Advances in dairy Research
Open Access
ISSN: 2329-888X
ISSN: 2329-888X
Research Article - (2019)Volume 7, Issue 2
Three types of caprine milk Cheddar cheeses as non-fortified control cheese (CC), regular ferrous sulfate (RFS) and large microencapsulated ferrous sulfate (LMFS) fortified cheeses were manufactured in three batches each to evaluate effects of iron addition on fatty acid compositions of two iron fortified cheeses compared to those of CC cheese. All manufactured experimental cheeses were subjected to storage treatments of two temperatures (4 and -18°C) and three periods (0, 2, and 4 months). Fatty acid profiles of the experimental cheeses were quantified using a gas chromatograph equipped with a fused silica capillary column, flame ionization detector, AOC-20s auto sampler and AOC-20i auto injector. Results showed that cheese type significantly (p<0.05 or p<0.01) affected levels of most fatty acids, except for C10:0, C14:1, C16:1, C18:1, and C20:0 acids, indicating that fatty acid contents of iron fortified cheeses were generally higher than control cheese especially at 4 months of storage. The level of C16:0 was highest, followed by C18:1, C18:0 and C10:0 acid, respectively, in all three types of the experimental goat milk cheeses for all storage treatment regimens. The main factors of cheese type, storage temperature and time had significant influences on several different fatty acids, and some of the 2-way and 3-way interactions also showed significant effects on levels of different fatty acids. It was concluded that iron fortification, storage temperature and period had significant influences on the levels of majority of tested fatty acids in the experimental caprine milk Cheddar cheeses.
Iron fortification; Goat cheese; Fatty acids; Storage temperature; Period
Iron deficiency anaemia is one of the most prevalent nutritional epidemics around the world [1,2]. Iron is deficient in milk and its products of most dairy species including cows and goats [3-5]. Furthermore, milk is reportedly a poor source of iron which cannot be significantly increased by oral administration of iron salts to lactating humans or animals [3]. Iron can further be a challenging nutrient to add to milk and dairy foods due to its potential to displace other divalent cations in the milk system [6].
Although iron has the potential for use in food vehicles, fortification with iron is technically more difficult than with other nutrients such as iodine or vitamin A, because iron reacts chemically with several food ingredients [3,7], and it also has potential to negatively affect the organoleptic properties of the fortified foods [8]. In order to prevent these negative effects of iron addition to dairy products, other avenues have been explored. Protected or microencapsulated iron salts have been used for iron fortification in bovine and caprine cheese products [9-11].
Since metal ions are prooxidants in a food system, iron fortification may cause a chance of increase in lipid oxidation in the fortified foods. Effects of iron fortification on changes in lipid moiety in bovine milk products such as fluid milk and cheese have been studied. While evaluating the effect of iron fortification on fatty acid profile especially in short chain fatty acids of cow milk Cheddar cheese, Kwak [11] found that the addition of microencapsulated and non-encapsulated iron salts to Cheddar cheese samples increased the fatty acid content as compared to non-fortified control cheeses. In a recent bovine Cheddar cheese study, Arce and Ustunol [10] reported that regardless of the particle size, iron fortification did not enhance lipid oxidation, and also consumer sensory panel results showed no negative effect on sensory attributes of the iron fortified cow milk Cheddar cheese.
There are some unique characteristics of lipid moiety in caprine milk which may have an influence in its lipid oxidation and lipolysis [12]. Nearly 20% of fatty acids of goat milk fat are in the category of short and medium chain length (4 to 12 carbons). Cow milk fat contains only 10 to 20% of fatty acids of this category [4,5,13], which is reportedly to lead more rapid digestion of goat milk fat than cow counterpart. Approximately 97% of the fat fraction of goat milk is triacylglycerol, which is composed of fatty acids of different carbon chain length (4 to 24 carbon atoms), degree of saturation and positional specificity on the glycerol backbone [14,15]. Other milk lipids are diacylglycerol (about 2% of the lipid fraction), cholesterol (less than 0.5%), phospholipids (about 1%), and free fatty acids (less than 0.5% of total milk lipids) [14,15]. Caprine milk fat exists as complex globules with structural properties distinct from other biological sources of fats. It is one of the most complex naturally-occurring fats, with more than 400 different fatty acids reported, however, only about 20 of these make up approximately 95% of the total fat [16].
