Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Research Article - (2019)Volume 7, Issue 2
Access to planting materials is one of the main challenges constraining the widespread adoption of the disease resistant Coffea arabica L. F1 hybrid variety Ruiru 11 in Kenya. Production of the planting materials for the variety relies on several cost-intensive methods including hand pollination for hybrid seed production and vegetative propagation through cuttings. These seed production methods are inefficient and costly and rely heavily on the weather conditions. Production and supply of planting materials for the commodity is therefore unable to meet the annual demand for the variety. In an attempt to bridge the gap between the supply and demand, tissue culture technique has been deployed at the Coffee Research Institute in Kenya. This however requires empirical tests to optimise in vitro mass propagation protocols for hybrid coffee varieties. The current study investigated the effects of genotype and plant growth regulators, auxins and cytokinins, on induction of embryogenic callus in two composite genotypes of Coffea arabica L. F1 hybrid variety Ruiru 11. Code 71 and Code 93. Leaf explants from the F1 hybrid were cultured on half-strength Murashige and Skoog (MS) media supplemented with varied concentrations of plant growth regulators. Callus formation was evaluated weekly until the 60th day. Genotypic effects were assessed based difference on callus induction rates, biomass fresh weights and callus formation. The genotypes tested showed highest callus induction 88% (Code 71) and 100% (Code 93) with respect to the formation of embryogenic calli. Highest fresh weight was obtained at 0.973 ± 0.011g in Code 71 and 0.649 ± 0.03 g in Code 93 in MS media supplemented with 2,4-D + BAP (2.5+0.5 mg/L). The observed results are useful in formulating the best growth regulator concentration suitable for mass in vitro propagation of genotypes of Arabica coffee hybrid Ruiru 11 through callus induction in vitro of leaf explants.
Somatic embryogenesis; Coffee arabica hybrid; Plant growth regulators; in vitro
Coffee is one of the most important commodities in the international trade and is cultivated in almost 80 countries where 70% of the total global production is by smallholder farmers [1]. The crop earns substantial foreign exchange earnings to the producer countries and contributes significantly to food security and employment and overall alleviates poverty [2]. In Africa, for example, the crop is a primary source of income for more than 12 million households and contributes a significant proportion of tax income in the producing countries. In Kenya, the coffee industry contributes about 1% to the national GDP, 8% of the total agricultural export earnings and up to 25% of the total labour force employed in agriculture [3]. The coffee sector supports around 700,000 households representing 4.2 million Kenyans. Improving coffee production is, therefore, paramount to solving income security challenges, at household, country, and regional levels.
Two main species of coffee dominate the global trade in the commodity, namely, Arabica coffee (Coffea arabica L.) which accounts for approximately 70% of the world’s production and Robusta coffee (Coffea canephora Pierre) accounting for 30% of the global trade [4]. C. arabica is preferred due to its high cup quality compared to other coffee species. However, Arabica coffee is highly sensitive to climate change and vulnerable to important diseases such as Coffee Leaf Rust (CLR) (Hemileia vastratix) and Coffee berry disease (Colletotrichum kahawae) (CBD) [5] which together greatly undermine its capacity for growth and fruiting and inflicts substantial economic losses to the resource-poor smallholder producers.
Kenya produces almost exclusively Arabica coffee. Production of the crop has declined considerably, falling from an average of 1.5 million bags in the 1970s to 790, 000 bags in by ICO in 2019 [6]. In light of the significance of the commodity to the Kenyan economy, substantial efforts have been directed towards the revitalisation of the coffee subsector in the country. The focus has been to increase coffee productivity with a focus on small-scale farmers. One of the pillars for delivering increased productivity is the large-scale adoption of the high yielding disease resistant F1 hybrid coffee variety Ruiru 11. Mass adoption of this improved variety is however challenged by the inadequate supply of hybrid seeds and seedlings, with the demand for planting materials of the variety more than doubling the current seed and seedling production capability of the country.
The conventional propagation methods of artificial hand pollination and cloning through rooted cuttings have proven insufficient in meeting demands for coffee planting materials in Kenya due to several limitations including the cumbersome nature of the methods, high dependence on weather conditions, high cost of labour, increased risk of spreading pests and diseases during transportation and low success rate with respect to rate of fruit set and rooting of cuttings [7]. In an attempt to mitigate the limitations associated with conventional production of planting materials for the hybrid coffee varieties, scientists have turned to in vitro techniques through somatic embryogenesis which has great potential for large scale multiplication of economically important species. The results have nevertheless been limited due to challenges inherent in the method when it comes to hybrid coffee varieties.
Several reports exist on the successful regeneration of plants using somatic embryogenesis techniques [8,9]. In coffee, two methods of somatic embryogenesis (SE) have been studied, direct somatic embryogenesis (DSE), from which embryos originate directly from the explants [4] and indirect somatic embryogenesis (ISE), from which embryos are derived through an intervening callus phase. The ISE approach is preferred for mass propagation in tissue culture [8,10,11] due to the production of a high number of somatic embryos per gram of callus compared to DSE. Despite the success associated with ISE, reports give caution on the specificity of genotype, and its interaction with plant growth regulators supplemented in the nutrient medium in coffee [12].
The efficiency of SE is not only attributed to the genotype but, also on plant growth regulators (PGR) concentration levels [13]. PGRs have exhibited effects on growth and development in a wide variety of woody species. As such, Evaluation of PGRs is therefore necessary when working with woody perennials such as coffee to determine their ability to control growth in vitro. Cytokinins promote cell division while Auxins promote both cell division and cell growth [14].
