Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2024)Volume 13, Issue 9
Background: The most important pathogenic risk factor for Obstructive Sleep Apnea (OSA) is obesity. Bariatric/ Metabolic Surgery (MS) is the most effective therapeutic strategy for severe obesity. This study aimed to determine the prevalence of severe obesity and OSA in patients who underwent MS and to identify the preoperative predictive factors associated with the effects of MS on OSA in Asians.
Methods: Polysomnography (PSG) was performed before and after surgery to determine the postoperative prevalence of OSA and evaluate the effects of MS on sleep architecture and sleep-disordered breathing indices. Apnea-hypopnea Index (AHI) of <15 events/h (omitted below) was defined as improvement, whereas an AHI of ≥ 15 was defined as no change. The preoperative factors that predict the effects of MS on OSA in the patients were analyzed to determine the prognostic factors associated with no change in preoperative data.
Results: The prevalence of OSA in patients with severe obesity in this study was 98.5%. However, rebound OSA was observed in some patients at 96-144 weeks after surgery. Only 31.1% of the patients showed improvement in AHI to <15. The preoperative predictive factors for no change in OSA were higher preoperative Apnea Index (AI) and lower mean preoperative %SpO2.
Conclusions: Surgical weight loss substantially improved OSA associated with obesity; however, AHI gradually rebounded over time. Long-term follow-up observation of OSA using PSG should be considered in these patients. AI may help to more precisely delineate MS outcomes.
Obstructive sleep apnea; Morbid obesity; Metabolic surgery; Apnea index; %SpO2 minimum; Polysomnography
Obstructive Sleep Apnea (OSA) is characterized by sleep fragmentation due to apnea and hypopnea caused by recurrent upper airway collapse during sleep. The prevalence of OSA in people aged 50-70 years is estimated to be 43% in males and 27% in females, whereas in people aged 30-49 years, it is estimated to be 9% in females and 26% in males [1,2].
Obesity is an important risk factor for OSA [3]. The estimated prevalence of OSA in adults with severe obesity ranges from 42% to 48% in males and from 8% to 38% in females [4,5]. Obese adults have a 10-times higher risk for OSA than adults with normal weight [6,7].
The standard therapy for OSA is Continuous Positive Airway Pressure (CPAP) therapy [8], which effectively improves Apnea- Hypopnea Index (AHI) and its associated symptoms and comorbidities [9].
MS is currently considered the most effective treatment modality for obesity. MS is associated with long-term maintenance of weight loss [10], and is the most effective method for markedly reducing the risk of obesity-related comorbidities and mortality [11,12]. Several studies and meta-analyses have reported improvement or resolution of OSA after MS in patients with severe obesity [9,13]. However, not all patients are cured of OSA after surgical weight loss. Indeed, the effects of weight loss on OSA vary widely among individuals [9,12-15].
Some Asians have micrognathia [16-18], and obesity simultaneously. Both conditions are risk factors for OSA; thus, Asians who have them concomitantly may develop severe OSA. This study aimed to determine the prevalence of OSA in Japanese individuals with severe obesity, assess the effects of MS on moderate to severe OSA, and identify the preoperative factors that predict improvement in OSA after MS.
Study design
The prevalence of OSA was investigated in patients who were scheduled for MS and underwent overnight PSG at the Department of Sleep Medicine, Uchimaru Medical Center, Iwate Medical University Hospital, Morioka, Japan and Yotsuya Medical Cube, Chiyoda, Tokyo, Japan or RESM Shin Yokohama Respiratory and Sleep Medical Care Clinic, Sinyokohama, kouhoku-ku, Yokohama, Japan. To be indicated for MS, patients with a primary objective of weight loss met a criterion of Body Mass Index (BMI) ≥ 35 kg/m2, whereas those with a primary objective of treatment of comorbidities met a criterion of BMI ≥ 32 kg/m2. All the patients provided informed consent for participation in this study. This study was approved by Iwate Medical University (Approval No: H27-22) and conducted according to the principles of the Declaration of Helsinki.
Data collection
Data on the age, gender, height, and weight of each patient were collected during outpatient visits at each institution. For patients who underwent MS and were regularly followed up using PSG, the masses of their abdominal visceral and subcutaneous adipose tissue were measured each week. The masses were calculated from preoperative and postoperative cross-sectional Computed Tomography (CT) images obtained at the level of the umbilicus using the dedicated software (Ziosoft, Inc., Japan) in the CT management system.
