Advances in dairy Research
Open Access
ISSN: 2329-888X
ISSN: 2329-888X
Research Article - (2017) Volume 5, Issue 4
Keywords: Powder goat milk; Storage; Stability; Physicochemical properties; Fatty acid profiles
The world total goat milk production was reported as 18,655,814 metric tons in 2014 [1]. Increasing popularity and interest in dairy goats and goat milk products are a part of the recent trend in health food demand in developed countries as well as a renewed interest in goat milk as a substitute for those who suffer from allergies against cow milk [2-4]. Goat milk products such as cheeses, yogurts and ice creams recently gained increasing demands among certain ethnic groups, health food lovers, connoisseur customers and private goat farmers in the U.S. [3,5].
Although a large volume of fluid goat milk has been consumed in many developing countries, various processed goat milk products, including cheeses and yogurts also have been produced in many parts of the world, especially in Mediterranean, Middle Eastern and eastern European regions [6,7]. In addition, powdered goat milk has attracted increasing interest from the dairy industries, since dehydration is a major alternative for fluid milk preservation and can extend the shelflife of caprine milk with minimal changes in its nutritional and sensory qualities [8,9].
There are two main benefits for producing powdered product from fluid goat milk, which are extending shelf life and easy transport of the product by volume reduction [10-12]. This dehydration technology can provide consumers with year-round marketable product, and expands the market to long distance regions with easier and lower-cost transportation [9]. Spray drying is the most common method used for the manufacturing of powdered milk [13]. Powdered caprine milk can be utilized to manufacture a variety of products, including bakery products, ice cream, chocolate, yogurt, infant formula, and cheeses [14].
During the storage of dehydrated milk products, some major problems can occur such as physical changes as caking and cohesion, as well as some chemical changes, such as Maillard reaction and oxidation of lipids [12]. Low moisture in powder milk may be considered to inhibit the growth of most microorganisms. However, changes in powder milk during storage can be caused not only by chemical reactions [15], but also by enzyme activities of microorganisms such as psychrotrophic bacteria [16]. The lipoprotein lipase (LPL) activity is lower in goat milk compared with cow milk, where LPL has higher affinity for the fat globules and higher correlation with spontaneous lipolysis in goat milk [17]. Lipases hydrolyze milk fat and release free fatty acids producing rancid and off flavors in dairy products [7,18].
Few scientific studies have been conducted on food stability, safety and physicochemical properties of powdered goat milk (PGM) products under controlled environmental storage conditions. Therefore, the objectives of our study were to: (1) evaluate the changes in physicochemical properties, including basic nutrient content, pH, water activity, and lipid oxidation of the commercial PGM, and (2) determine the changes in fatty acid profiles of experimental PGM products stored under two different temperatures (4°C and 22°C) for three different storage periods (0, 2, and 4 months).
Experimental design
This study was conducted in a 3 × 2 × 3 factorial experiment. Three batches of commercial whole powder goat milk (PGM) products were purchased from a local retail outlet at Warner Robins, GA, USA. The experimental caprine milk powder samples were placed in 125 mL amber glass bottle in triplicates for each batch of the dehydrated products, and stored at two different temperatures (4°C and 22°C) for three different storage periods (0, 2, and 4 months).
Nutritional and physicochemical properties were analyzed on all experimental PGM samples for basic nutrient contents, pH, water activity and peroxide value for lipid oxidation, as well as fatty acid compositions of all treatment group samples. All physicochemical parameters and fatty acid profiles were determined throughout the storage periods, while the basic nutrients contents were analyzed only for the initial samples from each batch of PGM products.
Analysis of basic nutrients
Moisture content: Moisture contents of the powder milk samples were determined in a laboratory drying oven at 105°C overnight using the AOAC [19] method.
Protein content: Nitrogen content of each PGM sample was determined using the vario MAX cube analyzer (Elementar Americas, Mt. Laurel, NJ, USA) in CNS mode. After preparation of blanks and standard, 250 mg of each sample was weighed into the carefully numbered ceramic crucibles. The sample crucibles were loaded into the carousel, and the automated machine ran each sample to determine the nitrogen content according to the manufacturer instructions. The actual protein contents of the powder milk samples were calculated by multiplying the factor of 6.38 by the N content of each unknown powder samples, and the factor of 6.25 was multiplied by the N content of the blank and standard.
