Journal of Pollution Effects & Control
Open Access
ISSN: 2375-4397
ISSN: 2375-4397
Research Article - (2017) Volume 5, Issue 2
Physical chemical analysis of the Bizerte’s landfill leachate showed high amounts of ammoniacal nitrogen. Its treatment with combined process of coagulation/flocculation, Fenton and powder zeolite adsorption allowed a treated effluent with low content of nitrogen. The microtoxicity of raw and treated leachate was monitored by LUMIStox and its phytotoxicity was examined by seed germination/root elongation tests using seeds of Lycopersicon esculentum, Lolium perenne, Helianthus annus and Medicago sativa. Seedlings of the three species (Lycopersicon esculentum, Helianthus annus and Medicago sativa) were grown in pots, which irrigated with treated leachate at the median effective concentration (EC50) levels, NPK fertilizer and tap water as a control. LUMIStox tests showed that combined process allowed a significant toxicity removal. Treated leachate played fertilizing effect on plants growth. An increase in median effective concentration from 18% to 25% was observed. However, raw leachate showed lower median effective concentration levels varied between 4% and 5%. Results indicated that plants receiving treated leachate and fertilizer grew better than those receiving water alone. The growth of plants with treated leachate irrigation did not differ significantly from plants treated with fertilizer. Treated Leachate irrigation also improved soil N content. Indeed, soils amended with treated leachate had more extractable N concentration in comparison with control soil.
Keywords: Germination index; Microtoxicity; Phytotoxicity; N contents; Physico chemical treatments
Landfill leachate is a heterogeneous mixture consisting of refractory organic compounds, inorganic contaminants, heavy metals, humic, fulvic acids and high nitrogen concentrations [1]. Studies have reported that leachate percolation contaminates nearby groundwater resources [2-4]. Leachates high load of organic matter, high nitrogen content and mass flux of transported contaminants impact plants heavily and can enter the food chain through vegetation around the site [5]. The high nitrogen levels may contaminate soil and phreatic groundwater [6]. It is important to note that nitrogen (N) is most imperative element for proper growth and development of plants which significantly increases and enhances the yield and its quality by playing a vital role in biochemical and physiological functions of plant. Soil nitrogen availability has been long known to influence crop and root growth thus to have a direct impact on crop yield, attributing increased death rates for young individuals and leaf yellowing to insufficient nitrogen uptake [7,8].
Nitrogen element is the nutrient that most frequently limits yield and plays an important role in quality of forage crops. It is almost deficient in most soils of Africa [9,10]. This calls for increasing the use efficiency of nitrogen fertilizers to enhance growth and development of plants.
The increasing agricultural reuse of treated effluent serves goals such as promoting sustainable agriculture in arid and semiarid region, preserving scare water resources, and maintaining environmental quality. Moreover, irrigating with wastewater may reduce purification levels and fertilization costs, because soil and crops serve as bio-filters, while wastewater contains nutrients [11]. Landfill leachates contains a number of favorable characteristic for agriculture since it contains nitrogen (N), potassium (K), magnesium (Mg), and very low load in heavy metals. For these reasons, considerable attention has been paid to adopt sustainable treatment methods allowing the use of the treated wastewater in agriculture.
An integrated technique that consisted of coagulation/flocculation, Fenton and adsorption methods was adopted for the treatment of raw leachate from a landfill site of Bizerte (Northern Tunisia). Activated carbon and powder zeolite were used as adsorbents. Nevertheless, the use of zeolite adsorption seems to be very efficient method to removal both organic and ammonium loads [12-14]. To allow the use of the treated leachate in agriculture, a treated effluent with no excessive amount of nitrogen should be produced.