However, there have been very little scientific reports available for effects of iron supplementation on goat milk products, including fluid goat milk and cheeses. Furthermore, no studies have been conducted on the effect of iron fortification and different types of iron supplementation on fatty acid profiles of caprine cheese products during different storage treatment regimens.
Therefore, the objectives of this study are to: (i) develop the iron-fortified goat milk cheeses by supplementation of regular ferrous sulfate (RFS) and Large micro-encapsulated ferrous sulfate (LMFS) salts in goat milk cheeses, and (ii) evaluate, changes in free fatty acid profile of iron fortified Cheddar type goat milk cheese in comparison with non-fortified control goat milk cheese.
Experimental design
The study was conducted in a 3 × 3 × 2 × 3 factorial experiment. Three batches of caprine milk Cheddar cheeses were manufactured for three Fe treatment groups as non-fortified control cheese (CC), regular ferrous sulfate (RFS) and large microencapsulated ferrous sulfate (LMFS) fortified cheese groups. All cheese groups were subdivided into two halves, and subjected to two temperature (4 and -18°C) treatments, and then stored for three storage periods of 0, 2 and 4 months. At each storage period, all experimental caprine Cheddar cheeses were evaluated for differences in basic nutrient contents and fatty acid profiles among cheese types (CC vs. Fe fortified), storage temperature and time treatments.
Reagents
All fatty acid standards were purchased from Supelco company (Bellefonte, PA, USA). Chromatography grade chloroform, methanol, hexane were obtained from Fisher Scientific (Waltham, MA, USA). Analytical grade NaOH, NaCl, anhydrous Na2SO4, and boron triflouride (12%, 1.5 M, ACROS organics) were also purchased from Fisher scientific (Waltham, MA, USA). Ultrapure water from Milli-Q system (18.2 MΩ, integral -3, Millipore, Bedford, MA, USA) was also used in this study.
Manufacture of control and iron fortified caprine milk Cheddar cheeses
Three batches of goat milk Cheddar cheeses were made for CC, RFS and LMFS cheeses each using the standard cow Cheddar cheese making procedure [17]. Twenty-five gallons (94.625 L) of goat milk were pasteurized and processed in a fifty gallon (188 L) size vat pasteurizer (Kusel Equipment Co. Model No. 66994p, Watertown, WI) at 62.8°C (145°F) for 30 min. Pasteurized milk was cooled to 31.1°C (88°F), and 4.16 grams of lyophilized Direct Vat Set culture (R-704, Chr. Hansen’s laboratory, Inc. Hoersholm, Denmark) was dissolved in deionized water and poured into the cheese vat (Kusel Equipment Co. Watertown, WI) with slow agitation for one hour. Thirty-five ml of single strength Chymax rennet (Chr. Hansen’s laboratory, Inc. Hoersholm, Denmark) was added to the 1-hr starter culture ripened milk, and allowed to coagulate the milk for 30-45 min without agitation. The cheese curd was cut by wire curd knives (1/2” × 1/2” × 3/4”) (Kusel Equipment Co. Watertown, WI). Ten minutes after cutting, curds were cooked by gradual increase in temperature (1°C every 5 minutes) to 39°C for 30 minute by the recommended heating schedule with mechanical stirring [17]. Whey was then drained, and cheese curds were stacked by two sides in the vat for Cheddaring process, and chunks of curds were turned over every 15 min for 3 times for further removal of whey (moisture). Before hooping, table salt and 0.8 gm of regular ferrous sulfate (FeSO4.7H2O; Fisher Scientific, Fair lawn, NJ, USA) and 0.90 gm of 700-800-micron medium particle size non-hydrogenated fat microencapsulated ferrous salt (Dr. Paul Lohmann Inc. Islandia, NY, USA) were added for iron fortification of the two experimental cheeses using the modified method used by Arce and Ustunol [10] and Siddique and Park [9]. After thorough mixing, the curds were pressed using a cheese press (Kusel Equipment, Model No.40, Watertown, WI) at 50 psi overnight. the pressed cheese was cut, placed in plastic pouches (Koch Inc. Kansas City, MO) and vacuum packaged (Hanchen Instrument, Model No.DZ-260, Watertown, WI), and stored at 4 and -18°C for storage treatments.