Fat Content: The fat content for the commercial whole goat milk powder was determined using the Folch et al. [20] extraction and direct volatilization of solvent methods. After fat extraction, 10 mL of the chloroform fat extract was measured into a clean 25 mL Erlenmeyer flask, and the flask was placed under a fume exhaust hood overnight for complete evaporation of the chloroform solvent. The fat content of each sample was calculated by the following equation:
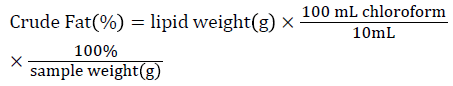
Ash content: All powder milk samples were dry ashed in a muffle furnace at 550°C overnight to determine ash content, according to the AOAC [19] procedures.
Analysis of physicochemical parameters
pH: The powder milk samples were first dissolved in double deionized water prior to pH analysis. A 12 gram of the PGM sample was reconstituted in 100 mL deionized water at room temperature, and pH was determined using a pH meter (Accumet AR10 pH meter; Fisher Scientific, Fair Lawn, NJ, USA). The pH meter was calibrated with two standard buffer solutions of pH 4 and pH 7.
Water activity: Water activity (aw) of the experimental PGM sample was analyzed at room temperature using an AquaLab water activity meter (cx-2; Decagon Devices, Pullman, WA, USA). A small amount (approximately 2 g) of dry sample was placed in the measuring sample cup, and loaded into the water activity meter. The water activity values for all experimental powder milk samples were determined by the aw readout values displayed in the activity meter.
Analysis of lipid oxidation: Lipid oxidation of all powder milk samples were determined by peroxide value (POV) using the AOCS [21] procedure. The lipids of powder milk samples were extracted using Folch et al. [20] method. Five grams of previously extracted fat sample was weighed into a 250 mL Erlenmeyer flask. Thirty milliliters of acetic acid-chloroform solution (3:2 v/v) was added to each sample, and contents were swirled until dissolved. Saturated potassium iodide (0.5 mL) was added to each sample, and shaken for 1 min. Thirty milliliters of distilled water was added to each sample, and the samples were shaken vigorously. One mL starch solution (1%) was added to each sample. The sample flask was titrated with 0.01 N sodium thiosulfate until the bottom layer appeared milky, which indicated the end point. During titrations, samples were shaken to ensure the release of iodide from the chloroform layer. A blank sample was run with actual unknown milk sample. The sample weight, mL used to titrate blank, and mL used to unknown sample were recorded for each prepared milk sample. Reagents were made fresh and the saturated potassium iodide was kept in a light proof container. Peroxide value was calculated using the following equation.
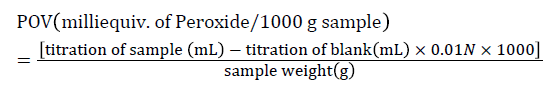
Fat extraction
The standard method of the AOCS [21] was used for the extraction of fat for fatty acid analysis. Two grams of powder milk sample was weighed into a 50 mL beaker. Eight mL methanol and 18 mL chloroform were added to each sample and homogenized for 30 s. Nine mL of chloroform was added to each sample, and homogenized again for 30 s. Nine mL zinc acetate (aq. solution: 0.115 g zinc acetate/5 mL H2O) was added to each sample and homogenized for 30 s. The content of each beaker was transferred to a 125 mL separatory funnel, and placed in a 4°C refrigerator until two distinct phases were separated. The bottom chloroform layer was drained into a 125 mL round bottom flask. The Büchi Rotavapor (R-200; BUCHI Corporation, New Castle, DE, USA) was used to dry the solvent of the fat extracted with dry ice to condense, and nitrogen gas was used to flush the samples. The dried samples were then closed and stored in the freezer (-18°C) until further analysis.
Preparation of fatty acid methyl esters
Five mL of 0.5 N methanolic NaOH and boiling beads were placed in each 125 mL flask, which contained the previously extracted fat samples. The sample flasks were attached to a cooling condenser assembly, and the contents were heated on a hot plate for 10 min. Through the condenser, 5 mL of boron triflouride- methanol reagent (12%, 1.5 M, ACROS organics; Thermo Fisher Scientific, Waltham, MA, USA) was added to each sample, and the samples then boiled for two min. Samples were removed from hot plate, but continued to be attached to the condenser and cooled. Seven mL hexane was added to each sample through the condenser, and then heated on hot plate for one min. Samples were cooled, removed one at a time from the condenser, and stoppered to ensure no loss of sample. Saturated NaCl solution was added to each sample flask, which floated the hexane solution to the neck of the flask, whereby this layer of fatty acid methyl esters (FAME) could be pipetted out. About 6 mL of this layer was transferred to a test tube containing a small amount of sodium sulfate (anhydrous) for removal of moisture from the hexane solution. The finally prepared FAME samples were stored in the freezer (-18°C) until gas chromatographic analysis.