The assessment of the potential impact of leachate should be determined to avoid and prevent both severe and continual toxicity. The phytotoxicity assessment on several test plants, such as Vicia faba [15], Zea mays [16] and Hordeum vulgare [17], have been used as receiving medium due to their sensitivity to a wide range of contaminants. Phytotoxicity assays can be used to measure putative environmental risks [18]. They are reliable, cost effective, quick, and simple [19]. The use of plants offers an advantage over others organisms because they can be more sensitive to environmental stresses [20].
The aim of this paper is to investigate the use of landfill leachates as a potential source of irrigated water. This effluent was previously analyzed and treated with coagulation/flocculation-Fenton-powder zeolite adsorption. The efficiency of the treatment on the seed germination, plant growth of a number of selected plant species was studied. The agronomic value of the treated effluent was also examined by comparison to a mostly used NPK fertilizer.
Leachate and soil origin
The soil was surface sampled from an agricultural area from Tunisia (Northern Africa). Crops usually cultivated in that area are Tomato, Lettuce and Cucumber. The climate of the region is typical Mediterranean, semi-arid to arid, with an average rainfall of 212 mm year-1 and an average annual temperature of 19° C. The field-moist soil samples were sieved (<2 mm), delivered in sealed plastic bags to the laboratory under refrigerated conditions and stored at 4° C until analysis.
The site used for this study was the sanitary landfill site of Bizerte, North Eastern Tunisia (37°16’N; 9°52’E) (Figure 1). A leachate management program has been applied, involving the collection of leachates through a drainage network and the continuous re-circulation through the deposited landfill. At the lowest point of landfill, leachate exits to the surface, forming an evaporation pond. About 60 m3d-1 of leachates are transferred to the main wastewater treatment plant for further treatment.
Leachate characterization
Leachate samples were collected from the evaporation pond in 40 l plastic carboys. Samples were transported to the laboratory, stored at 4° C and analyzed within two days. Physicochemical characteristics of leachate samples were validated according to French standard NF XPT 90-210 [21]. Biochemical oxygen demand (BOD5) was determined by the manometric method with a respirometer (BSB-Controlled Model OxiTop (WTW)) and the COD was estimated using the method described by Knechtel [22]. Total nitrogen contents (TKN) were measured by the Kjeldhal method using an automated apparatus (Buchi, Switzerland). Phosphorus was determined calorimetrically at 430 nm using a Shimadzu U 1000 spectrophotometer. The phosphorus content (TP) was measured calorimetrically by atomic absorption (ICE, 3000 model). The pH was measured using pH meter (INOLAB WTW720). Electrical conductivity was determined with an electronic conductivity meter (TACUSSEL, CD 6NG) equipped with an immersion measurement probe (cell constant Ksl-1=1 cm). The total concentrations of K, Ca, Mg, Na, K, Fe, Al, Cu and Mn were determined using atomic absorption flame emission spectroscopy AAS (Thermo scientific). Prior to analysis, 20 ml of the sample was transferred into the Teflon flask and then completely dissolved in HCl-HNO3 solution (30/70% in volume). After dissolution, the mixture was diluted with 100 ml of deionized water and analyzed by (AAS). The concentrations of heavy metals were also analyzed according to the standard methods for the examination of water and wastewater in order to validate/evaluate the produced results and they were found within accepted analytical error ( ± 7%). All chemicals used for the analytical determinations were of analytical grade. All analyses were run in triplicate for reproducibility of data and results were the average ones.
Leachates treatment description
Leachate was submitted to a coagulation/flocculation treatment with addition of Aluminum sulfate (Al2(SO4)3·18H2O, Merk) as coagulant (0.6 g Al3+ l-1) and P3-Ferrocryl 7223 (0.85 mgl-1) as flocculent. The process was carried out at pH 5.5. Afterwards, the leachate was treated by Fenton oxidation by the addition of Fenton reagents (1.2 gl-1) and hydrogen peroxide (2.8 gl-1). Because low pH favors Fenton oxidation, the pH was adjusted at pH 3. Finally, leachates treated by coagulation/ flocculation and Fenton oxidation were submitted to adsorption process using powdered zeolite PZ (zeolite 4A, Aluminosilicate, [Na12 (Al12Si12O48)] 27H2O, CEC, 5.47 meq/g) (30gl-1) as adsorbent carrier [14].
