Journal of Alcoholism & Drug Dependence
Open Access
ISSN: 2329-6488
ISSN: 2329-6488
Original Research Article - (2021)
Objective: Use of energy drinks is increasing now a days and red bull is common brand used by youth that causes nephrotoxicity. Therefore present study was conducted to observe conservative effect of vitamin D3 on renal histomorphological changes induced by EDs.
Study design: Laboratory based experimental study.
Place and duration of study: The research was carried out from 1st august to 30th September 2019 at National institute of health (NIH), Islamabad.
Material and Method: Total thirty adult male albino rats were divided into 3 groups, each group containing ten rats. Group A was control group while rats in group B received 3.57ml/kg body weight red bull that corresponds to one can of ED (250ml) in humans orally for 8 weeks. Rats in group C received 3.57ml/kg body weight red bull along with 5000 IU/kg body weight vitamin D3 orally for 8 weeks. After completion of 8 weeks rats were sacrificed their right kidneys were removed and Slides were prepared using PAS stain. Readings were taken through image j software and results were analyzed by SPSS.
Results: The results demonstrated that in group B use of red bull for 8 weeks resulted in decrease in radius and diameter of glomerular along with widening of bowman capsule space. In group C radius of glomerulus, diameter of glomerulus and bowman capsule space was near to control group. This showed that vitamin D3 conserved glomerulus histology significantly in group C and prevented renal damage (P≤0.05).
Conclusion: In conclusion, vitamin D3 is having noticeable conservative effect in preventing renal histomorphological changes caused energy drink.
Red bull; Conservation; Vitamin D3
Use of energy drinks is increasing now a day, they are popular in youth who use these drinks to boost energy. Energy drinks were originally introduced in UK in 1929 as hospital drink for aiding recovery; in 1980 they were promoted as energy drinks for replenishing lost energy. They are now available in > 140 countries as part of a multi- billion dollar business [1-4]. In 1960s they appeared in Asia and Europe [5]. There are diverse types of energy drinks available in Pakistan such as Power horse, Burn, Monster, AMP, and Sting. Red bull is the most popular and first energy drink introduce [6]. EDs claim to provide a burst of energy by using a combination of caffeine (the principal active ingredient), plant-based stimulants (e.g., guarana, yerba mate), simple sugars (e.g., glucose, fructose), glucuronolactone (a naturally occurring glucose metabolite), amino acids(e.g., taurine, carnitine, creatine), herbs (e.g., ginkgo biloba, ginseng), and vitamins [7]. Target of energy drink market is mainly youth. Majority of energy drink users are youngsters with age group of 13-35 years [8].
Kidneys are one of vital organs affected by reactive oxygen species produced by energy drinks. Vitamin D, which is fat soluble vitamin obtained from dietary sources and synthesized in the skin is biologically inactive. Kidneys play important role in vitamin D activation and metabolism. They convert it to 1, 25(OH)2D3 or calcitriol (active form of vitamin D), that is responsible for renal protection and regulation of many physiological processes. Renal diseases even in early stages are escorted by decrease level of 1, 25(OH)2D3, that impairs recovery and accelerates their further progression to chronic diseases. Therefore, vitamin D3 is considered generally safe and effective remedial in diverse kidney disorders. It is having nephro protective activity and can ameliorate renal injury. Vitamin D3 prevents inflammation through its antioxidant properties. Studies have proved that doses of 5000iu/ kg body weight vitamin D3 is having protective effect against oxidative stress.
Therefore this present study was conducted to see the role of vitamin D in case of damaged caused by energy dink on histo morphological structure of rat’s kidney.
This experimental study was conducted from 1st August to 30th September 2019 at national institute of health (NIH) after approval from ethical review committee (ERC).
Total thirty adult male healthy albinos Sprague Dawley rats (n=10/ group) weighing 250 ± 10 gm were used. They were housed in standard metallic cages (each cage length was 60 cm, width 45 cm, height,45cm and floor spacing 2444 sq.cm) at animal house of NIH Chak Shahzad Islamabad and were acclimatized to laboratory conditions with free access to food and water under natural dark and light rhythms prior to commencement of study. Female rats and rats with any disease or pathology were excludes. Total thirty rats were divided into three groups each group having ten rats selected by convenient random sampling and treated as follows:
| Group I Control group (n=10) |
Rats in this group received normal diet and water for 8 weeks. |
| Group II Energy drink group (n=10) |
Rats in ED group received 3.57ml/kg red bull orally for 8 weeks. |
| Group III Withdrawal group (n=10) |
Rats were given 3.57ml/kg red bull along with 5000 IU/kg body weight vitamin D3 orally for 8 weeks. |
Rats were dissected after 8 weeks of completion of experimental study. The right kidneys were removed weighted and fixed in formalin. Transverse sections of 5 μm thickness were obtained, stained with Periodic acid-Schiff stain (PAS). Under X40 slides were examined under light microscope. The parameters observed were glomerular radius, glomerular diameter and bowman capsule thickness. Readings were taken by using image j software and results were analyzed by SPSS.
