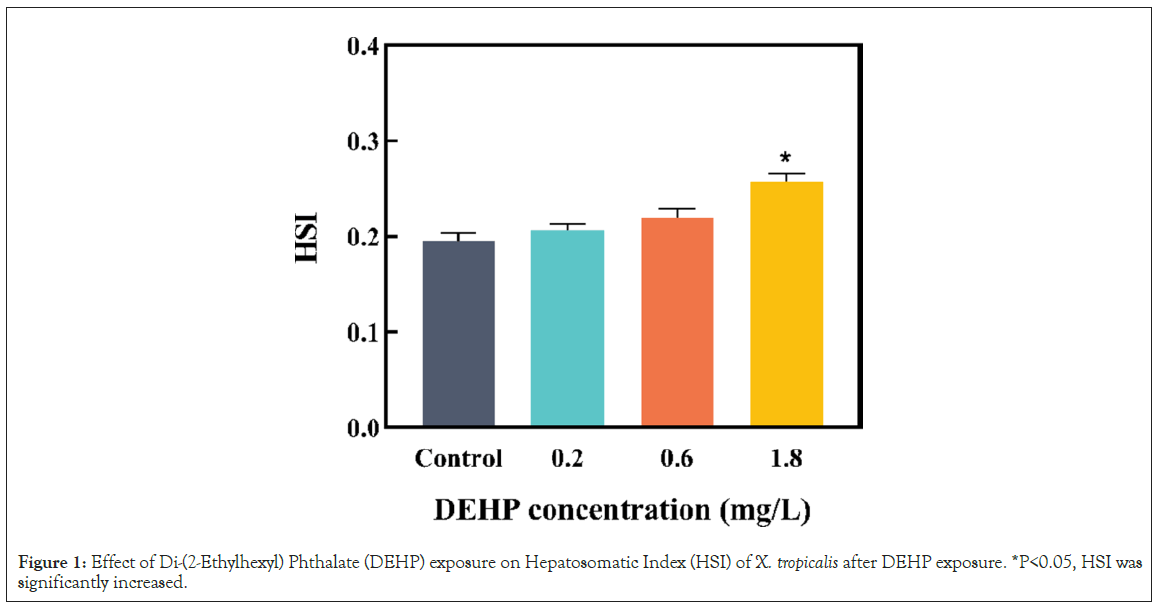
Figure 1: Effect of Di-(2-Ethylhexyl) Phthalate (DEHP) exposure on Hepatosomatic Index (HSI) of X. tropicalis after DEHP exposure. *P<0.05, HSI was significantly increased.
Effects of DEHP on the histopathology of livers
HE staining was applied to observe the cellular morphology, to determine whether DEHP caused structural damage to livers. On the other hand, Oil Red O stains the lipids, determining lipid content in livers. The toxic effects mediated by DEHP on livers were further explored through histopathological alterations.
The liver samples were subjected to HE staining (Figure 2). Normal hepatocytes, distributed in a concentric circle, with obvious and neat morphology, were observed in the control group (Figure 2a). Exposed to DEHP, the hepatocytes exhibited vacuolization of cytoplasm and lose cytoplasm (Figure 2b-2e). In the histopathological result of 5.4 mg/L DEHP, some hepatocytes were filled with nuclear shrinkage, swelled cells, and darkening of color. These results indicated that long-term exposure to DEHP can damage the structure of the liver. Destruction of hepatocytes caused by DEHP has been reported in other animals. In a previous study on quail, severe vacuolization, disorderly permutation of hepatocytes, and squeezed nucleus were found following exposure to a dose of 1000 mg/kg DEHP [18]. Our findings were in line with the outcome of a study by Ito Y, et al. [33], which showed that DEHP can cause fragmentation and necrosis of hepatocytes in Rattus norvegicus.
Figure 2: Histopathological images of Hematoxylin-Eosin (HE) staining in the livers of male X. tropicalis under (a1-e1) 100X and (a2-e2) 400X magnifications. (White arrows, black arrows, and yellow arrows show vacuolization of cytoplasm, loose cell cords, and solidification of hepatocytes, respectively; a, b, c, d and e represent livers from 0, 0.2, 0.6, 1.8 and 5.4 mg/L DEHP treatments, respectively).