Microtoxicity assay
Experiments were performed using raw and treated leachates samples. Microtoxicity was carried out with Vibrio fischeri (luminescent bacteria LCK 480) using LUMIStox 300 measuring instrument, according to ISO [23]. The inhibition of the bioluminescence of V. fischeri using the LUMIStox test kit was achieved by mixing 0.5 ml of tested water and 0.5 ml luminescent bacterial suspension. After a 15min exposure at 15° C, the decrease in light emission was measured. The toxicity of the tested water was expressed as percent bioluminescence inhibition (% IB) relative to a non-contaminated reference. A positive control (7.5% NaCl) was included for each test. The results were calculated using a correction factor (Cf), which is the monitor of the changes in intensity within control samples during the 15 min exposure.
Phytotoxicity assay
The phytotoxicity was assessed on seed germination of Lycopersicon Esculentum, Medicago sativa, Lolium perenne and Hélianthus annus. According to a modified Zucconi test [24] by measuring seed germination. Ten undamaged seeds with identical size were placed uniformly in 90 cm petri dishes, in a filter. Five dilutions of the sample and one control were prepared with three replicates. Each dish contained 5 ml sample dilution or 5 ml of distilled water (control). Three replicates were carried out for each sample, including the control. Dishes were then covered and incubated in the dark at 20 ± 2° C for 5 days. A germination index (GI) was calculated by counting the number of germinated seeds and the average root length observed in each sample compared to control seeds. Seeds were considered to have germinated when the radical penetrates the seed coat [25].
The median effective concentration (EC50) was calculated from the dose relationship between GI and leachates concentration by the brain cousens model according to the formula (Equation 1) [26].
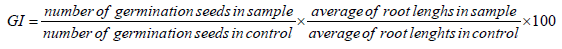
The effect results obtained with each toxicity test were transformed into toxic units according to the formula (Equation 2):
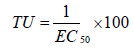
Irrigation experiment
Three seeds species: Helianthus annus, Lycopersicon esculentum and Medicago sativa were selected for irrigation experiment. Pot of 160 mm in diameter and 140 mm in height were filled with soil. Four seeds were sown uniformly in each pot in a depth of about 10 mm. An agricultural soil was used as growth media. The soil was passed through 2 mm mesh in order to remove large particles.
The soil pots were divided into three treatment groups: water with fertilizer added (F), diluted treated leachate (TL) and tap Water (C) as a control. The fertilizer group was treated with the NPK fertilizer (20; 20; 20) applied to the soil surface of leachate application, while the leachate treatment group received treated leachate irrigation at the EC50 levels as determined by the phytotoxicity test. Each pot received 100 ml of diluted treated leachate or tap water 3 times a week. The fertilizer was applied five times during irrigation experiment. Each treatment had five replicates arranged in completely randomized blocks in a greenhouse. Pesticide and fungicide were applied only when necessary. The irrigation experiment lasted for 90 days. The growth and health of plants were monitored qualitatively and quantitatively during the experimental period. Plant height and standing leaf number were measured every 4 weeks.
At the end of the irrigation period, the plants were harvested to determine leaf number, plant height (cm). Plant tissues were harvested and washed in deionized water. They were oven dried at 65° C for 7 days for the determination of dry weight of aboveground part and underground part (g) and N contents of all plants in each pot. Soils were collected; they were air-dried for 14 days and passed through a 2 mm sieve for Chemical analysis: pH, EC, NTK and extractable N. Extractable N in soil was accessed via a KCL extraction method.
Statistical analyses
All analyses were performed in tripliquates. Values of different parameters are expressed as the mean ± standard deviation. Statistical analyses were carried out with SPSS 17.0 for windows (SPSS Inc. USA). The results were analyzed by multiple ANOVA (Tukey’s and Duncan’s multiple range tests) to evaluate significant differences between means at the 95%.