Several plant growth regulators are used in indirect somatic embryogenesis, and available reports suggest auxins (2,4-D and IBA) and cytokinins (BAP and KIN) are ideal [11,13,15]. According to Maciel et al. [16] and [13] Etienne et al. PGR used in combinations and singly have successfully induced callus in Coffee species but, is specific to concentration levels and types of PGR in addition to coffee genotype. Improving the efficiency in induction protocols, therefore, requires a good understanding of the correct combinations of PGRs that illicit maximum callus induction for the genotypes under consideration. This would facilitate an understanding of the specific factors with respect to time, induction rates and callus characteristics necessary to design optimised callus induction protocols for varieties of woody species such as Arabica coffee hybrid Ruiru 11 under Temporary Immersion System (TIS). The current research was undertaken with these facts in mind and had, as an objective, to investigate the effects of different combinations of plant growth regulators on callus induction on two genotypes of C. arabica F1 hybrid, Ruiru 11.
Plant materials
The study was conducted at the Plant Tissue Culture Laboratory at the Coffee Research Institute (Ruiru, Kenya). Two genotypes of C. arabica F1 hybrid Ruiru 11, Code 71 and Code 93 were used as donor plants (Table 1). Tissue culture seedlings of the two Codes were then raised in a greenhouse for a period of six months prior to being used as donor plants.
| Coffee Hybrid | Pedigree | |
|---|---|---|
| Code 71 | SL28 | [ (N39 x HT) x (SL28 x RS)] x Catimor |
| Code 93 | SL28 | [ (SL34 x RS) x HT)] x Catimor |
Table 1: Pedigree on code 71 and code 93 C. arabica Ruiru 11.
Establishment of callus cultures
The third leaf-pairs were excised from the donor plants and placed in a beaker containing tap water and transferred to the tissue culture laboratory. The leaves were sterilized on both sides using cotton wool dipped in dilute Teepol detergent and rinsed under running tap water. In a sterile laminar flow hood, further surface sterilization was done using 20% commercial bleach (JIK) which contain 3.85% (w/v) sodium hypochlorite for 15 minutes and rinsed thrice using sterile distilled water. The leaf explants were subsequently immersed in 70% ethanol and rinsed three times with sterile distilled water [4]. These were then dissected into leaf discs measuring approximately 1 cm × 1 cm, excluding the main vein and edges (lateral apical and basal portions). The explants were inoculated in ½ [17,18] (MS) media basal media with the adaxial-side down in culture vessels containing three leaf discs per vessel.
Culture MS media containing ½ micro-salts, macro-salts and vitamins were supplemented with different plant growth regulators (2,4- D, IBA, BAP, and KIN) (Table 2), sucrose (30 g/L), 100 ml/L inositol, 30 mg/L cysteine-HCL and 40 g/L gelrite. Media pH was adjusted to 5.7 (using 1n NaOH and 1 nHCL). The media was sterilized at 121°C under 1.1 psi for 15 minutes prior to culture. Control experiments where MS media was not supplemented with plant growth regulators and inoculated with explants were used. The culture vessels containing explants were sealed and incubated in dark growth room at 25 ± 2°C.
| Plant growth regulator | Dilutions (mg/L) | ||||
|---|---|---|---|---|---|
| IBA+KIN/ IBA + BAP | 0.5+2.5 | 1.0+2.0 | 1.5+1.5 | 2.0+1.0 | 2.5+0.5 |
| 2,4-D + KIN / 2,4-D + BAP | 0.5+2.5 | 1.0+2.0 | 1.5+1.5 | 2.0+1.0 | 2.5+0.5 |
| 2,4-D/ IBA / KIN / BAP | 0.5 | 1 | 1.5 | 2 | 2.5 |
Table 2: Plant growth regulators used during experiments on C. arabica Ruiru 11 code 93 and code 71 leaf explants.
The frequency of callus induction was recorded every two weeks and calculated after 60 days, as shown below:
Callus induction (%) = [Total number of explants with callus induction/total number of explants inouculated for each treatment] × 100
Callus characteristics and growth parameters
The callus characteristics were graded based on morphology, callus score, and color. Callus morphology was characterized after 60 days of culture inoculation based on two characteristics: (i) friable (embryogenic) and (ii) compact (non-embryogenic) as shown in Figure 1. The study evaluated callus score based on growth viability under five categories: NC=No callus, + = Very poor, ++ = Poor, +++ = Good and ++++ = Very good for each of the plant growth regulator treatment.
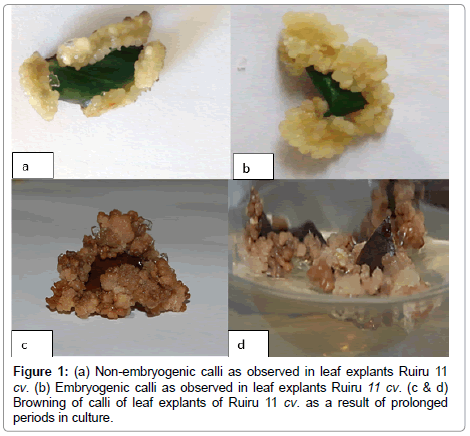
Figure 1. (a) Non-embryogenic calli as observed in leaf explants Ruiru 11 cv. (b) Embryogenic calli as observed in leaf explants Ruiru 11 cv. (c & d) Browning of calli of leaf explants of Ruiru 11 cv. as a result of prolonged periods in culture.
The callus growth period was recorded based on the time taken to initiate visible callus from leaf explants. The eye-ball technique was used to assess the callus induction time taken.
Callus weights were measured on a precision scale (g) in a sterile laminar flow hood as described by Balbaa et al. [19]. after 60 days from inoculation.
For growth curve, analysis of explant cultures was initiated on the first day of inoculation (day 0). Subsequently, analyses were done at intervals of 7 days until the 70th day. The growth rate was assessed as described by Dung et al. [20].
Average growth rate=[Wf-Wi]/t
where Wf= final weight of fresh callus matter, Wi= initial weight of fresh matter and t=cultivation period (days).
Effect of genotype on callus induction
The two genotypes, Code 71 and Code 93, were inoculated in MS Media to test for the callogenesis response. Effects were assessed based on the formation of embryogenic and non-embryogenic calli and callus induction rates (%).