Extraction of fat
The standard method of AOCS [18] was used for the extraction of fat for fatty acid profile analysis. Two grams of CC, RFS and LMFS cheese samples were weighed into a 50 mL beaker. Eight mL methanol and 18 mL chloroform were added to each sample and homogenized for 30s in Waring blender (Model No.CB15, Springfield, MO). Nine mL chloroform was added to each sample beaker, and homogenized again for 30s. Nine mL zinc acetate (aq. solution: 0.115 g zinc acetate/5 mL H2O) was added to each sample and homogenized for 30s. The content of each beaker was transferred to a 125 mL separatory funnel and placed in a 4°C refrigerator until two distinct phases were separated. The bottom chloroform layer was drained into a 125 mL round bottom flask. The Büchi Rotavapor (R-200; BUCHI Corporation, New Castle, DE, USA) was used to dry the solvent of the fat extracted with dry ice to condense, and nitrogen gas was used to flush the fat samples. The dried samples were then closed and stored in the freezer (-18°C) for further analysis.
Preparation of fatty acid methyl esters
Five mL of 0.5 N methanolic sodium hydroxide and boiling beads were added to each 125-mL flask which contained previously extracted fat samples. The sample flasks were attached to the cooling condenser and samples heated for 10 min on a hot plate. Then 5 mL of 12% boron triflouride (BF3) methanol reagent (12%, 1.5M, ACROS organics, Thermo Fisher Scientific, Waltham, MA, USA) was added, and the solution was again boiled for 2 min. Seven mL of hexane was added to each sample and then heated on hot plate for one min. Saturated sodium chloride solution was added to each sample flask, which brings the hexane solution up to the neck of the flask. About six mL of the hexane layer was transferred to a test tube containing a small amount of sodium sulfate (anhydrous) to remove moisture from the hexane extract. The fat extract samples were then stored in a freezer (-18°C) for fatty acids analysis by a gas chromatograph.
Gas Chromatographic (GC) analysis of fatty acids
Fatty acid profiles of experimental cheeses were quantified using a gas chromatograph (GC- 2010 Plus; Shimadzu Scientific Instruments, Canby, Oregon, USA), which was equipped with a fused silica capillary column of 100 m × 0.25 mm × 0.2 μm film thickness (SP- 2560; Supelco, Bellefonte, PA, USA), flame ionization detector, AOC-20s auto-sampler, and AOC-20i auto-injector. Injector and detector temperature were set at 250°C and 270°C, respectively. The initial oven temperature was kept at 140°C for 5 min., and the operating software was programmed to increase temperature up to 240°C at the rate of 4°C/min. One μL sample was injected to the injector with a split ratio of 1: 20, and helium was used as the carrier gas, and hydrogen was used as flame gas.
Statistical analysis
All experimental data collected from the CC and Fe fortified cheeses were analyzed for analysis of variance, least square means, Duncan’s multiple mean comparison using general linear model (GLM) of SAS program [19]. The effects of main factors (temperature and storage time) and their interaction effects on free fatty acid profiles were also analyzed by the procedures of Steel and Torrie [20].
Fatty acid profiles of control and Fe fortified caprine milk Cheddar cheeses
The results of fatty acid profiles of the control and iron fortified goat milk Cheddar cheeses during four months storage are shown in Table 1. Concerning main factors, palmitic acid (C16:0) content was the highest among all fatty acids tested with regard to effects of storage period and temperature treatments. Oleic acid (C18:1) was the second highest content among all the fatty acids in all three-experimental goat milk Cheddar cheeses. Stearic acid (C18:0) followed as the third highest level of fatty acid among all fatty acids analyzed Table 1, Figures 1 and 2. These outcomes were somewhat unexpected, since C18:1 content has been reportedly higher than that of C16:0, and results of the present study may indicate that the goat milk used in our study may had higher C16:0 than that of C18:1 (Figure 2). The levels of nutrient compositions can be affected by different factors, including species, breed within species, feeds, stage of lactation and management conditions of the lactating animals [5,13,15] With respect to the composition of short and medium chain fatty acids levels in the experimental goat Cheddar cheeses, Capric acid (C10:0) content was the highest among those short and medium chain fatty acids (MCT) (Table 1). Goat milk has been known to contain significantly higher levels of small and medium chain fatty acids compared to cow or human milk [5,15,16]. The higher levels of these short chain acids may be attributable to the differences in polymerization of the acetate produced by the rumen bacteria in goats [21]. These MCT are highly beneficial to the growing children [4,5,15]. Nearly 20% of fatty acids of goat milk fat are in the category of short and medium chain length (4 to 12 carbons), which is in agreement with the results of our present study. Cow milk fat contains only 10 to 20% of fatty acids of this category [4,5,13], which is reportedly attributable to more rapid digestion of goat milk fat than cow counterpart.