Leachates
The results of the physical-chemical analysis relating to the raw and treated leachate are given in Table 1. The raw leachate had high COD and soluble N-NH4+ contents. The major fraction of the TKN was in ammonical form. Electrical conductivity as high as 19.6 ms cm-1 reflects their high salt contents. Although leachates contained elevated levels of major cations such as K+ and Na+, the concentration of heavy metals (except Fe and Al) was relatively low (<1 mg L-1). Heavy metals may be retained in the landfill body as they precipitate out under reducing and alkaline conditions. The treated leachate is free from excessive amount of N and rich in major elements such as K, Ca, Na and Mg. The high strength of electrical conductivity (16.1 ms cm-1), K+ and Na+ contents may inhibit its use without dilution [27].
| Parameters | Unit | RL | TL |
|---|---|---|---|
| pH | - | 7.74 ± 0.4 | 6.5 ± 0.22 |
| Electrical Conductivity | (mS cm-1) | 19.6 ± 1.5 | 16.1 ± 1.3 |
| Chlorides | (mg L-1) | 3300 ± 120 | 2935 ± 135 |
| COD | (mgO2 L-1) | 26200 ± 130 | 620 ± 22 |
| BOD5 | (mgO2 L-1) | 5200 ± 65 | 391.3 ± 12 |
| TNK | (mg L-1) | 1770 ± 19 | 127 ± 31 |
| TOC | (mg L-1) | 21000 ± 51 | 501 ± 17 |
| N-NH4+ | (mg L-1) | 1628 ± 21 | 107 ± 27 |
| Total Phosphorus | (mg L-1) | 13.64 ± 1.2 | 7.23 ± 1.4 |
| Phenols | (g L-1) | 3.52 ± 1.2 | 0.8 ± 0.1 |
| K | (mg L-1) | 465 ± 3.2 | 420 ± 5.4 |
| Ca | (mg L-1) | 520 ± 5.6 | 385 ± 4.3 |
| Na | (mg L-1) | 420 ± 8.6 | 393 ± 19 |
| Mg | (mg L-1) | 32 ± 1.4 | 26 ± 2.5 |
| Fe | (mg L-1) | 6.87 ± 1.2 | 0.08 ± 0.005 |
| Al | (mg L-1) | 1.73 ± 0.05 | 0.05 ± 0.002 |
| Cu | (mg L-1) | 0,087 ± 0.05 | -a |
| Mn | (mg L-1) | 0.16 ± 0.02 | 0.6 ± 0.31 |
Table 1: Physico chemical characteristics of raw leachate (RL), treated leachate (TL) samples.
Soil analysis
Selected characteristics of the soil used for the experiment are shown in Table 2. The soil is typical Tunisian sandy soil. The sand provided excellent drainage and aeration [28]. The soil organic carbon and nitrogen was very low (<1%). The lack of organic matter and clay results in poor nutriment retention. The soil materials were bases such as Ca and Mg. The inherited nutriment reserve was also limited and the supply of macronutrients was inadequate. Its overall quality could be improved by adding fertilizers or soil ameliorants.
| Parameters | Mean ± SD |
|---|---|
| pH | 7.9 ± 0.1 |
| Electrical conductivity (µS cm-1) | 495 ± 5.40 |
| CEC (cmol.Kg-1) | 11 |
| TKN (%) | 0.03 |
| TP (mgg-1) | <1 |
| Organic carbon (%) | 0.65 ± 0.09 |
| Texture | |
| Sand (%) | 74.85 ± 6.9 |
| Silt (%) | 10.11 ± 1.2 |
| Clay (%) | 15 ±1.9 |
| Texture class | Sandy |
| Total metals (mg g-1) | |
| Na | 4.99 ± 1.01 |
| K | 20.41 ± 4.85 |
| Mg | 29.45 ± 4.36 |
| Ca | 211.27 ± 33.8 |
| Mn | 1.099 ± 0.11 |
| Fe | 7.06 ± 1 |
Table 2: Properties of soil used for the irrigation experiment.