PSG
All patients underwent PSG with an Alice PDx system (Philips Respironics; Murrysville, PA) in a dedicated examination room at Iwate Medical University Hospital. PSG was initiated at 20:00 and concluded at 6:00 AM the following morning, with test conditions kept as consistent as possible. PSG was performed before surgery and at 4, 24, 48, 96, 144, 192, and 240 weeks after surgery. The results of PSG performed at these time points were analyzed to determine the effects of MS on OSA according to the Scoring Manual (version 2.5) issued by the American Academy of Sleep Medicine [19].
Bariatric surgery
The patients underwent Laparoscopic Sleeve Gastrectomy (LSG) under general anesthesia at Iwate Medical University Hospital. The surgeries were performed by board-certified endoscopic and gastrointestinal surgeons with extensive experience in bariatric surgery.
Outcomes
Postoperative “improvement” in OSA was defined as AHI of <15 events/h, which is the target in CPAP therapy, whereas “no change” after MS was defined as AHI of ≥ 15 events/h. To investigate factors associated with “no change” in preoperative data, the patients, excluding those with mild OSA, were divided into an “improvement” group, which included those with AHI of <15 events/h, and a “no change” group, which included those with AHI of ≥ 15 events/h. The PSG data used for the improvement group were obtained in the weeks when the patients achieved an AHI of <15 events/h, whereas the PSG data used for the no change group were obtained in the last week of the study period.
Statistical analysis
Statistical Package for the Social Sciences (SPSS) version 29.0 (IBM Corp., Armonk, NY, USA) was used to calculate the overall and sex-stratified prevalence of severe obesity among the patients. The unpaired t-test was used to compare the preoperative and postoperative BMI, subcutaneous adipose tissue mass, visceral adipose tissue mass, and PSG data of the patients. Tukey’s honestly significant difference test was performed for comparison of homoscedastic variables, whereas the Games-Howell test was used for the analysis of heteroscedastic data.
Bivariate logistic analysis of the results of preoperative examinations (sex-stratified differences, age, severity, preoperative BMI, preoperative subcutaneous adipose tissue mass, preoperative visceral adipose tissue mass, preoperative Total Sleep Time (TST) preoperative Sleep Efficiency (SE) preoperative percentage of stage N1 (%stage N1), preoperative %stage N2 (%stage N2), preoperative %stage N3 (%stage N3), preoperative percentage of rapid eye movement stage (%Stage R), preoperative Arousal Index (ArI), preoperative Apnea Index (AI), preoperative Hypopnea Index (HI), preoperative Rapid Eye Movement (REM)-AHI, preoperative Non-REM (NREM)-AHI, preoperative desaturation index, preoperative mean Oxygen Saturation (SpO2 mean), and preoperative minimum Oxygen Saturation (SpO2 minimum) obtained with PSG was performed to determine the factors associated with “improvement” and “no change” in OSA after MS.
One-way Analysis Of Variance (one-way ANOVA) was performed using the data obtained in the weeks when “improvement” was achieved and in the last week when PSG confirmed “no change” to calculate the differences in AHI (δ AHI), AI (δ AI), HI (δ HI), BMI (δ BMI), and visceral adipose tissue mass (δ visceral adipose tissue mass) and determine to what extent AHI, AI, and HI would decrease if BMI decreases by 10 kg/m2 and visceral adipose tissue mass by 10 cm2. Bell curve for excel version 4.04 (Social Survey Research Information Co. Ltd., Tokyo, Japan) was used for this analysis.
PSG data obtained in the weeks when patients in the “improvement” group achieved an AHI of <15 events/h and in the last week when the AHI of patients in the “no change” group was ≥ 15 events/h were used to calculate the ratio of improvement in the patients, excluding those who had rebound OSA after surgery (those who were previously classified into the “improvement” group but were later moved into the “no change” group), and the rate of improvement in all the patients, including those who had rebound OSA. A two-sided p value of 0.05 was considered significant in all analyses.