The liver samples were subjected to Oil Red O (Figure 3) staining. DEHP caused pathological changes in the form of larger melanin and larger lipids (Figure 3a-3e). Very small lipid drops were observed in the control group (a). Conspicuous and larger melanin was observed in the 0.2 and 0.6 mg/L DEHP treatments, because the important role of melanin is an important scavenger of xenobiotics and a powerful antioxidant for the prevention of oxidative stress (Figure 3b-3c); [33-35]. The melanin in livers was probably present in macrophage aggregates [36,37]. On the contrary, the amount of melanin decreased in the 1.8 and 5.4 mg/L DEHP treatments, possibly due to the occurrence of oxidative stress and severe damage to the antioxidant system; (Figure 3d-3e). Figure 3f shows that the area of lipid droplets in different DEHP-treated (0.2, 0.6, 1.8, and 5.4 mg/L) livers samples markedly increased in a dose-dependent manner by 0.61%, 0.69%, 0.83%, and 1.04% (P<0.01), respectively, compared with the controls. The results reflected the accumulation of lipids following DEHP exposure. Our study agreed with a previous study that indicated DEHP could increase the lipid content in the livers of Zebrafish (Danio rerio). The histopathological alterations of liver caused by DEHP, such as abnormalities of hepatocyte and accumulation of lipids, may be attributed to the disturbed metabolism of toxicants in the liver [18]. Furthermore, the alterations of the structure could possibly cause liver dysfunction and lead to necrosis of cells, which emphasized the importance of the ultrastructural analysis of livers [19].
Figure 3: Histopathological images of Oil Red O staining in the livers of male X. tropicalis under (a1-e1) 100X and (a2-e2) 400X magnifications. (f) Oil Red O-stained area in the livers. a, b, c, d and e represent livers from 0, 0.2, 0.6, 1.8 and 5.4 mg/L DEHP treatments, respectively; **P<0.01, there is a significant difference from the control group; n=6).
Effects of DEHP on the ultrastructure of livers
The liver slices were further scanned using Transmission Electron Microscopy (TEM), to observe the hepatocyte microstructure of the hepatic cell membrane and organelles. The control treatment showed an integral structure of hepatocytes, including a welldefined nucleus, a large number of oval-shaped mitochondria and endoplasmic reticulum, and tiny lipid droplets. Moreover, the nuclear membrane and chromatin of the nucleus were evident and closely arranged, with no diffusion of chromatin (Figure 4a).
Figure 4: The ultrastructure of livers of male X. tropicalis after DEHP exposure. (Blue arrow: Normal mitochondria; white arrow: Normal nucleus; red arrow: Chromatin margination; yellow circle: Lipid drops; blue circle: Swollen mitochondria; a, b, c, d and e represent livers from 0, 0.2, 0.6, 1.8 and 5.4 mg/L DEHP treatments, respectively; scale bar: 10.0 μm).
A reduction of mitochondria and the larger lipid droplets within the same field of view were observed in the 0.2 and 0.6 mg/L DEHP treatments (Figure 4b-4c). With exposure to 1.8 and 5.4 mg/L DEHP treatments, swollen and deformed mitochondria were observed, and the chromatin margination dispersing the nuclear membrane has slightly occurred (Figure 4d-4e). As a result, DEHP could cause mitochondrial abnormalities and accumulation of lipids. Lipid accumulation caused by DEHP corroborated the histopathological findings about the production of lipid droplets. Mitochondria are the target of exogenous toxic substances [19], which clarifies that the liver is the target of DEHP in male X. tropicalis. Previous studies have demonstrated that the mechanism of lipid accumulation was related to damage to hepatocyte mitochondria, and mitochondria contained proteins involved in lipid metabolism and regulated energy generation by decomposing lipids [38-40]. In addition, mitochondrial damage is related to changes in the antioxidant system [19]. Antioxidants, existing in the mitochondrial inner membrane, efficiently inhibited lipid peroxidation in cells and prevented oxidative damage. To some extent, damage to hepatocyte mitochondria induced by DEHP may be harmful to the antioxidant capacity of livers. Therefore, the results suggest that DEHP may induce hepatotoxicity by inhibiting mitochondrial function, leading to the accumulation of lipids and inhibition of antioxidant capacity.