Toxicity test
The use of V. fischeri bioluminescence test to estimate the toxicity of treated wastewater before their discharge into the environment was reported by Hao et al. [29]. Our results showed that although the raw leachate (RL) was extremely toxic according to the method using V. fischeri, as the most sensitive bacterium. Raw leachate caused 100% of bioluminescence inhibition (IB) of V. fischeri (Figure 2). Although, the treated leachate (TL) showed a very low microtoxicity (IB=19.2%). This is in accordance with the findings of Marttinen et al. [30] and Silva et al. [31] who indicated that all samples of landfill leachates exhibited acute toxicity to the bacterium V. fischeri. Isidori et al. [32] demonstrated that toxicity increased at higher pH levels and toxic compounds could be characterized as cations, basic chemicals, suspended solids and a polar compounds. These results showed that the combined process of coagulation/flocculation, Fenton and powder zeolite adsorption allowed a significant toxicity removal.
Germination test
Plant bioassay evaluated the response of selected plant indicators to the complex leachate where inhibitory factors such as high salinity and toxicity coexist with plant nutrients. The dose response relationships of Lycopersicon esculentum, Lolium perenne, Helianthus annus and Medicago sativa seeds are shown in Figure 3. Raw leachate was also very toxic to plants since it caused inhibitory effects on the germination seeds and plant growth. As shown in Figure 3 and 4 the EC50s of raw leachate varied from 4% to 5% for the four species studied. However, an increase in EC50s of treated leachate was observed. Their values ranged from 20% to 25%. The results thus obtained are compatible with the results acquired from toxicity test.
Germination tests confirmed the toxicity of the raw leachate. This fact can be attributed to the high nitrogen content and the high salinity of the effluent [33,34]. In addition, various toxic organics have been detected in leachates [35-37] and their presence would inevitably lead to phytotoxicity depending on their forms, molecular weights and concentrations. However, the use of the treated leachate had a positive effect on seed germination. The increase in GI was probably due to longer root length when compared with the control. Such growth promotion can be contributed to the presence of nutrients such as NH4+ and K+ in the treated effluent [38]. The seeds germination test has served as a reliable surrogated model for finding the most effective concentration (dilution) for treated leachate irrigation.
As can be seen from Figure 4, that toxicity unit of the raw leachate reached between 20 and 30. The results showed toxicity reduction of treated leachate for the four species studied (toxicity unit<5). The toxicological assays showed a reduction of toxicity after the treatment of landfill leachates by the integrated method, indicating that the combined process corresponds to an actual detoxification of the leachate.
Irrigation experiment
All seedlings tested survived until harvest. No massive leaf fall or significant changes in the ecophysiology were observed during irrigation experiment. In general, plants amended with treated leachate (TL) and fertilizer (F) have better growth performance than those receiving water alone (control) in terms of plant height, leaf number, aboveground dry weight and underground dry weight after 90 days (Figure 5). There were no significant difference (p>0.05) between plant receiving treated leachate and fertilizer solution. The increase in the number of leaves could possibly be ascribed to the fact that nitrogen often increases plant growth and plant height and this resulted in more nodes and internodes and subsequently more production of leaves [39-41]. The use of treated leachate in irrigation experiment, confirmed its beneficial effect on plant growth. Ammoniacal nitrogen could be regarded as alternative source nitrogen for plants. Plants assimilation of N plays an important role in the accumulation of N during ecological succession. The N from symbiotic fixation and, to a lesser extent, atmospheric deposition was taken up and stored in plant biomass. It is then returned to the soil via litter fall [42]. The process is accelerated when there is more biomass formed with higher N content in each growing season.