Statistical Analysis
The experiments were laid out in completely randomized design and repeated three times with 20 replicates. The results were assessed by a standard analysis of two-way ANOVA variance (Duncan Multiple Range Test) using SAS software 9.11. Each genotype was assessed individually.
Callus induction
The effects of plant growth regulators, genotype, and callus characteristics were investigated. The results indicated that control experiments that lacked plant growth regulators did not induce callus even after 40 days of inoculation in basal culture media thereby affirming that plant growth regulator (PGR) are crucial to induction of callus in C. arabica F1 hybrid Ruiru 11 varieties. PGR used singly, auxin 2,4-D induced callus whereas, auxin IBA and cytokinin (BAP and KIN) did not induce callus even after 40 days of culture. Highest induction frequency was observed for 2,4-D (2.5 mg/L) in Code 93 at 97% and 2,4-D (1.5 mg/L) in Code 71 at 88% (Tables 3 and 4). The induction frequency, however, varied within concentrations. Low concentration levels of 2,4-D in Code 71 was observed to have increased induction rates, whereas higher concentration levels recorded reduced induction rates (Table 3). The opposite was true for Code 93, which recorded increased induction rates with increased concentration levels of 2,4-D (Table 4). The results suggest that genotype plays an important role in callus induction. Both the type of PGR and the concentration of the same are therefore important in callus induction in Arabica coffee hybrids.
| Plant Growth Regulators | Concentration (mg/L) | Degree of Callus formation | Duration of Calli initiation | Color and morphology of callus | % Callus induction | Callus FW (g ± SE) |
|---|---|---|---|---|---|---|
| 2,4-D | 0 | - | - | NC | - | - |
| 0.5 | ++ | 6 weeks | FW | 78% | 0.178 ± 0.026c | |
| 1 | ++ | 6 weeks | FW | 85% | 0.320 ± 0.006ab | |
| 1.5 | ++ | 5 weeks | FW | 88% | 0.490 ± 0.013b | |
| 2 | ++ | 5 weeks | FW | 50% | 0.0962 ± 0.011d | |
| 2.5 | ++ | 5 weeks | FW | 45% | 0.0672 ± 0.002d | |
| 2,4-D + BAP | 0.5 + 2.5 | +++ | 5 weeks | FW | 68% | 0.237 ± 0.017c |
| 1.0 + 2.0 | +++ | 5 weeks | FW | 80% | 0.294 ± 0.023b | |
| 1.5 + 1.5 | +++ | 4 weeks | FW | 75% | 0.298 ± 0.011b | |
| 2.0 + 1.0 | ++++ | 4 weeks | FW | 76% | 0.593 ± 0.03a | |
| 2.5 + 0.5 | ++++ | 4 weeks | FW | 88% | 0.649 ± 0.026a | |
| 2,4-D + KIN | 0.5 + 2.5 | ++ | 6 weeks | CW | 53% | 0.088 ± 0.002b |
| 1.0 + 2.0 | ++ | 5 weeks | CW | 60% | 0.080 ± 0.052a | |
| 1.5 + 1.5 | +++ | 5 weeks | FW | 68% | 0.238 ± 0.016a | |
| 2.0 + 1.0 | +++ | 5 weeks | FW | 73% | 0.200 ± 0.002b | |
| 2.5 + 0.5 | +++ | 5 weeks | FW | 76% | 0.037 ± 0.013b |
Table 3: Callus induction from leaf explants of C. arabica code 71 in MS media supplemented with different concentration of auxins and cytokinins.
| Plant Growth Regulator | Concentration (mg/L) | Degree of Callus formation | Duration of calli initiation | Color and morphology of callus | % Calli Induction | Callus FW (g ± SE) |
|---|---|---|---|---|---|---|
| 2,4-D | 0 | - | - | NC | - | - |
| 0.5 | ++ | 4 weeks | FW | 75% | 0.190 ± 0.01d | |
| 1 | +++ | 4 weeks | FW | 88% | 0.394 ± 0.008b | |
| 1.5 | +++ | 3 weeks | FW | 86% | 0.636 ± 0.025c | |
| 2 | ++++ | 3 weeks | FW | 96% | 1.069 ± 0.151a | |
| 2.5 | ++++ | 3 weeks | FW | 97% | 0.961 ± 0.02a | |
| 2,4-D+ BAP | 0.5+2.5 | +++ | 4 weeks | FW | 75% | 0.273 ± 0.006b |
| 1.0+2.0 | +++ | 4 weeks | FW | 80% | 0.291 ± 0.022ab | |
| 1.5+1.5 | +++ | 3 weeks | FW | 93% | 0.174 ± 0.032b | |
| 2.0+1.0 | ++++ | 3 weeks | FW | 100% | 0.341 ± 0.011a | |
| 2.5+0.5 | ++++ | 3 weeks | FW | 100% | 0.348 ± 0.017b | |
| 2,4-D + KIN | 0.5+2.5 | ++ | 5 weeks | CW | 50% | 0.273 ± 0.052b |
| 1.0+2.0 | ++ | 5 weeks | CW | 55% | 0.291 ± 0.058ab | |
| 1.5+1.5 | +++ | 4 weeks | FW | 63% | 0.174 ± 0.032b | |
| 2.0+1.0 | +++ | 4 weeks | FW | 73% | 0.341 ± 0.03a | |
| 2.5+0.5 | +++ | 4 weeks | FW | 76% | 0.248 ± 0.045b |
Table 4: Callus induction from leaf explants of C. arabica code 93 in MS media supplemented with different concentration of auxins and cytokinins.