Analysis of FAME with gas chromatograph
Fatty acid profiles were quantified by injecting 1 μL of FAME sample into a gas chromatograph (GC-2010 Plus; Shimadzu Scientific Instruments, Canby, Oregon, USA), equipped with a fused silica capillary column of 100 m × 0.25 mm × 0.2 μm film thickness (SP-2560; Supelco, Bellefonte, PA, USA), FID (flame ionization detector), AOC-20s auto sampler, and AOC-20i auto injector. Injector and detector temperatures were set at 250°C and 270°C, respectively. The initial oven temperature was set to 140°C for 5 min, and increased to 240°C at a rate of 4°C /min. A 1 μL sample was injected with a split ratio of 1:20, and helium was used as the carrier gas.
All collected experimental data were statistically analyzed using the GLM procedure of SAS program version 9.4 [22] and Steel and Torrie [23] methods for analysis of variance, least square means, and Duncan’s multiple mean comparison. The differences between treatments were assessed for effects of batch, storage temperature, and storage time on chemical changes and bacterial survival in three batches of commercial whole goat milk powder.
Basic nutrient composition
The respective mean basic nutrient contents (%) of the commercial powdered goat milk stored at 4°C and 22°C were: 23.5, 24.5, fat; 2.10, 2.09, moisture; 27.5, 27.4, protein; and 6.20, 6.20, ash, indicating that there were no differences in basic nutrient compositions between the powder goat milk samples stored at two different temperature treatments (Table 1). In addition, batch effect on basic nutrient levels was not significant, as displayed in Figure 1. Since these proximate values were expected not to be affected by storage period, only initial 0 months PGM samples of the three batches were analyzed for the basic compositions.
| Storage Temp (°C) | Fat | Protein | Moisture | Ash | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 4 | 23.5 | 1.35 | 27.5 | 0.09 | 2.1 | 0.26 | 6.2 | 0.06 |
| 22 | 24.5 | 0.55 | 27.4 | 0.05 | 2.09 | 0.36 | 6.2 | 0.07 |
SD: standard deviation
Table 1: Summary of basic nutrient contents (%) of powdered goat milk at two different storage temperatures.
These basic nutrient contents of the commercial powder goat milk products of the current study are in agreement with those of other previous studies [7,24]. Park [24] reported that the average basic nutrient content of U.S. caprine whole milk powder contained 94.1% total solids, 27.0% protein, 28.2% fat, 32.0% carbohydrates, and 6.77% ash, indicating that these data are fairly close to the results of our study.
Changes in physicochemical properties of PGM during storage
pH: There were no significant differences in pH values of the experimental whole goat milk powder samples between two storage temperatures. Both temperature treatments revealed the same trend of change in pH during 4 months period (Figure 2). However, there was a significant (P<0.05) elevation in pH at two months storage for both temperature treatments. The pH values of the powder milk samples at 4 months storage were also higher than those of initial samples, but lower than those of 2 months stored samples.
Water ativity: Changes in water activity (aw) during 4 months storage for the commercial powder goat milk samples are shown in Figure 3. The average aw for PGM samples stored at 4 and 22°C for 0, 2, and 4 months storage were 0.251, 0.224, 0.249; 0.268, 0.229, 0.221, respectively. There were no differences in aw between the two temperature treatments, nor among the three storage periods for the experimental powder goat milk samples. Slight differences in aw were observed among the three batches of the commercial PGM. However, all water activity values remained at around 0.2, indicating that the aw values in our study are similar to those of previous reports for dehydrated milk samples [25-27]. The value of aw at 0.2 indicates that there was little chance of microbial growth for the PGM samples during 4 months storage period, suggesting stability and food safety of the commercial goat milk powder goat milk products would have minimal problems.
Variability and higher values of aw have been known as a direct contributor to the poor shelf life and higher possibility of spoilage of food products, since water activity is an important index to measure the stability and shelf life of foods during storage. Several parameters involved in the production and storage of whole milk powder have been shown to affect the shelf life of the products, which include water activity, storage temperature, and preheat treatments of the fluid milk during processing [12].