Figure 5: Plant growth of Lycopersicon esculentum, Medicago sativa and Helianthus annus in aboveground dry weight (a), underground dry weight (b), plant height (c) and leaf number (d) with water only (C), NPK fertilizer (F) and treated leachate (TL) after 90 days treatment. When compared within the same species, bars followed the same letter are not significantly different at P=0.05 according to the Tukey’s and Duncan’s tests.
The application of treated leachate increased significantly (p<0.05) the aboveground and underground content of N in all plants species. Plants irrigated with treated leachate accumulated more N in their tissues than corresponding controls, due to the higher load of nitrogen provided during the experiment [43]. For all species, the aboveground N contents increased two times of the control (Table 3). The ammoniacal nitrogen of leachates was rapidly assimilated and incorporated in the vegetative tissue. The fine, massive root system of crops may have facilitated the uptake and volatilization of exchangeable N from the soil. The N in biomass would become a nutriment reserve of the ecosystem in the long therm. It would be released through the decomposition of litter and provide N for plant growth [44].
| Species | Treatment | Tissue N content (%) (w/w) | |
|---|---|---|---|
| Aboveground | Underground | ||
| Helianthus annus | Water | 1.02 ± 0.11a | 0.41 ± 0.06a |
| Treated Leachate | 3.6 ± 0.127c | 1.43 ± 0.11b | |
| NPK Fertilizer | 2.92 ± 0.14b | 1.01 ± 0.11b | |
| Lycopersicon esculentum | Water | 0.79 ± 0.06a | 0.53 ± 0.05a |
| Treated Leachate | 5.33 ± 0.18c | 0.91 ± 0.04a | |
| NPK Fertilizer | 2.6 ± 0.3b | 0.57 ± 0.06a | |
| Medicago sativa | Water | 1.37 ± 0.09a | 0.43 ± 0.05a |
| Treated Leachate | 4.12 ± 0.29c | 1.59 ± 0.09b | |
| NPK Fertilizer | 3.6 ± 0.07b | 1.38 ± 0.16b | |
When compared within species, means followed by the same letters are not significantly different at P=0.05 according to the Tukey’s and Duncan’s tests.
Table 3: Tissue N content after 90 days of irrigation with water (C), treated leachate (TL) and NPK fertilizer (F).
Results showed that there is no significant pH change in all soil samples during the treatment (p>0.05). The soil used in this study was moderately basic (Table 2). Despite the acid character of the treated leachate, the pH value reached neutrality in all treated soils (Table 4). These results could be explained by the strong buffer capacity of the calcareous soils [45,46] and the nitrification process by which ammonia is converted to nitrites and then nitrates [47], this reaction was accompanied with the release of H ions [48-50]. The ammonia production resulting from degradation of the organic matter contained in leachates. Results showed that soil color and texture was also similar.
| Species | Treatment | pH | EC | NTK | Extractable N |
|---|---|---|---|---|---|
| (µScm-1) | (mg kg-1) | (mg kg-1) | |||
| Helianthus annus | Pré-irrigation | 7.97 ± 0.4 | 495 | 300 ± 23 | 138 ± 11.9 |
| Post-irrigation | |||||
| Water | 7.91 ± 0.91a | 490 ± 20a | 351 ± 11.2a* | 94,7 ± 7.6a | |
| Treated Leachate | 7.10 ± 0.3a | 820 ± 75c*** | 570 ± 19b*** | 456 ± 29b*** | |
| NPK Fertilizer | 7.15 ± 0.23a | 620 ± 32b** | 573 ± 25b*** | 567,2 ± 37b*** | |
| Lycopersicon esculentum | Water | 7.95 ± 0.96a | 490 ± 24a | 390 ± 17a** | 54.6 ± 4.3a |
| Treated Leachate | 7.15 ± 0.26a | 620 ± 54b** | 525 ± 41b*** | 267.7 ± 15b*** | |
| NPK Fertilizer | 7.25 ± 0.51a | 500 ± 79a | 530 ± 32b*** | 418 ± 36c*** | |
| Medicago sativa | Water | 7.91 ± 0.45a | 490 ± 13a | 320 ± 15a | 70 ± 9a |
| Treated Leachate | 7.10 ± 0.12a | 750 ± 80c*** | 675 ± 25b*** | 418.5 ± 35b*** | |
| NPK Fertilizer | 7.07 ± 0.37a | 590 ± 56b** | 480 ± 26b*** | 384 ± 28b*** | |
When compared within species, means followed by the same letters are not significantly different at P=0.05 according to the Tukey’s and Duncan’s tests. Significant difference when compared with the initial value (pre-irrigation) using Student’s T-test. *P<0.05, **P<0.01, ***P<0.001.