Mean radius of glomerulus in in group A was found to be 80.25um. The mean radius of glomerulus in group B was 61.5um that was significantly less than group A. In group C mean radius was 80.3, this shows that vitamin D have conserved renal histo morphological structure significantly (p=0.00).
Mean diameter of glomerulus in group A was 161um while it was significantly reduced to 123um in group B. The mean difference in diameter of glomerulus in group C was 160um. This shows that vitamin D has conserved renal histo morphology in rat renal tissue as compared to group B (Figure 1 and Table 1).
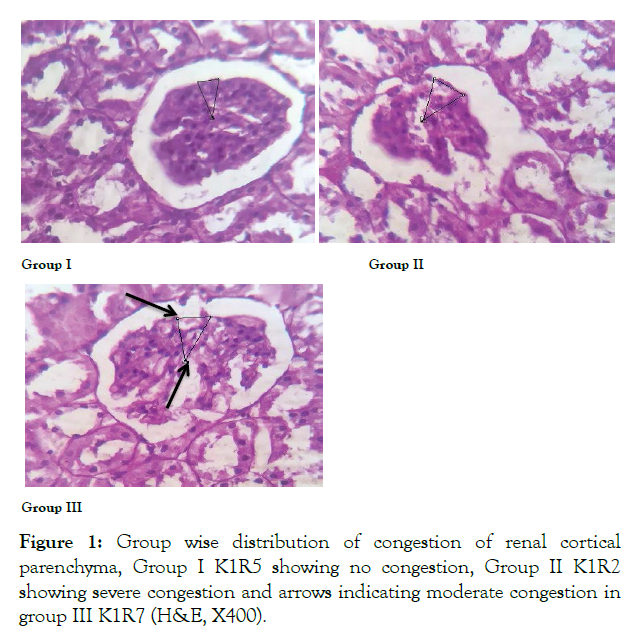
Figure 1: Group wise distribution of congestion of renal cortical parenchyma, Group I K1R5 showing no congestion, Group II K1R2 showing severe congestion and arrows indicating moderate congestion in group III K1R7 (H&E, X400).
| Grades | Group1 | Group2 | Group3 | P value |
|---|---|---|---|---|
| Normal | 100%* | 0% | 0% | 0.000* |
| Minimal | 0% | 0% | 0% | |
| Mild | 0% | 0% | 25% | |
| Moderate | 0% | 37.5% | 62.5%* | |
| Severe | 0% | 62.5%* | 12.5% |
P value ≤ 0.05
Table 1: Group wise distribution of grades of congestion among control and experimental groups of albino rats.
In 100% rats of control group 1 no hemorrhage was observed in renal cortex while in ED group severe hemorrhage (grade 4) was noted in 75% of rats. In withdrawal group 75% lesions were mild (grade 2) showing significant reduction in hemorrhage grade when compared to ED group (Figure 2 and Table 2).
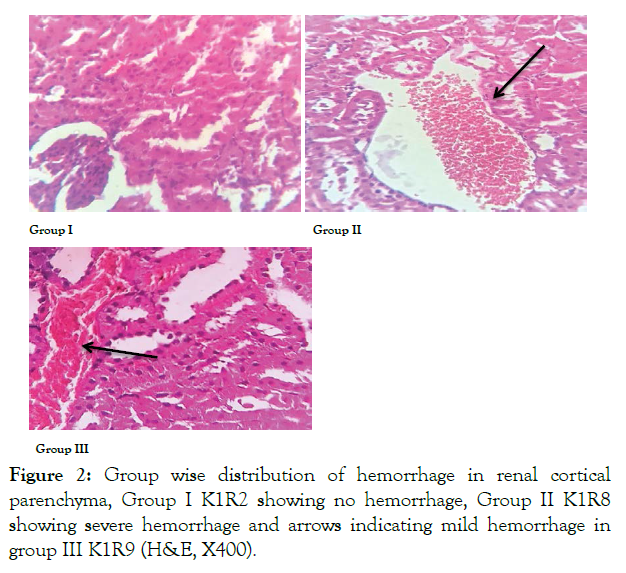
Figure 2: Group wise distribution of hemorrhage in renal cortical parenchyma, Group I K1R2 showing no hemorrhage, Group II K1R8 showing severe hemorrhage and arrows indicating mild hemorrhage in group III K1R9 (H&E, X400).
| Grades | Control group | ED group | Withdrawal group | P value |
|---|---|---|---|---|
| Normal | 100%* | 0% | 0% | 0.000* |
| Minimal | 0% | 0% | 12.5% | |
| Mild | 0% | 12.5% | 75%* | |
| Moderate | 0% | 12.5% | 12.5% | |
| Severe | 0% | 75%* | 0% |
P value ≤ 0.05
Table 2: Group wise distribution of grades of hemorrhage among control and experimental groups of albino rats.