Auxin and cytokinin combinations significantly affected callus induction in both Code 93 and Code 71. 2,4-D+BAP overall recorded highest induction rates in both Code 71 (Table 3) and Code 93 (Table 4). 2,4-D+BAP (2.5+0.5 mg/L) resulted in highest induction rates at 100% in Code 93 (Table 4) and 88% in Code 71 (Table 3). The study also observed that higher concentration levels of auxins (2,4-D and IBA) combined with lower concentrations of cytokinins (BAP and KIN) improved induction rates in both Code 93 (Tables 5 and 6) and Code 71 (Table 3 and Table 5).
| Plant Growth Regulator | Concentration (mg/L) | Degree of Callus formation | Duration of calli initiation | Color and morphology of callus | % Calli formation | Callus FW (g ± SE) |
|---|---|---|---|---|---|---|
| IBA+ BAP | 0.5+2.5 | ++ | 6 weeks | CW | 65% | 0.124 ± 0.022d |
| 1.0+2.0 | ++ | 6 weeks | CW | 68% | 0.183 ± 0.028c | |
| 1.5+1.5 | ++ | 6weeks | CW | 69% | 0.258 ± 0.012ab | |
| 2.0+1.0 | ++ | 5 weeks | CW | 75% | 0.304 ± 0.011a | |
| 2.5+0.5 | ++ | 5 weeks | CW | 71% | 0.249 ± 0.012b | |
| IBA + KIN | 0.5+2.5 | ++ | 6 weeks | CW | 61% | 0.123 ± 0.022c |
| 1.0+2.0 | ++ | 6 weeks | CW | 68% | 0.190 ± 0.02b | |
| 1.5+1.5 | +++ | 5 weeks | CW | 60% | 0.236 ± 0.018b | |
| 2.0+1.0 | +++ | 5 weeks | CW | 75% | 0.342 ± 0.045a | |
| 2.5 + 0.5 | +++ | 5 weeks | CW | 81% | 0.248 ± 0.048b |
Table 5: Callus Induction from leaf explants of C. arabica Code 71 in MS media supplemented with different concentration of auxins and cytokinin.
| Plant Growth Regulator | Concentration (mg/L) | Degree of Callus formation | Duration of calli initiation | Color and morphology of callus | % Calli induction | Callus FW (g ± SE) |
|---|---|---|---|---|---|---|
| IBA+BAP | 0.5+2.5 | ++ | 5 weeks | CW | 60% | 0.280 ± 0.015ab |
| 1.0+2.0 | ++ | 5 weeks | CW | 64% | 0.302 ± 0.011a | |
| 1.5+1.5 | ++ | 4 weeks | CW | 68% | 0.279 ± 0.011ab | |
| 2.0+1.0 | ++ | 4 weeks | CW | 75% | 0.304 ± 0.011a | |
| 2.5+0.5 | ++ | 4 weeks | CW | 70% | 0.249 ± 0.012b | |
| IBA+ KIN | 0.5+2.5 | ++ | 6 weeks | CW | 61% | 0.273 ± 0.015b |
| 1.0+2.0 | ++ | 6 weeks | CW | 68% | 0.291 ± 0.013ab | |
| 1.5+1.5 | +++ | 5 weeks | CW | 58% | 0.274 ± 0.015b | |
| 2.0+1.0 | +++ | 5 weeks | CW | 73% | 0.341 ± 0.044a | |
| 2.5+0.5 | +++ | 5 weeks | CW | 80% | 0.248 ± 0.009b |
Table 6: Callus induction from leaf explants of C. arabica code 93 in MS media supplemented with different concentration of auxins and cytokinins.
Callus characteristics
Preliminary callus structures were observed at the cut edges of the leaf discs after 7 days of culture and callus induction was observed for a period of 8 weeks (Figures 1a and 1b). A white callus morphology was recorded across all treatments in Code 71 and Code 93. The color of resultant calli did not affect proliferation rates across all treatments.
Media supplemented with 2,4-D induced friable callus (Figure 1b) with poor formation (++) across all treatments in Code 71 (Table 3) and Code 93 (Table 4). Higher concentration levels of 2,4-D combined with lower concentration levels of BAP induced very good friable calli (++++) in Code 71 and Code 93 whereas, lower levels of 2,4-D and higher levels of BAP recorded good friable callus formation (+++) in Code 71 (Table 3) and Code 93 (Table 4). On the other hand, IBA combined with KIN and BAP induced compact calli (Figure 1a) across all treatments in Code 71 (Table 5) and Code 93 (Table 6). The browning of callus was observed after extended periods in culture, which could indicate the onset of cell death (Figures 1c and 1d).
Callus induction time
The shortest period for maximum callus proliferation in Code 71 was recorded in MS media supplemented with 2,4-D + BAP (Table 3) and Code 93 (Table 4). 2,4-D recorded increased initiation time with a difference of 5-6 weeks in Code 71 (Table 3) but, reduced initiation time (3-4 weeks) in Code 93 (Table 4). These observations indicate that PGRs treatments had an effect both on the time to and rate of callus induction. Auxin-cytokinin combinations (2,4-D, IBA) and (BAP and KIN) recorded longer initiation periods in Code 71 and Code 93. Time is a critical factor in the determination of efficient induction protocols. However, accompanying factors including biomass, callus morphology, and induction frequency, are important.
Callus growth curve
The callus growth curve is necessary to identify the suitable point of transfer in coffee tissue culture. The growth curve of calli indicated three distinct phases: lag phase, exponential phase, and linear phase [21,22]. Lag phase and exponential phase represent cell growth ideal for cell division and cell multiplication of callus. Linear phase is characterized by reduced cell division [21]. Prolonged linear phase promotes phenolic compound production, which is not amenable for callus proliferation. This may explain the browning observed in cultures. Figures 2 and 3 indicates growth curves recorded on embryogenic calli obtained from 2,4-D+BAP (Figure 4a), 2,4-D (Figure 4b) and 2,4-D+KIN (Figure 4c) in Code 71 and Code 93. Dedifferentiation of leaf calli was evident through a lag phase from the day of inoculation to the 14th day of culture inoculation; exponential phase from the 21st day to the 42nd day of exposure and linear phases from the 42nd day of culture. The growth curves showed a peak in growth from the 28th day to the 42nd day with a subsequent decrease in biomass. The prolonged linear phase suggests that a decrease in growth rate results from the scarcity of nutrients or drying of solidifying agent or accumulation of toxic substances [22]. The results, therefore, suggest that transfer to new growth media is necessary to increase survival rates (cell viability) and sustain callus growth after the 42nd day.