Effects of DEHP on oxidative stress
Antioxidants include antioxidant enzymes and non-enzymatic scavengers, which adjust the production and removal of ROS to maintain dynamic homeostasis [41]. However, excessive ROS production is stimulated by external pollutants [42], which may attack the biological macromolecules of the cell and produce lipid peroxidation products, such as MDA. Due to the inhibition of antioxidant functions, there is an occurrence of oxidative stress and damage [43]. To examine whether DEHP caused oxidative stress in the livers of male X. tropicalis, we determined the ROS content (Figure 5), activities of antioxidants, and MDA content (Figure 6a- 6f) in the livers subjected to different DEHP treatments.
Figure 5: Reactive Oxygen Species (ROS) production in the livers of male X. tropicalis after DEHP exposure. (a-e) Fluorescence images of the livers under 100X magnification. (f) Fluorescence intensity in the livers of the different DEHP groups. (a, b, c, d and e represent livers from 0, 0.2, 0.6, 1.8 and 5.4 mg/L DEHP treatments, respectively; n=6). **P<0.01, DEHP treatments were significantly increased.
As shown in Figure 5a-5e, the amount of ROS in the liver was determined by measuring the intensity of red fluorescence. The fluorescence intensity in 0.2, 0.6, 1.8, and 5.4 mg/L DEHP treatments were significantly increased in a dose-dependent manner by 6.4%, 56%, 71%, and 76%, respectively, relative to the controls (P<0.05 and P<0.01) (Figure 5f). Huang Y, et al. [44], noted that the ROS in Mus musculus livers increased following exposure to DEHP at doses of 125, 250, and 375 mg/kg/day. In another relevant study, zebrafish juveniles, which were aquatic vertebrates along with X. tropicalis, showed a 1.13-fold increase in ROS levels after DEHP exposure at 9.75 mg/L, compared to the control group [45]. Compared to zebrafish juveniles, adult frogs showed a significant increase in ROS levels at lower DEHP concentrations (0.2 mg/L). It suggests that adult X. tropicalis may be more sensitive to DEHP than zebrafish larvae and more suitable for studies of DEHP pollution in the water environment, especially in the case of lower concentrations of DEHP. Moreover, this result indicated that excessive ROS was produced after DEHP exposure and it is possible that a lack of antioxidants were used to eliminate the ROS [46].
Antioxidants, including antioxidant enzymes (SOD, CAT, GST, and GPX) and non-enzymatic scavengers, (GSH), are known biomarkers of oxidative stress [47]. Figure 6a shows that the activity of SOD treated with 0.2, 0.6, 1.8, and 5.4 mg/L DEHP increased by 30%, 29%, 14%, and 2%, respectively, as compared to that in the controls. Similarly, an increase was also found in CAT and GST activities. Significant increase in CAT and SOD (P<0.05 and P<0.01) was observed at 0.2, 0.6, and 1.8 mg/L DEHP, and a decrease was found at 5.4 mg/L DEHP (Figure 6b-6c). The GPX activity displayed an upward trend in all of the DEHP groups, compared to the control group (Figure 6d). The decrease in antioxidant enzymes following DEHP exposure was in line with the findings in Mus musculus and human endometrial stromal cells [48,49]. As an important scavenger of ROS, GSH is highly correlated with the antioxidant capacity of the liver [41]. In our study, the GSH content of 0.2, 0.6, 1.8, and 5.4 mg/L DEHP-treated groups markedly decreased by 27%, 56%, 63%, and 69%, respectively, relative to the controls (P<0.01) (Figure 6e). MDA content directly reflects the degree of oxidative damage, including lipid peroxidation [50]. The MDA content increased at 5.4 mg/L DEHP (Figure 6f, P<0.05), however, decreased slightly after exposure to 0.6 mg/L DEHP, because enough antioxidants removed excessive ROS and maximized protection against oxidative stress [12]. Under the exposure at 1.8, and 5.4 mg/L DEHP, the antioxidants may be saturated which resulted in the increase of MDA.
Figure 6: The content of antioxidants and indicator of oxidative damage in the livers of male X. tropicalis post-DEHP exposure. (a-d) The activity of antioxidant substances in the livers of male X. tropicalis. (f) Contents of malondialdehyde of the livers of male X. tropicalis. *P<0.05, **P<0.01, shows that the activity of SOD treated with 0.2, 0.6, 1.8, and 5.4 mg/L DEHP.