| Fatty Acid | Storage Temp (oC) | 0 month | 2 month | 4 month | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| C4:0 | 4 | 2.36ax | 0.21 | 2.39ax | 0.04 | 2.42ax | 0.17 |
| -18 | 2.30bx | 0.07 | 2.35bx | 0.15 | 2.40ax | 0.14 | |
| C6:0 | 4 | 2.27bx | 0.26 | 2.52ax | 0.03 | 2.57ax | 0.15 |
| -18 | 2.26bx | 0.05 | 2.44ay | 0.13 | 2.49by | 0.17 | |
| C8:0 | 4 | 2.89ax | 1.18 | 3.04ax | 0.07 | 3.09ax | 0.67 |
| -18 | 2.67by | 0.06 | 2.96ax | 0.31 | 2.99ax | 0.74 | |
| C10:0 | 4 | 6.78bx | 0.01 | 7.08bx | 0 | 7.91ax | 0.01 |
| -18 | 6.65bx | 0.02 | 6.97bx | 0.02 | 7.54ay | 0.01 | |
| C12:0 | 4 | 2.72bx | 0.04 | 2.82bx | 0.02 | 3.38ax | 0.02 |
| -18 | 2.66ax | 0.21 | 2.67ay | 0.4 | 2.98ay | 0.32 | |
| C14:0 | 4 | 8.68cx | 0.02 | 9.15bx | 0.03 | 10.01ax | 0.15 |
| -18 | 8.60by | 0.01 | 8.78by | 0.01 | 9.92ax | 0.15 | |
| C14:1 | 4 | 0.33by | 0.05 | 0.36by | 0.05 | 0.43ay | 0.04 |
| -18 | 0.35bx | 0.08 | 0.44ax | 0.03 | 0.58ax | 0.03 | |
| C16:0 | 4 | 26.6bx | 0.03 | 27.3bx | 0.02 | 31.8ax | 0.07 |
| -18 | 26.2ax | 0.04 | 26.9ay | 0.05 | 29.8ay | 0.06 | |
| C16:1 | 4 | 0.68ax | 0.03 | 0.69ax | 0.01 | 0.65bx | 0.25 |
| -18 | 0.67ax | 0.02 | 0.68ax | 0.03 | 0.67ax | 0.33 | |
| C18:0 | 4 | 12.4bx | 0.7 | 12.6ax | 0.01 | 12.9ax | 0.01 |
| -18 | 12.1ax | 0.17 | 12.3ax | 0.02 | 12.7ay | 0.02 | |
| C18:1 | 4 | 21.5bx | 1.07 | 21.9bx | 0.12 | 27.9ax | 1 |
| -18 | 21.3bx | 0.92 | 21.5ay | 0.35 | 25.8ay | 1.04 | |
| C18:2 | 4 | 2.29ax | 0.05 | 2.01bx | 0.05 | 2.36ax | 0.22 |
| -18 | 2.20ax | 0.05 | 2.12ax | 0.01 | 2.21ax | 0.23 | |
| C18:3 | 4 | 0.33bx | 0.07 | 0.67ax | 0.01 | 0.31bx | 0.05 |
| -18 | 0.47bx | 0.04 | 0.53ax | 0.08 | 0.40bx | 0.19 | |
| C20:0 | 4 | 0.05bx | 0.06 | 0.03bx | 0.01 | 0.13ax | 0.15 |
| -18 | 0.30ax | 0.02 | 0.43abx | 0.01 | 0.52ax | 0.18 | |
| C22:0 | 4 | 0.31ax | 0.01 | 0.31ax | 0.01 | 0.11bx | 0.03 |
| -18 | 0.38ax | 0.01 | 0.10bx | 0.1 | 0.11bx | 0.02 | |
| C24:0 | 4 | 0.40ax | 0.07 | 0.24bx | 0 | 0.42ax | 0.01 |
| -18 | 0.24ax | 0.01 | 0.26ax | 0 | 0.13bx | 0 | |
1All mean fatty acid values are the means of pooled data across three types of cheeses.