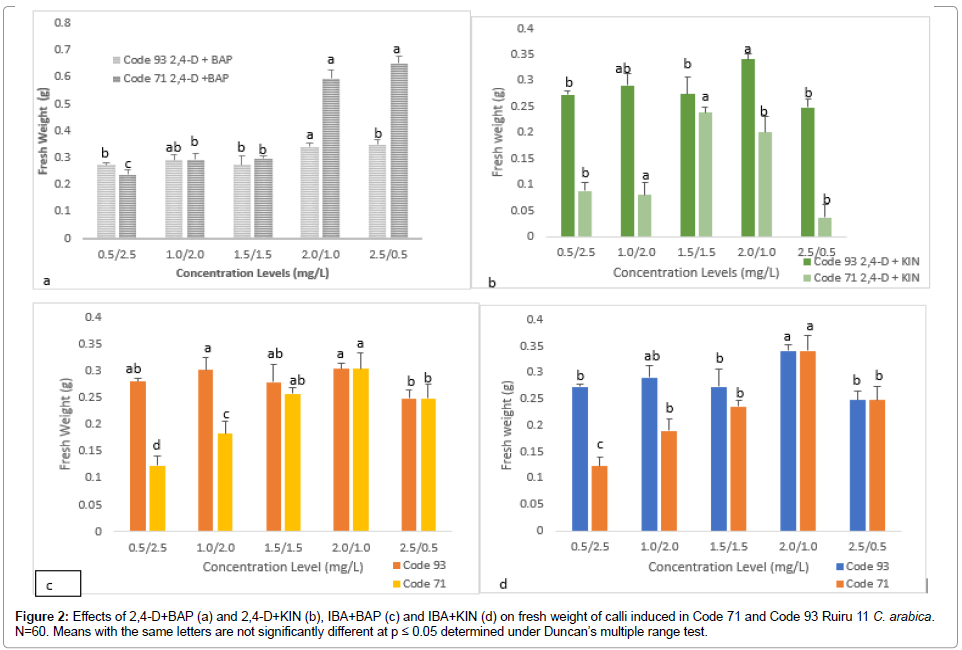
Figure 2. Effects of 2,4-D+BAP (a) and 2,4-D+KIN (b), IBA+BAP (c) and IBA+KIN (d) on fresh weight of calli induced in Code 71 and Code 93 Ruiru 11 C. arabica. N=60. Means with the same letters are not significantly different at p ≤ 0.05 determined under Duncan’s multiple range test.
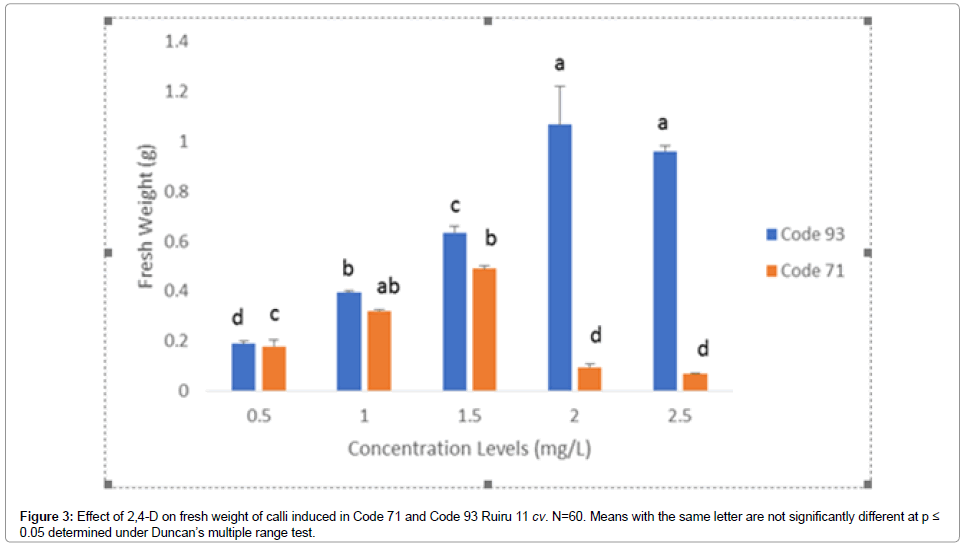
Figure 3. Effect of 2,4-D on fresh weight of calli induced in Code 71 and Code 93 Ruiru 11 cv. N=60. Means with the same letter are not significantly different at p ≤ 0.05 determined under Duncan’s multiple range test.