Prevalence of OSA complicating severe obesity The prevalence of OSA was investigated in 203 patients (103 males and 100 females) who were scheduled for MS for severe obesity and underwent PSG. The analysis showed that 200 (98.5%) of the 203 patients had OSA. Of these 203 patients, 22 (10.8%) had mild OSA (5<AHI<15), 37 (18.2%) had moderate OSA (15<AHI<30), and 141 (69.5%) had severe OSA (AHI>30). Of the 103 male patients, 3 (2.9%) had mild OSA, 14 (13.6%) had moderate OSA, and 86 (83.5%) had severe OSA. Of the 100 female patients, 19 (19.0%) had mild OSA, 23 (23.0%) had moderate OSA, and 55 (55.0%) had severe OSA (Figure 1).
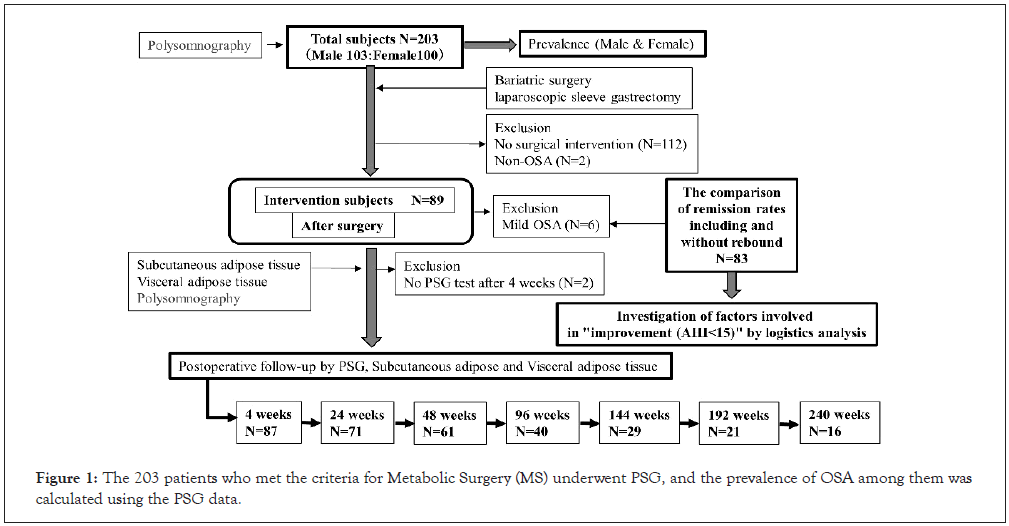
Figure 1: The 203 patients who met the criteria for Metabolic Surgery (MS) underwent PSG, and the prevalence of OSA among them was calculated using the PSG data.
Effects of bariatric/metabolic surgery on obstructive sleep apnea complicating severe obesity
Table 1, shows the effects of MS for severe obesity on physical findings, sleep indices, and sleep-disordered breathing indices. A total of 89 patients underwent surgery. Of these, 83 patients, excluding those with mild OSA, were divided into the improvement group (AHI<15 events/h) and the no change group (AHI ≥ 15 events/h). The 89 patients who underwent MS underwent PSG before surgery, 87 underwent PSG at 4 weeks after surgery, 71 at 24 weeks, 61 at 48 weeks, 40 at 96 weeks, 29 at 144 weeks, 21 at 192 weeks, and 16 at 240 weeks.