a, b Means with different superscript in a same row are significantly (p<0.05) different.
x, y Means with different superscript in a same column are significantly (p<0.05) different.
Table 1: Comparison of mean fatty acid contents (mg/g cheese sample) in experimental goat milk Cheddar cheese during four months of storage at 4 and -18°C.
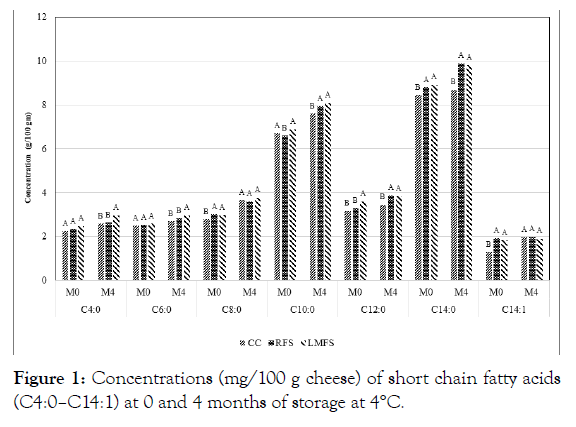
Figure 1: Concentrations (mg/100 g cheese) of short chain fatty acids (C4:0–C14:1) at 0 and 4 months of storage at 4°C.
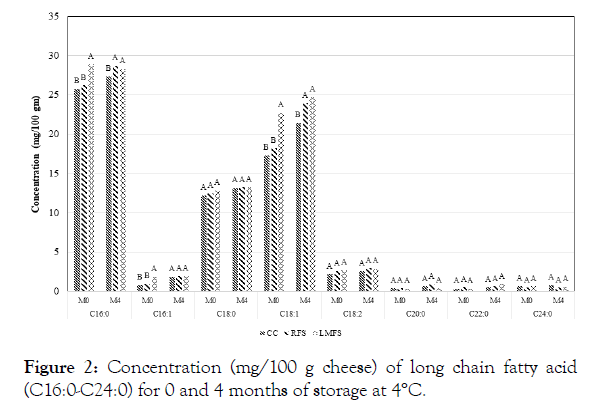
Figure 2: Concentration (mg/100 g cheese) of long chain fatty acid (C16:0-C24:0) for 0 and 4 months of storage at 4°C.
In addition, caprine milk has a unique characteristic in the lauric: capric acid (C12:C10) ratio, where goat milk has a significantly lower C12:C10 ratio than cow milk (0.46 vs. 1.16) [22]. This same phenomenon of low C12:C10 ratio, or even lower ratio (0.38 vs. 0.41) was observed in our experimental goat Cheddar cheeses.
The differences in fatty acid contents between storage periods were gradually increased for both C10:0 and C14:0, and the difference in these fatty acids at 4 months of storage were significant (p<0.05) at both (4 and -18°C) temperatures (Table 1 and Figure 1). Approximately 97% of the fat fraction of goat milk is triacylglycerol, which is composed of fatty acids of different carbon chain length (4 to 24 carbon atoms), degree of saturation and positional specificity on the glycerol backbone [14,15].