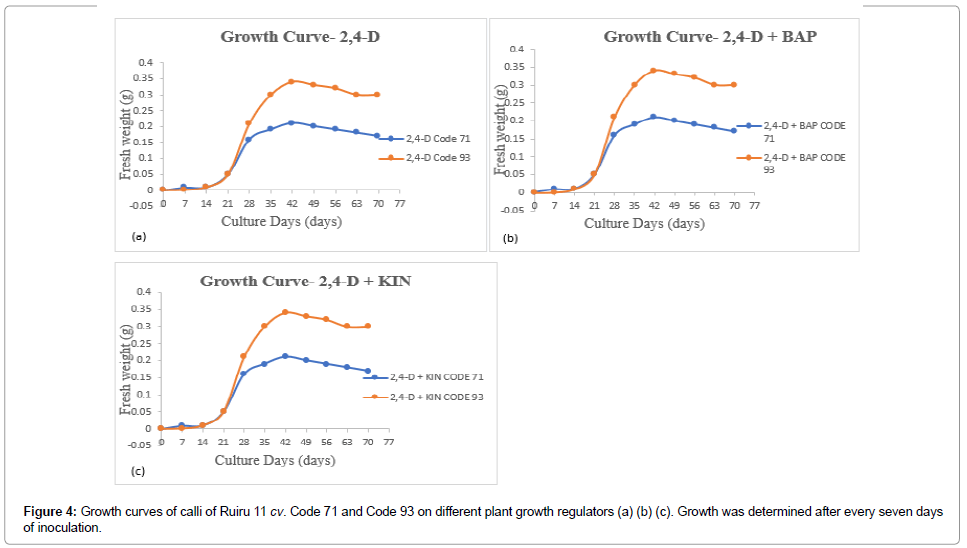
Figure 4: Growth curves of calli of Ruiru 11 cv. Code 71 and Code 93 on different plant growth regulators (a) (b) (c). Growth was determined after every seven days of inoculation.
Genotypes
Genotype played a significant role in callus induction in cultures. Code 93 culture response in induction rates, fresh weight biomass and time taken for callus induction was significantly better compared to Code 71. Code 93 recorded callus induction at a 50-100% rate in the different plant growth regulators supplemented in MS media (Table 4 and Table 6). Code 93 (Table 4 and 6) was observed to have better callus formation compared to Code 71 (Table 3 and Table 5) across all treatments. The explanation to this could be attributed to genealogy influences.
Callus induction
From the results observed on the control experiments with no plant growth regulators in culture media, there was no callus induction in all the trials. Similar results were obtained by Ahmed et al. [13] in C. arabica leaf explants and [21] in Capsicum annum. This is expected given that both auxins and cytokinins are paramount in promoting both cell division and cell growth, with auxins being necessary for both parameters and cytokinins being crucial in cell division. Callus induction is the product of both cell division and growth. Ideally, the results indicate those plant growth regulators are necessary for callus induction. Plant growth regulators are important for regulation of growth and development in plant cells and tissues specifically with callus induction [23] elucidates that effective callus induction is as a result of positive interaction relationship between endogenous PGR and exogenous PGR. Exogenous PGR biological activity can be equivalent to or be in excess of endogenous PGR to influence physiological activity including callus induction. The present observations, therefore, support the results obtained hitherto by other researchers in coffee and other crop species. Auxins are plant growth regulators that promote cell division, cell growth and organization of meristems for callus formation [23,24].
The PGR used singly, auxin 2,4-D induced callus, whereas, auxin IBA and cytokinin (BAP and KIN) did not induce callus even after 40 days of culture. This agrees with and reemphasize the observations by (Maciel et al. [16] in Coffee Arabica L., [25], in Nerium odorum (Apocynaceae), [21] Dos Santos and De Souza in Capsicum annum, Gopitha et al. [26] in Saccharum offinarum (Sugarcane), and Lee et al. [14] in Morus alba (Mulberry) all whom observed the importance of 2,4-D in callus formation.
Unlike auxins, the single treatment with cytokinins did not yield any embryogenic response. This is expected from the observation because Gapsar et al. [23] point to the fact that cytokinins are suitable for cell division, with BAP and KIN being the most commonly used. The authors further observed that cytokinins are effective in callus formation when combined with auxins; this was observed to be true for the coffee genotypes investigated through this study. The reason is that cell division is a joint action that requires a synergistic relation between auxins and cytokinins [27]. In plant cells, for auxins to be effective, the PGR has to be protected from oxidative denaturization through molecular conjugation enabling storage and consequential, gradual release for enzymatic action [23]. 2,4-D, therefore, is ideal for callus formation compared to IBA when used singly.
The level of concentration of the respective PGRs was observed to have an important impact on the rate of callus induction, with different genotypes displaying different reaction patterns with low concentration levels of 2,4-D (0.5, 1.0 and 1.5 mg/L) resulting in higher induction rates in code 71 whereas a linear increase in induction rates was observed in Code 93 with increased concentration levels. This is in line with the observations by Molina et al. [28] and Rezende et al. [29] in C. arabica. Overall, Code 93 recorded highest induction rates compared to Code 71 which indicate that induction rates are strongly influenced by genotype which, supporting the observations by Jiménez [30] that embryogenic response to PGR is directly related to genotype.
Auxin and cytokinin combinations significantly affected callus induction in both Code 93 and Code 71. 2,4-D+BAP overall recorded highest induction rates in both Code 71 (Table 3) and Code 93 (Table 4). 2,4-D+BAP (2.5+0.5 mg/L) resulted in highest induction rates at 100% in Code 93 (Table 4) and 88% in Code 71 (Table 3). The results suggest the complementarity between auxins and cytokinins in callus formation. Similar observations have indeed been made in other studies including Gatica-Arias et al. [31], Maciel et al. [16] and Aga et al. [32] in C. arabica callus induction using auxin and cytokinin combinations.
Auxins (2,4-D and IBA) combined with cytokinin (BAP and KIN) obtained callus formation across all treatments in Code 71 and Code 93. However, significant differences were observed within the treatments. The differences were specific to auxin and cytokinins concentration and type along with genotype.
Cytokinin BAP combined with 2,4-D obtained highest induction rate compared to BAP combined with IBA. For instance, 2,4-D + BAP (2.5+0.5 mg/L) induced 88% in Code 71 and 100% in Code 93 whereas, IBA + BAP (2.0+1.0 mg/L) induced 75% in Code 71 and 75% in Code 93. A similar study by Aga et al. [32] in C. arabica report 2,4-D + BAP to induce best compared to IBA+BAP. However, combination 2,4-D+KIN and IBA+KIN obtained lower induction rates compared to 2,4-D+BAP. Studies by Etienne et al. [13] Gatica-Arias et al. [31] report of best callus induction was obtained in treatments supplemented with 2,4-D + KIN. Therefore, the results indicate that the induction rate in relation to the combination of PGR (auxin and cytokinin) greatly depends on the type of combination which varies alongside genotype. This is attributed to the different roles of auxins and cytokinins in plant cells division and growth. Reports on the role of PGR indicate that auxins regulate DNA replication while cytokinins control cell processes, including mitosis and cytokinesis [14,33].