Table 4: pH, EC, N concentrations in soil before and after 90 days irrigation with water (C), Treated leachate (TL) and NPK fertilizer (F).
The change in soil salt content is a preoccupation in leachate irrigation because leachate contains high amount of dissolved salts such as Cl-, K+ and Na+ (Table 1), and its application to soil increased soil electrical conductivity. Our results suggest that application of treated leachate increased significantly the electrical conductivity of all treated soils (p<0.05) (Table 4). Although growth inhibition was not observed in this study, it should be noted that the potential hazard of Cl- as well as osmotic stress may be exacerbated when the salts are left behind by evapotranspiration [38]. Application with diluted treated leachate can relieve water stress and thus increase the stomatal conductance of leaves for water vapor [51].
Treated leachate application had significantly affected soil N content (P<0.05). There were marked increases in the soil N content in the treated leachate treatments. Soils fertilized with treated leachate had significantly higher extractable N concentrations compared with control ones (P<0.05) (Table 4). The increase of total N in the treated soils could be attributed to the nitrogen load of the treated leachate. Being the N concentration of the residue, the most influencing factor in the dynamics of soil mineral N [52,53]. Landfill leachate contained large amounts of organic and inorganic N, which might have been retained in soil after landfill leachate application [38,54]. The fresh input of easily available N substrates led to a rapid increase of soil respiration and accelerated organic N decomposition. This could be attributed to the high contents of organic nitrogen and the light texture of the sandy soil, which is characterized by a good aeration and permeability. When applied to soil, the C and N from leachate and the good aeration in the sandy soil provided a favourable condition for mineralization and nitrification [28]. Hernandez et al. [55] showed that sandy soil favouring more than clayey loam and loams soils mineralization processes.
The analysis indicates that there were no significant differences between the soils irrigated with treated leachate (LT) and fertilizer (F) for all species tested (p>0.05). The available N derived from treated leachate replaced an equivalent amount of fertilizer (Table 4). The finding suggests that the treated leachate addition increased the soil N content and led to a progressive enrichment of this fraction at the end of experiment. This fact improves once again the beneficial effect of the application of this treated effluent as fertilizer.
Based on the results of the microtoxicity, germination and irrigation experiments, one can conclude that the application of the treated leachate as fertilizer can improve plant growth. The positive effects of the treated leachate in ferti-irrigation seemed evident, especially when compared with the NPK fertilizer.
Landfill leachate constitutes a serious environmental problem. Several physico-chemical and biological processes to reduce their contaminant impacts have been proposed. Many researchers have established that this wastewater have a high fertilizer value when applied to the soil. Soils in semi-arid and arid areas are known to have low organic matter levels, a low fertility and a high exposure to degradation, desertification and pollution. Currently, organic wastes of various origins and nature are widely used as amendments to increase soil fertility and crop productivity. Treated landfill leachates still contains relatively high organic matter and nitrogen amounts in an important volume of water and could be used as a potential fertilizer, especially for soils and crops.
The authors would like to thank the Ministry of Higher Education and Scientific Research (Tunisia) for financial support.