The analysis of variance of all experimental data for the effects of main factors and their interactions on fatty acids compositions are presented in Table 2. The effect of cheese type was significant (p<0.05 or p<0.01) for the most of fatty acids determined, except C10:0, C14:1, C16:1, C18:1, and C20:0 acids. These results imply that iron fortification in the experimental goat Cheddar cheeses may have affected the compositional changes in different fatty acids by possible biochemical structural changes in fatty acid chains, due to the oxidation of fat moieties entrapped in the cheese matrix. In addition, most of the fatty acids contents were increased as storage period advanced. This fact was suggested in the previous report by Collins [23]. The increases in several fatty acids were significant (P<0.05 or 0.01<P) at 4oC, while the increases were less or minimal at -18°C treatment (Table 2, Figures 1 and 2). The effect of storage period revealed significant effect (p<0.05 or p<0.01) on the levels of many fatty acids, including C4:0, C6:0, C8:0, C10:0, C12:0, C14:1, C18:0, C18:1, C22:0, whereas the storage effect was not significant for the levels of C14:0, C16:0, C16:1, C18:2, C18:3, C20:0, and C24:0 (Table 2, Figures 1 and 2). However, storage temperature showed significant effect only on the levels of C10:0, C12:0, C14:0, C14:1 and C18:1 (p<0.05 or p<0.01), and levels of all other fatty acids were not affected by temperature treatments, whereas the differences in temperature effect became minimal at the later period of storage (Table 2). The reasons for the variability of effects of storage period and storage temperature on the levels of each individual fatty acid is not clear. However, more fatty acids were affected in their compositions by storage period than those of storage temperature.
| Parameters | DF | C4 | C6 | C8 | C10 | C12:0 | C14:0 | C14:1 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C22:0 | C24:0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT | 2 | 28.20** | 59.08** | 36.45** | 0.97 | 2.19* | 2.59* | 1.57 | 18.11** | 1.6 | 43.92** | 1.21 | 8.07** | 4.35* | 2 | 3.09* | 4.07* |
| Ba | 2 | 0.55 | 0.49 | 2.26 | 0.93 | 0.93 | 7.45** | 2.64 | 2.45 | 0.65 | 0.51 | 0.78 | 0.34 | 0.7 | 0.5 | 0.22 | 0.72 |
| SP | 2 | 20.04** | 15.57** | 20.75** | 17.23** | 7.11** | 0.44 | 7.75* | 1.57 | 0.21 | 16.49** | 3.00* | 0.02 | 1.61 | 2 | 6.99** | 2.72 |
| ST | 1 | 0.78 | 0.55 | 0.01 | 23.51** | 9.82** | 9.83** | 2.08* | 1.52 | 0.19 | 0.12 | 3.00* | 0.13 | 0.5 | 2.09 | 0.86 | 0.04 |
| CT × ST | 2 | 0.64 | 1.1 | 0.52 | 4.11* | 3.30** | 1.55 | 0.22 | 0.45 | 2.15 | 0.1 | 0.96 | 1.03 | 0.35 | 2.1 | 0.74 | 0.15 |
| SP × ST | 2 | 2.82* | 3.68* | 3.45* | 1.41 | 1.92 | 1.1 | 1.78 | 1.85 | 1.98 | 1.93 | 2.02 | 2.86* | 1.39 | 2.2 | 0.37 | 1.24 |
| CT × SP | 4 | 2 | 3.81** | 3.38* | 1.62 | 1.97 | 2.43* | 1.77 | 1.85 | 2.01 | 1.97 | 1.9 | 2.77 | 1.4 | 0.56 | 1.37 | 1.24 |
| CT × ST × SP | 4 | 1.23 | 2.16* | 2.40* | 1.96* | 2.5 | 2.02* | 1.05 | 1.04* | 1.95 | 1.16 | 1.22 | 2.09* | 0.63 | 2.50* | 1.77 | 0.69 |
CT: cheese type
Ba: Batch
ST: Storage Temperature
SP: Storage Period
*P < 0.05, **P < 0.01
Table 2: Analysis of variance (F value) for effects of main factors and their interactions on levels of fatty acids (C4:0 -C24:0) in the experimental goat milk Cheddar cheeses.
As far as interaction effects of main factors on fatty acids profiles are concerned, 2-way interactions of cheese type x storage temperature affected only C10:0 (p<0.05) and C12:0 (p<0.01) contents, and all other fatty acids levels were not affected by cheese type x storage temperature. For the other 2-way interaction effects, the storage period x storage temperature interaction showed significant (p<0.05) influence on levels of C4:0, C6:0, C8:0 and C18:2 only, and all other fatty acids (C10:0, C12:0, C14:0, C16:0, C18:0 and C18:1) were not affected by this 2-way interaction of storage period x temperature. For third interaction effect of cheese type × storage period, it had significant effects on C6:0, C8:0 and C14:0 fatty acid only, but majority of fatty acid contents were affected for the experimental goat milk Cheddar cheeses. With regard to 3-way interaction of cheese type × storage temperature x storage period effect, it had significant effects on several of the fatty acids, such as C6:0, C8:0, C10:0, C14:0, C16:0, C18:2 and C20:0, only at p<0.05 level of significance.