Renal cortex appeared to be normal in 100% rats in control group 1. In ED group severe (grade 4) loss of brush border was seen in PCT of 87.5% rats. In group E mild loss of brush border in 50% of rats was seen that showed significant reversal of histological alteration after withdrawal of ED (Figure 3 and Table 3).
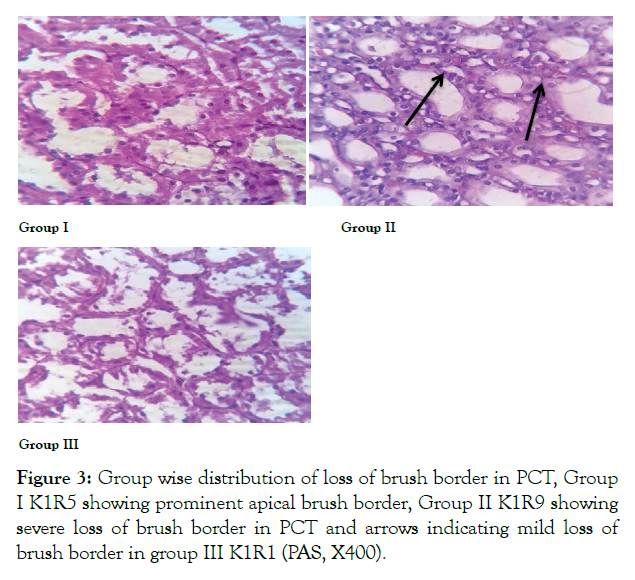
Figure 3: Group wise distribution of loss of brush border in PCT, Group I K1R5 showing prominent apical brush border, Group II K1R9 showing severe loss of brush border in PCT and arrows indicating mild loss of brush border in group III K1R1 (PAS, X400).
| Grades | Control group | ED group | Withdrawal group | P value |
|---|---|---|---|---|
| Normal | 100%* | 0% | 0% | 0.000* |
| Minimal | 0% | 0% | 12.5% | |
| Mild | 0% | 0% | 50%* | |
| Moderate | 0% | 12.5% | 37.5% | |
| Severe | 0% | 87.5%* | 0% |
P value ≤ 0.05
Table 3: Group wise distribution of grades of loss of brush border among control and experimental groups of albino rats.
In control group 100% rats showed normal renal cortical architecture. In ED group severe (grade 4) necrosis was observed in 100% of rats. In group 3 moderate (grade 3) necrosis was seen in 75.5% rats. In this way significant reduction in grades of necrosis was observed in withdrawal group showing changes were reversible in 8 weeks duration (Figure 4 and Table 4).
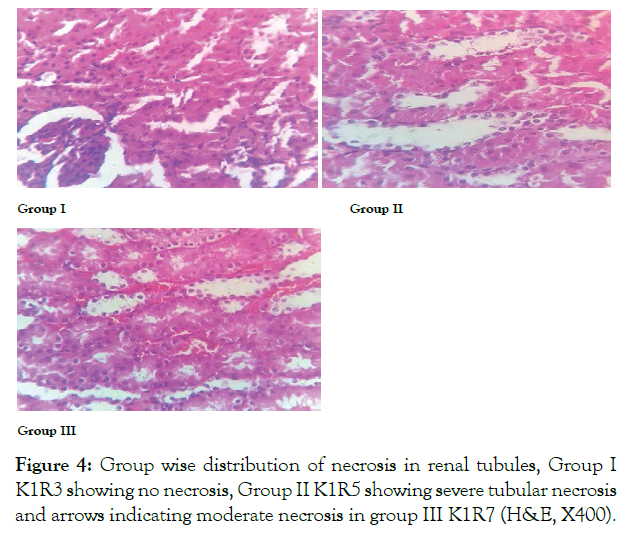
Figure 4: Group wise distribution of necrosis in renal tubules, Group I K1R3 showing no necrosis, Group II K1R5 showing severe tubular necrosis and arrows indicating moderate necrosis in group III K1R7 (H&E, X400).
| Grades | Control group | ED group | Withdrawal group | P value |
|---|---|---|---|---|
| Normal | 100% | 0% | 0% | 0.000* |
| Minimal | 0% | 0% | 12.5% | |
| Mild | 0% | 0% | 12.5% | |
| Moderate | 0% | 0% | 75% | |
| Severe | 0% | 100% | 0% |
P value ≤ 0.05
Table 4: Group wise distribution of grades of necrosis among control and experimental groups of albino rats.