Moisture content: Results of the mean moisture contents of the PGM stored at 4 and 22°C during four months of the experimental period are shown in Figure 4. There were no differences in aw among the commercial milk samples in regard to both storage temperature and storage time. The average moisture content (%, wet basis) for the PGM samples stored for 0, 2, and 4 months at 4 and 22°C were 2.10, 2.07, 2.35; 2.09, 2.17, 2.29, respectively. Because there were little fluctuations in moisture contents of the PGM samples, the moisture effect on the stability of the powder goat milk was minimal, which was reported in previous reports [27,28].
Lipid oxidation (peroxide value: POV): Peroxide value is a measure of the level of hydroperoxides - the primary product of lipid oxidation in a food sample. The results of POV values of the experimental PGM samples at 2 storage temperatures for 4 months storage are shown in Table 2. There were significant (P<0.05) differences in POV of the experimental powdered goat milk samples between storage temperatures and among storage periods. Samples stored at room temperature (22°C) were found to have a significantly (P<0.05) higher peroxide value than those stored at 4°C, indicating that the higher storage temperature (22°C) caused greater lipid oxidation than the refrigeration storage. For the perspective of the effect of storage period on POVs of the experimental PGM samples, those of 4 months storage had significantly (P<0.05) higher POVs than those of shorter storage periods, suggesting that the rate of lipid oxidation would be increased by extended storage periods. However, the POV values observed in this study are still below the unacceptable level of peroxide value, which is less than 1 milliequivalent peroxide/1000 g sample [29,30]. The lipid oxidation of the commercial PGM samples at 4 months storage was still acceptable for consumption of the product.
| Storage Temp (°C) | 0 month | 2 month | 4 month | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| 4 | 0.099by | 0 | 0.115by | 0.04 | 0.148ay | 0.05 |
| 22 | 0.107by | 0.02 | 0.166abx | 0.05 | 0.182ax | 0.08 |
a,bMeans with different superscript within a same row are significant (P<0.05)
x,yMeans with different superscript within a same column are significant (P<0.05)
SD: Standard Deviation
Table 2: Comparison of average peroxide values (milliequiv. of Peroxide/1000 g sample) in powdered whole goat milk during four months storage at 4 and 22°C.
Elevation of lipid oxidation in a dry food product can cause unfavorable outcomes in flavor, color, and a reduction in vitamin levels [12,31]. The rate of lipid oxidation that takes place in milk powders is determined by several storage conditions, including oxygen, exposure to UV light, temperature, moisture, water activity, and the amount of unsaturated fatty acids [12,32]. Thus, lipid oxidation can be an important parameter to assess the storage stability of dehydrated milk products.
Fatty Acid Profiles: The summary of fatty acid compositions of the experimental commercial whole goat milk powder products stored at 4°C and 22°C for 0, 2 and 4 months are listed in Table 3. In addition, a statistical summary for analysis of variance (F values) on main factors and their interaction effects on fatty acid profiles of the PGM products are shown in Table 4. The effects of main factors (batch, storage temperature, storage period) and their 2-way and 3-way interactions on fatty acid concentrations of the commercial PGM samples revealed that C18:1 (oleic acid) was the highest content among all the fatty acids quantified in the experimental goat milk samples. The C8:0 (caprylic acid) followed as the second highest level of fatty acid among all fatty acids determined (Table 3). Linoleic (C18:2) acid content was the third highest fatty acid.