| Parameters | Preoperative | Postoperative | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 (n=89) | 4 (n=87) | 24 (n=71) | 48 (n=61) | 96 (n=40) | 144 (n=29) | 192 (n=21) | 240 (n=16) | ||
| BMI (kg/m2) | 41.6 (5.0) | 35.6 * (3.9) | 32.0† ¶ (4.3) | 31.2‡∫ (4.5) | 31.4 ⁑∬ (4.9) | 31.3 ⧺♭ (4.9) | 32.3⧻ (5.6) | 32.7# (5.9) | |
| SAT (cm2) | 517.4 (128.0) | 434.6* (119.0) | 346.2† ¶ (120.4) | 334.3‡∫ (126.4) | 342.3⁑∬ (148.6) | 354.5⧺ (161.7) | 376.5⧻ (142.3) | 375.3# (176.9) | |
| VAT (cm2) | 260.7 (83.0) | 195.4* (78.3) | 162.0† (70.9) | 143.1‡∫ (62.8) | 141.4⁑∬ (62.5) | 156.0⧺ (49.2) | 168.3⧻ (56.7) | 176.1# (60.5) | |
| %EWL (%) | 0.0 (0.0) | 30.8* (9.5) | 48.7 † ¶ (15.3) | 54.0‡∫ (16.9) | 53.4⁑∬ (18.9) | 52.0⧺♭ (17.7) | 49.2⧻♮ (16.1) | 44.2#§ (18.0) | |
| TST (mins) | 439.7 (75.2) | 470.2 (57.1) | 478.5† (64.2) | 478.4† (61.2) | 477.1 (69.3) | 456.5 (61.4) | 459.0(80.0) | 460.3 (60.4) | |
| SE (%) | 79.9 (11.3) | 84.2(10.8) | 85.9† (10.9) | 84.6 (11.6) | 84.4 (10.9) | 82.4 (10.1) | 81.3 (14.2) | 83.0 (10.7) | |
| N1 (%) | 25.3 (17.0) | 17.7* (12.0) | 16.0† (9.6) | 15.5† (9.8) | 15.6⁑ (11.4) | 17.1 (13.1) | 10.6⧻♮ (6.2) | 14.5# (6.6) | |
| N2 (%) | 50.1 (14.6) | 58.2* (10.9) | 58.4† (9.3) | 57.9† (11.3) | 59.9⁑ (11.0) | 59.1⧺ (11.9) | 62.2⧻ (9.0) | 60.8# (8.6) | |
| N3 (%) | 9.3 (9.2) | 7.0 (7.2) | 6.9 (4.8) | 8.0 (9.0) | 6.5 (5.2) | 6.7 (6.0) | 6.3 (5.1) | 6.4 (5.2) | |
| REM (%) | 15.0 (6.7) | 16.9 (5.7) | 18.2† (6.4) | 18.6† (5.6) | 17.9 (5.7) | 17.2 (6.1) | 19.5 (6.4) | 18.7 (5.2) | |
| ArI (events/h) | 32.6 (24.4) | 21.0*(16.3) | 18.1† (12.7) | 16.3† (10.3) | 16.7⁑ (10.3) | 18.4⧺ (12.5) | 15.0⧻ (8.0) | 19.2# (10.1) | |
| AHI (events/h) | 54.5 (30.2) | 34.9* (25.6) | 26.2† (21.2) | 23.1†∫ (20.2) | 21.3⁑∬ (18.3) | 25.5⧺ (19.5) | 21.8⧻ (14.9) | 25.5# (16.4) | |
| AI (events/h) | 22.5 (23.2) | 9.9* (19.8) | 7.9† (14.0) | 7.0† (13.8) | 5.1⁑ (11.6) | 6.5⧺ (13.2) | 3.9⧻ (7.0) | 3.8# (7.8) | |
| AHI (events/h) | 32.1 (23.9) | 24.9*(17.9) | 19.0† (14.5) | 16.3‡∫ (13.0) | 16.1⁑∬ (11.6) | 18.6⧺ (12.8) | 17.9⧻ (9.9) | 21.6# (11.2) | |
| REM-AHI (events/h) | 56.4 (28.7) | 41.8*(24.4) | 30.8† (20.9) | 29.0‡∫ (19.8) | 26.5⁑∬(18.7) | 30.1⧺ (15.4) | 29.5 ⧻ (14.7) | 37.5 # (22.3) | |
| NREM-AHI (events/h) | 51.2 (31.1) | 33.0* (26.4) | 24.8† (22.6) | 21.2† (21.2) | 18.7⁑∬ (18.5) | 22.6⧺ (20.8) | 18.5⧻♮(16.3) | 21.5# (17.8) | |
| 3% ODI (events/h) | 54.9 (31.7) | 33.0*(24.6) | 24.2† (20.2) | 21.5‡∫ (19.2) | 20.5 ⁑∬ (18.2) | 24.9⧺ (19.4) | 21.4⧻ (16.1) | 25.7# (18.6) | |
| Mean SpO2 (%) | 91.6 (3.6) | 93.6* (2.2) | 94.4† (2.0) | 93.7 (6.1) | 94.6⁑ (1.9) | 94.1⧺ (1.8) | 94.4⧻ (1.8) | 93.6# (1.7) | |
| Minimum SpO2 (%) | 72.9 (12.1) | 78.2* (9.1) | 80.2† (8.2) | 80.5† (9.3) | 83.6⁑∬ (7.2) | 81.8⧺ (7.0) | 79.9⧻ (7.1) | 79.9# (7.6) | |
Note: p value of <5% was considered statistically significant; *Preoperative vs. 4 weeks after surgery; †Preoperative vs. 24 weeks after surgery; †preoperative vs. 48 weeks after surgery; ⁑: Preoperative vs. 96 weeks after surgery; ⧺: Preoperative vs 144 weeks after surgery; ⧻: Preoperative vs 192 weeks after surgery; #: Preoperative vs. 240 weeks after surgery; ¶: 4 vs. 24 weeks after surgery; ∫: 4 vs. 48 weeks after surgery; ∬: 4 vs. 96 weeks after surgery; ♭: 4 vs. 144 weeks after surgery; ♮: 4 vs. 192 weeks after surgery; §: 4 vs. 240 weeks after surgery; (Parentheses) indicatethe standard deviation. Abbreviations: BMI: Body Mass Index; SAT: Subcutaneous Adipose Tissue; VAT: Visceral Adipose Tissue; %EWL: %Excess Weight Loss; TST: Total Sleep Time; SE: Sleep Efficiency; ArI: Arousal Index; AHI: Apnea Hypopnea Index; AI: Apnea Index; HI: Hypopnea Index; REM: Rapid Eye Movement; NREM: Non-Rapid Eye Movement; 3% ODI: 3% Oxygen Desaturation Index; SpO2: Oxygen Saturation of peripheral artery. <
Table 1: Comparison of the preoperative and postoperative body mass index, adipose tissue mass and PSG parameters.
Changes in physical findings after bariatric/metabolic surgery