Excessive ROS, imbalance in antioxidants (SOD, CAT, GPX, GST, GSH), and MDA content are used as major biomarkers of oxidative stress in organisms [47,51]. Our study suggested that DEHP induced hepatotoxicity through oxidative stress. In addition, oxidative stress is strongly related to the toxic effects of DEHP on the liver, especially in terms of the histopathological biomarkers and accumulation of lipids [19,52]. Therefore, oxidative stress was associated with hepatocyte structural and microstructural changes, which is the finding in HE stains, Oil Red O staining and mitochondrial abnormalities.
Effect of DEHP on genes related to oxidative stress and lipid metabolism
To further elucidate the molecular mechanism of oxidative stress and lipid accumulation, the transcription of related genes was investigated in the livers of male X. tropicalis (Figure 7). Longterm exposure to DEHP (0.2, 0.6, 1.8, and 5.4 mg/L) significantly decreased the expression of nrf2 by 30%, 31%, 33%, and 39%, respectively, of that in the controls (P<0.01) (Figure 7a). Upon exposure to 0.2, 0.6, 1.8, and 5.4 mg/L DEHP, the mRNA expression of SOD increased by 124%, 106%, 30%, and 28%, respectively, of that in the control treatment (Figure 7b). The mRNA level of CAT is significantly induced in 0.6 and 1.8 mg/L treatments (P<0.01), while slightly inhibited in 5.4 mg/L DEHPexposed group, as compared to that in the control group (Figure 7c). Gene expression of GST was significantly increased in the 0.2 and 0.6 mg/L DEHP-exposed groups, while it decreased in the 1.8 and 5.4 mg/L DEHP-exposed groups, when compared to the control group (P<0.01) (Figure 7d). The mRNA level of GPX showed an obvious increase in all the DEHP treatments (P<0.05 and P<0.01) (Figure 7e). From these results, it can be seen that the expression patterns of antioxidant genes correspond to the previous results of the antioxidants. Nuclear factor erythrocyte 2-related factor 2 (encoded by nrf2) is a nuclear transcription factor that plays a crucial role in activating the antioxidant defense system in response to oxidative stress and excessive ROS. Downstream genes of antioxidants (CAT, SOD, GPX, and GST), regulated by nrf2, also have an outstanding protective effect against oxidative stress [53]. Many studies have revealed that the activation of the nrf2 signaling pathway and downstream antioxidant genes prevent lipid peroxidation, structural damage, and disease in the livers [54,55]. Therefore, the present study indicated that the livers of X. tropical males had a certain degree of resistance to the toxic effects of low concentrations of DEHP through activating genes related to oxidative stress and highly stimulating the antioxidant activities of the organism. However, the antioxidant activities were suppressed because of the decreased antioxidant genes at higher concentrations.
Figure 7: The relative expression of genes in the livers of male X. tropicalis post-DEHP exposure. (a-e) Genes related to oxidative stress. (f) Gene related to lipid metabolism. *P<0.05, **P<0.01, shows that the activity of SOD treated with 0.2, 0.6, 1.8, and 5.4 mg/L DEHP.
Based on the histopathological and ultrastructural findings of the study, it can be speculated that lipid accumulation occurred following DEHP exposure. Lipid accumulation contributes to the variation in lipid metabolism [56-59]. In this study, the transcription levels of genes related to lipid metabolism were used to determine the molecular mechanism of variation in lipid metabolism. Downregulation of PPARα expression was observed in all the DEHP-exposed groups. DEHP concentrations of 0.2, 0.6, 1.8, and 5.4 mg/L caused a marked decrease in the expression, by 16%, 21%, 27%, and 40%, respectively, as compared to the controls (P<0.05 and P<0.01) (Figure 7f). Peroxisome proliferator activated receptor-α (encoded by PPARα) is a type of peroxisome proliferatoractivated receptor that plays an important role in enhancing fatty acid β-oxidation capacity in the liver [60-62]. The promotion of fatty acid β-oxidation is associated with the release of free fatty acids and inhibition of lipogenesis [63]. This study supported our results, indicating that there was a decrease in the expression of PPARα in Clarias gariepinus, after dealing with similar concentrations of DEHP (200 and 400 μg/L). In addition, published studies have indicated that the restrained activity of fatty acid β-oxidation could lead to excessive lipid accumulation and oxidative stress in the liver of Rattus norvegicus [64-66]. According to the expression of PPARα, it may be determined that the mechanism of disordered fatty acid β-oxidation finally induced lipid accumulation and oxidative stress in the livers exposed to DEHP.