This study investigated the reversal of renal histo morphological features after withdrawal of ED in kidneys of male rats. In this study we observed no congestion with normal renal cortical parenchyma in control group. In ED group severe congestion in renal cortical parenchyma was observed. In withdrawal group, moderate congestion was seen. Impairment of venous outflow due to inflammatory mediators results in a localized increase in blood to different areas of kidney, which is demonstrated histologically as congestion [9]. Taiwo et al. documented congestion after administration of red bull in different doses to rabbits [10]. He documented that changes observed were reversible in 28 days duration of study [10]. Similarly study conducted by Adel et al. indicated severe congestion in kidneys of groups receiving Coca-cola, Pepsi, 7-Up for 12 weeks (chronic use) [11]. Comparable results were shown by Josiah who used two brands of soda drinks liberally to group of rats for 4 weeks; disruption of membranes due to toxic effects of these drinks was implicated in renal parenchymal damage [12]. Our results are consistent with Kassab, who used similar dose of energy drinks and observed histological changes on submandibular gland. The reported changes were reversible in that study [13].
In our study severe hemorrhage was observed in ED group. In rats of group 3 mild hemorrhages was seen. Hemorrhage observed was due to the effect of inflammation leading to expansion of blood vessels, as result vessels rupture and blood flows out. A previous study showed similar findings after administration of Tiger-type ED 1 ml/animal/day orally for 4 weeks [14]. In another study, comparable histological findings were observed in liver of rats after administration of high dose of EDs [15]. Akande observed the nephrotoxic effects of power horse ED in different doses in rats and proved that damaged caused by ED was reversible in 28 days duration [7].
The present study shows severe loss of brush border of PCT in ED group, while in withdrawal group mild loss of brush border was observed this showed that after withdrawal of ED histological changes caused by red bull in renal cortical parenchyma were reversible with this duration of study and with low doses used in this study that were equivalent to one can in humans. Loss of brush border in ED group might be explained by vulnerability of cellular membranes to toxins leading to decrease cellular production of ATP. This causes accumulation of reactive oxygen species, which further aggravates cell damage, destabilization of cell contact areas, and detachment of epithelial cell from basement membrane. Similar results were shown in albino rabbits by Salih after they were given different doses of red bull, results were ascribed to high level of caffeine in EDs [16].
Acute tubular necrosis is most common cause of acute kidney injury; it leads to death of tubular epithelial cells resulting in renal failure. In withdrawal group moderate necrosis was seen which was significantly reverted after withdrawal of ED [17]. Coagulate pattern of necrosis was observed in various experimental groups which is more common in toxic or ischemic injury [18]. The observed necrosis may be due to carbon dioxide present in energy drinks that damages membranes of mitochondria, change in ATP production, hypoxia and cell death [14]. In another study after administration of red bull, severe necrosis was observed in seminiferous tubules of male rats [19].
Kidneys play an important role in the excretion of toxic substances. High caffeine content in EDs adversely affects renal function. In this study we observed the severity of renal injury in relation to time duration for which EDs were used. In ED group significant difference was observed in all parameters; weight of kidneys, congestion, hemorrhage, loss of brush border and necrosis. This indicates that EDs leads to renal damage that is duration or time dependent, since more damage was observed in ED group when compared to group 3, it showed reversal of these histological features after withdrawal of ED. Our findings are consistent with the results of study that proved that effects of caffeine on cell survival are highly time and dose dependent; in low doses it increases cell survival and at higher doses it increases super oxide production [20,21]. Also the study proved that chronic caffeine intake has age dependent effects on brain [22].
In conclusion with low doses used in this study, corresponding to one can of ED (250ml) in humans and with this duration of study histo morphological changes caused by EDs are reversible.
Citation: : Bano SS, Bokhari HA (2021) Effects of Energy Drink on Glomerular Parameters and its Conservation by Vitamin D3. J Alcohol Drug Depend9: 362. doi: 10.35248/2329-6488.21.9.362
Received: 04-Oct-2021 Accepted: 20-Oct-2021 Published: 26-Oct-2021 , DOI: 10.35248/2329-6488.21.9.362
Copyright: ©2021 Bano SS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.