BMI, as well as subcutaneous and visceral adipose tissue masses of the patients significantly decreased after surgery (p<0.05); however, no significant differences were observed at 24 weeks after surgery and each week thereafter. The BMI of the patients decreased significantly between 4 and 192 weeks (p<0.05); however, no significant differences were observed at 192 and 240 weeks. The subcutaneous adipose tissue mass of the patients at 24, 48, and 96 weeks were significantly decreased compared with that at 4 weeks after surgery (p<0.05); however, no significant decrease was observed after the latter weeks. The visceral adipose tissue mass of the patients did not significantly decrease between 4 and 24 weeks after surgery. However, the visceral adipose tissues mass of the patients at 48 and 96 weeks were significantly decreased compared with that at 4 weeks (p<0.05). The percentage of excess weight loss (%EWL) values recorded up to 240 weeks after surgery were significantly increased compared with the preoperative values (p<0.05). The %EWL recorded at and after 24 weeks were significantly increased compared with that recorded at 4 weeks after surgery (p<0.05); however, there were no significant differences between the %EWL recorded at 24 weeks and that recorded each week thereafter. Although BMI and subcutaneous and visceral adipose tissue masses significantly decreased after surgery, they increased between 96 and 144 weeks; however, the differences were not statistically significant. In addition, although %EWL significantly increased after surgery, it tended to decrease at and after 96 weeks after surgery; however, the difference was not statistically significant as well.
Changes in sleep architecture after bariatric/metabolic surgery
The TST of the patients at 24 and 48 weeks after surgery were significantly increased compared with the preoperative values (p<0.05). Although the TST recorded in the subsequent weeks showed an increasing trend compared with that recorded before surgery, no significant differences were observed. In addition, no significant differences were noted between the TST recorded at 4 weeks and that recorded each week thereafter. The SE recorded at 24 weeks after surgery was significantly increased compared with that recorded before surgery (p<0.05). Although the SE recorded at 4 and 48 weeks and thereafter showed an increasing trend compared with that recorded before surgery, no significant differences were observed among the values recorded each week after surgery. In addition, no significant differences were noted in the SE recorded at 4 weeks and that recorded each week thereafter. The %stage N1 recorded at 4, 96, 192 and 240 weeks after surgery were significantly decreased compared with that recorded before surgery (p<0.05). However, no significant difference was noted between the preoperative %stage N1 and that recorded at 144 weeks. Although %stage N1 significantly decreased between 4 and 192 weeks after surgery (p<0.05), it tended to decrease without significant differences at other weeks. There were no significant differences between the %stage N1 recorded at 24 weeks and that recorded each week thereafter. %stage N2 significantly increased each week after surgery compared with that record before surgery (p<0.05). However, no significant difference was observed between the %stage N2 recorded at 4 weeks and that recorded each week thereafter. %stage N3 did not significantly change each week. The %stage REM recorded before surgery was not significantly different from that recorded at 4 weeks after surgery. However, the %stage REM recorded at both 24 and 48 weeks were significantly increased compared with the preoperative value (p<0.05). In addition, %stage REM tended to increase at and after 96 weeks compared with the preoperative value, but no significant differences were noted between the values recorded at these time points. Moreover, there were no significant differences among the values recorded each week.