| Fatty Acid | Storage Temp (°C) | 0 month | 2 month | 4 month | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| C4:0 | 4 | 1.25 | 0.21 | 1.18 | 0.04 | 1.14 | 0.17 |
| 22 | 1.14 | 0.07 | 1.12 | 0.15 | 1.03 | 0.14 | |
| C6:0 | 4 | 1.42 | 0.26 | 1.33 | 0.03 | 1.24 | 0.15 |
| 22 | 1.31 | 0.05 | 1.3 | 0.13 | 1.2 | 0.17 | |
| C8:0 | 4 | 6.87 | 1.18 | 6.44 | 0.07 | 5.99 | 0.67 |
| 22 | 6.33 | 0.06 | 6.33 | 0.31 | 6 | 0.74 | |
| C10:0 | 4 | 0.11 | 0.01 | 0.12 | 0 | 0.12 | 0.01 |
| 22 | 0.12 | 0 | 0.12 | 0.01 | 0.12 | 0.01 | |
| C12:0 | 4 | ND | ND | 0.21 | 0.02 | 0.19 | 0.02 |
| 22 | 0.01 | 0.21 | 0.19 | 0.04 | 0.18 | 0.03 | |
| C14:0 | 4 | 0.15 | 0.02 | 0.16 | 0 | 0.26 | 0.15 |
| 22 | 0.16 | 0.01 | 0.16 | 0.01 | 0.26 | 0.15 | |
| C14:1 | 4 | 0.73 | 0.05 | 0.76 | 0 | 0.7 | 0.09 |
| 22 | 0.74 | 0.03 | 0.75 | 0.03 | 0.71 | 0.09 | |
| C16:0 | 4 | 1.43 | 0.03 | 1.43 | 0.02 | 1.35 | 0.07 |
| 22 | 1.42 | 0.04 | 1.39 | 0.05 | 1.38 | 0.06 | |
| C16:1 | 4 | 0.78 | 0.03 | 0.79 | 0.01 | 0.65 | 0.25 |
| 22 | 0.79 | 0.02 | 0.78 | 0.03 | 0.57 | 0.33 | |
| C18:0 | 4 | 0.17 | 0.17 | 0.02 | 0.01 | 0.02 | 0.013 |
| 22 | 0.17 | 0.17 | 0.03 | 0.02 | 0.03 | 0.02 | |
| C18:1 | 4 | 9.6 | 1.07 | 8.74 | 0.12 | 8.02 | 1 |
| 22 | 9.09 | 0.92 | 8.59 | 0.35 | 8.08 | 1.04 | |
| C18:2 | 4 | 2.03 | 0.05 | 2.01 | 0.01 | 1.87 | 0.22 |
| 22 | 1.99 | 0.05 | 2.02 | 0.01 | 1.86 | 0.23 | |
| C18:3 | 4 | 0.47 | 0.07 | 0.48 | 0.01 | 0.46 | 0.05 |
| 22 | 0.47 | 0.04 | 0.53 | 0.08 | 0.4 | 0.19 | |
| C20:0 | 4 | 0.05 | 0.06 | 0.03 | 0.01 | 0.13 | 0.15 |
| 22 | 0.01 | 0.02 | 0.03 | 0.01 | 0.15 | 0.18 | |
| C22:0 | 4 | 0.01 | 0.01 | 0.02 | 0.01 | 0.03 | 0.03 |
| 22 | 0 | 0.01 | 0.06 | 0.1 | 0.03 | 0.02 | |
| C24:0 | 4 | 0.05 | 0.07 | 0.01 | 0 | 0.01 | 0.01 |
| 22 | 0.03 | 0.01 | 0.01 | 0 | 0.01 | 0 | |
ND: Non-Detectable; SD: Standard Deviation
Table 3: Profiles of fatty acid composition (mg/g powder milk) of commercial whole goat milk powder samples stored less than two temperatures for four months period.
More importantly, the short chain fatty acids (C4:0, C6:0, C8:0) of the experimental PGM showed the next high levels of fatty acids among others tested in this study. Previous reports have shown that the high levels of short chain and medium chain fatty acids (MCT) in goat milk fat observed in this study have special significance in human nutrition. Those are: (i) goat milk fat may be more rapidly digested than cow milk fat because lipase attacks ester linkages of short or medium chain fatty acids more easily than those of longer chains [33-35], (ii) these fatty acids have the unique metabolic ability to provide energy in growing children, and also exhibit beneficial effects on cholesterol metabolism, such as hypocholesterolemic action on tissues and blood via inhibition of cholesterol deposition and dissolution of cholesterol in gallstones [36-38], and (iii) they also have been therapeutically used for treatment of various cases of malabsorption patients suffering from steatorrhea, chyluria, hyperlipoproteinemia, and in case of intestinal resorption, coronary bypass, childhood epilepsy, premature infant feeding, cystic fibrosis and gallstones [4,34-39].
Concerning the effects of main factors on the fatty acid profiles, batch effect turned out to be significant (P<0.05, 0.01, 0.001) in majority of the fatty acid concentrations (Table 4), indicating that there were significant variations in fat composition of the commercial goat powder products between different batches of the PGM products. About half of fatty acids examined in the PGM samples also showed significant (P<0.05) differences in concentrations between storage periods., whereas storage period did not have any influence on levels C4:0, C10:0, C12:0, C14:1, C18:3, C22:0, and C24:0 fatty acids. On the other hand, the effect of storage temperature on levels of fatty acids was found to be insignificant except for butyric acid (C4:0).