For PGR to be effective, [23] Gaspar et al. elucidate that endogenous PGR interacts with exogenous PGR by which exogenous PGR biological activity can either be equivalent to or in excess to endogenous PGR. The interaction with endogenous PGR is specific, and cell and tissue responses greatly rely on plant species, the genotype of species and explant source. In most cases, auxin and cytokinin interaction can either be synergistic or antagonistic, whereby, auxins inhibit cytokinin action and vice versa. This may explain the significant difference in induction rates within the treatments supplemented with auxin and cytokinin in Code 71 and Code 93, including callus morphological response.
Callus morphology
Investigation of callus morphology is crucial in the assessment of the formation of embryogenic and non-embryogenic calli. Embryogenic calli are preferred due to characteristic loosely aggregated cells of low densities which have high cell viability for embryogenesis [21,34]. Auxin 2,4-D and auxin-cytokinin, 2,4-D+BAP obtained embryogenic calli across all treatments in Code 71 and Code 93. Similar observations are reported by Molina et al. [28] Maciel et al. [16] and Aga et al. [32] in C. arabica; Durrani et al. [33] Solanum spp, and Gopitha et al. [26] Saccharum officinarum. Low concentration levels of 2,4-D (0.5 and 1.0 mg/L) combined with KIN resulted in compact calli whereas, 2,4- D (1.5, 2.0 and 2.5 mg/L) combined with KIN induced embryogenic calli in Code 71 (Table 3) and Code 93 (Table 4). Etienne et al. [9] reported inducing embryogenic calli using 2,4-D+KIN (1.0+1.0 mg/L) while, Gatica-Arias et al. [31] reported the same results at 2,4-D+KIN (4.52 μM+18.56 μM) in C. arabica leaf explants. The present study also observed induction of non-embryogenic calli in auxin-cytokinin IBA+BAP and IBA+KIN. [11] on C. arabica leaf explants callus induction using IBA + KIN reported induction of embryogenic call whereas Mohajer et al. [35] on Onobrychis sativa, and [36] Wahyuni et al. on Justicia gendarussa reported non-embryogenic callus induction in media supplemented with IBA+BAP. Code 71 and Code 93 are obtained from similar coffee variety and similar explant, but, the embryogenic and non-embryogenic response is different, probably due to their genotypic difference. Jiménez [30] suggests that embryogenic and non-embryogenic competence in callus formation in similar explants from genetically identical cells and tissues respond differently to varying stimuli which could also be the case with respect to Codes 71 and 93 in this study.
Varying callus scores were observed across all treatments in Code 71 and Code 93. 2,4-D was observed to have poor callus induction calli (++) across all treatments in Code 71 (Table 3) but, significant differences were observed in Code 93 (Table 4) with increased levels of 2,4-D recording improved callus score (++++). Auxin-cytokinin (2,4- D + BAP) treatments recorded an improved callus score compared to 2,4-D used singly across all treatments in Code 71 (Table 3) and Code 93 (Table 4). 2,4-D+BAP was also observed to induce better callus score compared to 2,4-D+KIN and IBA combinations with BAP and KIN. The results suggest that callus score did not have any direct influence on embryogenic response rates but, presented a direct relationship with concentration and type of PGR used singly and in combination.
With regard to callus induction time, the results suggest that time taken to induce callus is directly influenced by genotype as well as PGR type and concentration level. 2,4-D was observed to induce callus within a shorter period of time in Code 93 (Table 4) compared to Code 71 (Table 3). Embryogenic calli obtained in 2,4-D+BAP in Code 71 (Table 3) was observed to induce calli at a shorter period of time compared to 2,4-D but, no difference observed in Code 93 (Table 4). However, combinations of 2,4-D +KIN in Code 93 and Code 71 recorded callus induction time longer compared to 2,4-D +BAP with a one-week interval. Similarly, IBA combined with KIN and BAP induced callus within a longer period of time in Code 71 (Table 5) and Code 93 (Table 6). Time is a critical factor when optimizing micropropagation protocols for high-frequency somatic embryogenesis for large-scale propagation. As such, shorter induction periods are superlative compared to longer induction periods. This parameter is often coupled with embryo formation yield, which should emphasize on high-frequency somatic embryogenesis.
Callus growth curve
Investigation on callus growth curve is paramount to determine the deceleration phase [21]. A growth curve encompasses phases that include lag phase, exponential phase, linear phase, and deceleration phase. The pattern of the growth curve is dependent on plant species [21,22]. The present study observed three growth stages; lag, exponential, and linear phase. The growth curve of the study did not obtain the deceleration phase, but, phenolic compound production (synonymous with browning in coffee) was reported. Browning is tantamount with activation of secondary metabolites; phenolic compounds [37] considered to be a severe problem in indirect somatic embryogenesis where it inhibits growth resulting in reduced regeneration potential and recalcitrance [38]. Data in Figure 3 indicate that peak growth competence was achieved in embryogenic calli on the 42nd day with a subsequent slight deceleration in the growth curve. This peak indicates that probably optimal growth was achieved at the 42nd day, which consequently should be the ideal time to transfer calli to new media for callus proliferation hence increase survival rate and improve on callus growth. A similar conclusion was reached by Dos Santos and De Souza [21] working on C. canephora calli. which which suggest that genotype has no influence over callus growth curve.