Potential mechanisms of DEHP-induced hepatotoxicity in X. tropicalis
Based on previous results and analyses, Figure 8 demonstrates the potential mechanisms of hepatotoxicity to X. tropicalis. Excessive ROS is the primary factor of toxicity, and the potential mechanism of hepatotoxicity by excessive ROS is divided into the following three parts. Firstly, the attack of DEHP on hepatocytes disrupted the balance of ROS and induced excessive ROS production (Figure 5). Part of the ROS attacked the structure of hepatocytes, causing an increase in MDA content (Figure 6f) with structural damage to hepatocytes (Figure 2). Secondly, a portion of ROS interfered with antioxidant-related pathways (NRF2 pathway, Figure 7), thereby inducing oxidative stress, such as inhibition of antioxidant activity (Figure 6), lipid peroxidation (Figure 6f) and mitochondrial abnormalities (Figure 4), and the occurrence of oxidative stress may further exacerbate the imbalance in ROS levels. Finally, a portion of ROS inhibited the expression of the lipid metabolism gene (Figure 7f), which may cause a disturbance in fatty acid metabolism. Mitochondrial abnormalities also potentially affect the process of catabolism of fatty acids which ultimately induces lipid accumulation (Figures 3 and 4).
Figure 8: Potential mechanism of DEHP-induced hepatotoxicity on X. tropicalis.
Our study is the first to investigate the growth and developmental toxicity caused by DEHP at environmentally relevant concentrations in male X. tropicalis. At environmentally relevant concentrations, DEHP induced hepatotoxicity in X. tropicalis, including histopathological damage, morphological changes in mitochondria, lipid accumulation, and oxidative stress (excessive ROS and inhibition of antioxidants). In addition, DEHP disturbed the expression of genes related to oxidative stress and lipid metabolism. In summary, the above results suggested that liver damage may arise from oxidative damage caused by the downregulation of oxidative stress genes, while lipid accumulation in the liver is mediated by the downregulation of lipid metabolism-related genes. There is a need for further studies focusing on other toxic end-points of hepatotoxicity caused by DEHP in X. tropicalis (e.g., alterations in metabolites and proteomics), as well as, a significant understanding of the mechanisms of toxicity in other aquatic models.
Conceptualization: Li Zheng, Xufeng Qi, Yanbin Xu; Review and Editing: Xinying Pan, Li Zheng; Methodology: Li Zheng, Xufeng Qi, Yanbin Xu, Guangyan Xie, Qingxia Qiao; Writing-original draft preparation: Xinying Pan; Formal Analysis: Xinying Pan; Validation: Xinying Pan, Yishen Ding, Zhuo Dai, Xiaochun Zhang; Funding acquisition: Li Zheng; Supervision: Li Zheng; Visualization: Visualization; Data curation: Xinying Pan. All authors have read and approved the published version of the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (No. 31802025, 4197070130), and the Key Laboratory of Regenerative Medicine, Ministry of Education, Jinan University (No. ZSYX-M-2019-00009), China.
The author declared no conflict of interest.
The research protocol and experiments were approved by the Committee on the Ethics of Animal Experiments of The Guangdong University of Technology (No. GDUTXS002).
The research leading to these results received funding from the National Natural Science Foundation of China under Grant Agreement No. 31802025, 4197070130.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Pan X, Zheng L, Ding Y, Dai Z, Qi X, Zhang X, et al. (2023) Effects of Di-(2-Ethylhexyl) Phthalate on Oxidative Stress and Lipid Metabolism in Livers of Male Xenopus tropicalis. J Clin Toxicol. 13:528.
Received: 13-Jan-2023, Manuscript No. JCT-23-21400; Editor assigned: 16-Jan-2023, Pre QC No. JCT-23-21400 (PQ); Reviewed: 30-Jan-2023, QC No. JCT-23-21400; Revised: 06-Feb-2023, Manuscript No. JCT-23-21400 (R); Published: 13-Feb-2023 , DOI: 10.35248/2161-0495.23.13.528
Copyright: © 2023 Pan X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.