| Parameter | DF | C4:0 | C6:0 | C8:0 | C10:0 | C12:0 | C14:0 | C14:1 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C22:0 | C24:0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Batch | 2 | 6.16** | 6.71** | 6.67** | 4.13* | 3.17 | 5.53* | 3.25 | 5.53* | 3.48* | 10.21*** | 21.14*** | 5.01* | 0.39 | 6.33** | 2.93 | 0.65 |
| Storage Temp | 1 | 4.70* | 1.88 | 0.9 | 0.13 | 0.82 | 0 | 0.01 | 0.06 | 0.23 | 0.01 | 0.73 | 0.05 | 0.07 | 0 | 0.53 | 0.61 |
| Storage period | 2 | 3.77 | 5.84** | 5.28* | 1.59 | 1.99 | 8.26** | 3.04 | 7.98** | 5.04* | 7.15** | 19.44*** | 7.16** | 1.48 | 8.92** | 2.18 | 1.4 |
| Batch × Storage period | 2 | 2.26 | 2.79 | 3.76* | 56.72*** | 2.77 | 1239*** | 76.24*** | 8.08** | 29.37*** | 259.0*** | 13.40*** | 80.09*** | 7.21** | 38.86*** | 0.54 | 0.68 |
| Batch × Storage Temp | 2 | 0.42 | 0.77 | 0.68 | 0.35 | 1.09 | 0.01 | 0.22 | 0.45 | 0.24 | 0.05 | 0.22 | 0.02 | 0.17 | 0.01 | 0.45 | 0.26 |
| Storage Temp × Storage period | 2 | 0.11 | 0.19 | 0.44 | 0.31 | 1.14 | 0 | 0.07 | 0.71 | 0.15 | 0.01 | 0.26 | 0.05 | 0.55 | 0.13 | 0.73 | 0.15 |
| Batch × Storage Temp × Storage period | 8 | 1.54 | 1.58 | 1.77 | 19.57*** | 1.52 | 343.7*** | 18.96*** | 3.10* | 8.69*** | 66.69*** | 4.67** | 15.15*** | 2.21 | 7.77*** | 0.69 | 0.33 |
(*P<0.05, **P<0.01, ***P<0.001)
Table 4: Summary of Analysis of Variance (F-Values) for effects of main factors (batch, storage temperature and period) and their interactions on levels of individual fatty acids (C4:0-C24:0) in the experimental whole goat milk powder.
With regard to interaction effects, two-way interactions of storage temperature x storage period and batch x storage temperature had no significant influence on the fatty acid levels of the PGM samples. However, the 2-way interaction of batch x storage period showed significant effects (P<0.05, 0.01, 0.001) on the majority of fatty acids, including C8:0, C10:0, C14:0, C14:1, C16:0, C16:1, C18:0, C18:1, C18:2, C18:3, and C20:0. Furthermore, 3-way interaction of batch x storage temperature x storage period also had significant effects on levels of several fatty acids, such as C10:0, C14:0, C14:1, C16:0, C16:1, C18:0, C18:1, C18:2, C20:0.
Interestingly, batch effects were significant (P<0.05, 0.01, 0.001) for majority of fatty acid concentrations, suggesting that the original milk used for manufacturing powder the commercial PGM products probably had significantly difference between batches in fat chemical compositions. It has shown that breeds, stage of lactation, feeding, animal management, and environmental conditions would have significant influences on milk composition [4,33,35,39]. In addition, pasteurization and spray drying conditions can make differences in milk processing and alteration of milk fat moiety and its composition.
The results of our study revealed that lipid oxidation and shelf-life of the commercial powder goat milk products were affected by both storage temperature and storage period, suggesting that lower temperatures and shorter storage periods are desirable for consumption of dehydrated goat milk products in order to ensure the supply of high quality powder products for the consumers at the time of consumption.
Fatty acids profiles of the commercial PGM products were influenced by mainly batch, storage period and some interaction effects. These results may imply that, in addition to the storage effects, the original milk used for manufacturing the commercial PGM products probably affected compositions of different batches of the milk, which are influenced by goat breeds, stage of lactation, feeding, animal management, and environmental conditions. Further studies may be necessary to examine more numbers of storage temperature and period treatments in order to make more inclusive, convincing and generalized scientific conclusions on storage effects on physicochemical properties and fatty acid profiles of commercial powdered goat milk products.
This research was supported by the Agricultural Research Station project GEOX-3225 at Fort Valley State University, Fort Valley, GA, which was originally funded by the USDA/NIFA Evan-Allen funds.