Previous reports on bovine milk fatty acid had shown that storage and environmental conditions would have significant influences on cheese composition [24]. In addition, pasteurization and cheese making conditions can make differences and alteration of milk fat composition and cheese property. The results of this study on goat milk Cheddar cheeses were somewhat different from those of McGhee et al. [25] on fatty acid profiles of C10:0, C14:1 and C16:0 acids in goat milk ice cream examined during 8 weeks storage period. Furthermore, storage time had significant (p<0.05 or p<0.01) effects on fatty acid concentrations except for C14:0, C16:0, C16:1, C18:2, C18:3, C20:0, and C24:0 fatty acids in our present study.
Effect of Fe fortification on fatty acid composition
Figures 1 and 2 show that LMFS fortified cheeses had highest contents of fatty acids among three types of goat Cheddar cheeses at 0 and 4 months of storage. The possible major reason for high levels of fatty acids in the LMFS cheeses may likely be attributed to the materials used for microencapsulation, which was made up of vegetable oils, such as palm oil. Cheeses fortified with RFS salts generally revealed the second highest contents of fatty acids among the cheese groups. There were no differences observed in fatty acid levels above C18:1 acid among the cheese groups (Figure 2). The present study is in agreement with the previous report on short chain fatty acids of bovine Cheddar cheese by Kwak [11]. The effect of iron fortification on changes in fatty acid profile of goat milk Cheddar cheese has not been reported in the past.
However, the effect of Fe fortification on fatty acid levels of bovine milk Cheddar cheese had been partially reported by Kwak et al. [11]. They evaluated the microencapsulated and non-encapsulated iron fortification in Cheddar cheeses, and found that the addition of microencapsulated and non-encapsulated salts to Cheddar cheese samples increased the fatty acid content as compared to non-fortified control cheeses. They also found that there were no significant differences in the effect of storage temperature on short (C4:0-C8:0) and long (C16:0-C24:0) chain fatty acids, except for the medium chain (C10:0–C14:0) acids, which are somewhat different from the results of our goat milk Cheddar cheese study.
The study revealed that palmitic acid (C16:0) was the highest fatty acid among all the experimental caprine Cheddar cheeses, followed by oleic acid (C18:1) and then stearic acid (C18:0), which is somewhat different from the previous reports. Among short chain fatty acids, C10:0 was the highest. The C12:C10 ratio (0.38 vs. 0.41) in our goat cheeses was even lower than the previously reported values, which can be used to detect the adulteration of other species milk in goat milk and its products. Iron fortification resulted in significant differences in fatty acids contents between cheese types (Fe fortified vs. control cheeses). Storage period also had significant influence on many fatty acids determined, while storage temperature had minimal effect, except for C10:0, C12:0, and C14:0 fatty acid. Some interaction effects among cheese types, storage temperatures and storage periods also made differences in fatty acid profiles of the goat Cheddar cheeses. Further studies may be necessary to examine effects of different types of iron supplementation and more numbers of storage temperature and time treatments in order to have more comprehensive, conclusive and convincing scientific data, applicable to the production of iron fortified caprine cheeses to enhance the benefits of consumers.
This research was supported by the Agricultural Research Station project number USDA/GEOX-3225 of the USDA Evan-Allen Grant Funds. The authors greatly appreciate Mr. Ralf Dieckhoff (Dr. Paul Lohmann Inc., Islandia, NY) for donation of the nonhydrogenated LMFS salt for this study.
Citation: Park YW, Siddique A (2019) Effects of Iron Fortification on Fatty Acid Profiles of Caprine Milk Cheddar Cheeses under Different Storage Treatment Regimens. Adv Dairy Res 7:225.
Received: 10-Apr-2019 Accepted: 26-Apr-2019 Published: 06-May-2019 , DOI: 10.35248/2329-888X.19.7.225
Copyright: © 2019 Park YW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.