Biomass yield
The embryogenic potential is determined by biomass yield, which is an important factor in coffee somatic embryogenesis. Auxin, 2,4-D used singly in Code 71 (Table 3) showed an inverse relation with increased concentration levels to reduced biomass yield. Contrary, Code 93 (Table 4) was observed to increase biomass yield with increased concentration levels. Similar results were obtained with 2,4-D + BAP in Code 93 and Code 71 across all treatments. However, biomass yield presented no effect to induction rates across all treatments in Code 71 and Code 93 (Figure 5). The results suggest that genotype and type and concentration levels of PGR affect biomass yield in Arabica coffee hybrids. This observation is important given that optimized micropropagation protocols rely on high-frequency somatic embryogenesis, which is directly related to induction rates; time is taken to induce callus and biomass yield.
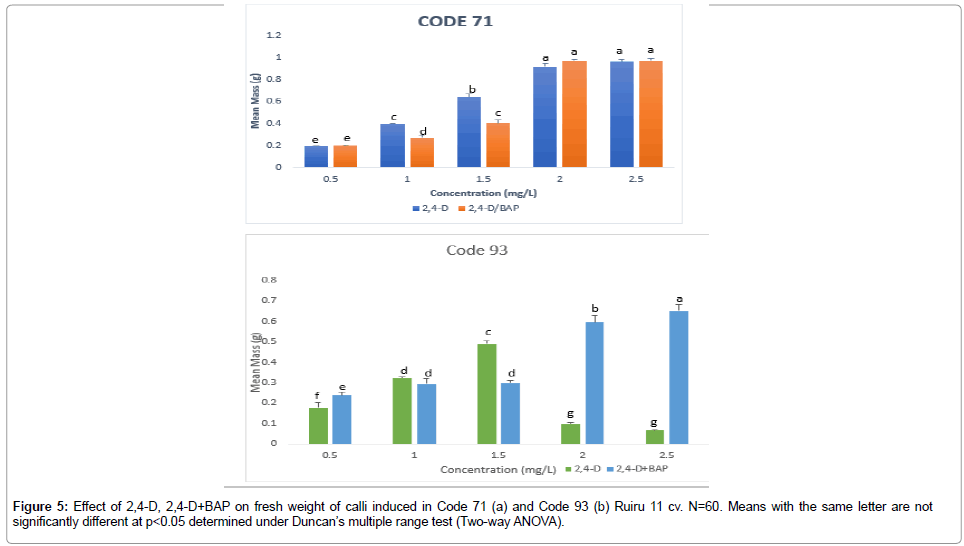
Figure 5: Effect of 2,4-D, 2,4-D+BAP on fresh weight of calli induced in Code 71 (a) and Code 93 (b) Ruiru 11 cv. N=60. Means with the same letter are not significantly different at p<0.05 determined under Duncan’s multiple range test (Two-way ANOVA).
For Code 71, 2,4-D+BAP was recorded to have the highest biomass yield compared to 2,4-D used singly. The combination of 2,4-D + BAP (2.5+0.5 mg/L) induced the highest biomass yield with very good (++++) embryogenic calli at 0.649 ± 0.026g FW and induction rate of 88% in four weeks. For Code 93, the highest biomass yield with very good embryogenic calli (++++) of 0.348 ± 0.017g FW at the induction rate of 100% was achieved in three weeks in similar combination concentration.
The difference in embryogenic response in Code 93 and Code 71 was significantly different in the assessed parameters in the present study. This further confirms the conclusion by Nic-can et al. [12] that somatic coffee embryogenesis is highly dependent on the genotype of coffee, explant source, and type and concentration of plant growth regulator. The results obtained have shown a strong inclination on the influence of different types and concentration of PGR (auxin and cytokinin) on callogenesis, meaning that both PGR choice and concentration levels are an important consideration in the optimization of protocols for somatic embryogenesis in the species. The results suggest that the difference in growth response is also attributed to the diversity in the genealogy of F1 hybrid Ruiru 11 Code 71 and Code 93 [28] Molina et al. observed that coffee genealogy plays a crucial role in embryogenic capacity in genotypes which is based on the genealogy of progenies and embryogenic response is under a strong genetic control [28,39-42]. Ruiru 11 is a composite Arabica coffee that comprises of hybridization of diverse coffee species including Robusta coffee and difference in results observed for the two Clones may be a reflection of the differences in the residual backgrounds of the respective progenitors for the two Clones.
The study established that combined use of plant growth regulators 2,4-dichlorophenoxy acetic acid [2,4-D] and 2,4-dichlorophenoxy acetic acid [2,4-D] + Benzyl amino purine [BAP] are ideal induction protocol for C. arabica F1 hybrid, Ruiru 11. The study observes that the presence of genotypic difference with respect to response to treatments with plant growth regulators leading to differences in induction rates, response rates and callus morphology. It is therefore recommended that further studies be undertaken using representative sample of codes of Ruiru 11 given that the current study had limited number of codes.
The authors aregrateful to the European Union for the financial support through the ACP-EU Project - Boosting coffee productivity in Kenya and Malawi through better access to and use of modern technologies and innovations (Contract number: FED/2013/330-219). The authors would also like to acknowledge the technical staff at the Coffee Research Institute- Breeding Department for their assistance in the completion of the study. The publication of this paper has been made possible through the financial support provided by the Value Chains and Trade theme of CAB International (CABI). This paper has been published with the permission of Institute of Biotechnology Research (IBR)- Jomo Kenyatta University of Agriculture and Technology (JKUAT).
Citation: Irene WM, Alumiro HL, Asava KK, Agwanda CO, Anami SE (2019) Effects of Genotype and Plant Growth Regulators on Callus Induction in Leaf Cultures of Coffea arabica L. F1 Hybrid. J Plant Biochem Physiol 7:236. doi: 10.35248/2329-9029.19.7.236
Received: 19-Apr-2019 Accepted: 21-May-2019 Published: 28-May-2019
Copyright: © 2019 Irene WM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.