Changes in sleep-disordered breathing indices after bariatric/metabolic surgery
The ArI, AHI, AI, REM-AHI, NREM-AHI, and desaturation index of the patients significantly decreased each week after surgery (p<0.05). The %SpO2 minimum of the patients significantly increased each week after surgery (p<0.05). We found no significant differences between the ArI and AI recorded at 4 weeks and the values recorded each week thereafter. The AHI recorded at 24, 48, and 96 weeks after surgery were significantly decreased compared with that recorded at 4 weeks (p<0.05). However, no significant differences were observed between the AHI recorded at 4 weeks and that recorded each week after 96 weeks. In addition, there were no significant differences between the AI recorded at 4 weeks and that recorded each week thereafter.
There was no significant difference in the preoperative HI and that recorded at 4 weeks after surgery, but the HI recorded at 24 weeks and each week thereafter were significantly decreased compared with the preoperative value (p<0.05). In addition, the HI recorded at 24 and 96 weeks after surgery were significantly decreased compared with that recorded at 4 weeks (p<0.05). However, there was no significant difference between the HI at 4 weeks and that at 144 weeks after surgery.
The REM-AHI recorded at 24 and 96 weeks after surgery were significantly decreased compared with that recorded at 4 weeks (p<0.05). However, the REM-AHI recorded at 144 weeks and thereafter were not significantly different from that recorded at 4 weeks after surgery. NREM-AHI were significantly decreased at 4, 96, and 192 weeks after surgery (p<0.05). We noted no significant differences between the HI, REM-AHI, and NREM- AHI recorded at 24 weeks after surgery and the values recorded each week thereafter.
The desaturation index of the patients did not significantly decrease between 4 and 24 weeks after surgery (p<0.05). In addition, the desaturation indices recorded at 48 and 96 weeks after surgery were not significantly decreased compared with that recorded at 4 weeks. Furthermore, there were no significant differences between the values recorded at 24 weeks after surgery and each week thereafter.
As with BMI and visceral adipose tissue mass, AHI, HI, REM- AHI, NREM-AHI, desaturation index, and ArI tended to increase from 96 weeks to 144 weeks and thereafter, but without any statistically significant differences (Figures 2A-2I). The %SpO2 mean measured at 4, 24, 96, 144, 192, and 240 weeks after surgery were significantly increased compared with the preoperative value. There was no significant difference between the preoperative %SpO2 mean and that recorded at 48 weeks. In addition, there were no significant differences between the %SpO2 mean recorded 4 weeks after surgery and that recorded each week thereafter. The %SpO2 minimum recorded at 96 weeks and thereafter were significantly increased compared with that recorded at 4 weeks after surgery (p<0.05). There was no significant difference between the %SpO2 minimum recorded at 24 weeks and the values recorded in the weeks thereafter.
Figure 2: Changes in PSG parameters after surgery, (A): Body mass index; (B): Visceral adipose tissue mass; (C): AHI; (D): AI; (E): HI; (F): Rapid eye movement-AHI; (G): Non-rapid eye movement-apnea-hypopnea index; (H): Desaturation index and (I): Arousal index; p value of ≤ 5% was considered statistically significant.
Preoperative factors associated with the effects of bariatric/metabolic surgery on OSA
The improvement group was defined as patients who achieved a postoperative AHI of <15 events/h at least once during the follow-up period, whereas the no change group was defined as patients whose AHI was ≥ 15 events/h during the follow- up period. Bivariate logistic analysis of the preoperative baseline characteristics and PSG data of 83 patients, excluding those without OSA and those with mild OSA, was performed to determine the factors associated with no change in OSA after MS (Table 2). The variables were selected using a stepwise procedure with a p value of 0.200 for entry and removal. The preoperative AI, NREM-AHI, and %SpO2 mean were selected as variables, using this process, and were used for analyses (Table 2). The results of the analyses using variables, including the preoperative AI, NREM-AHI, and %SpO2 mean, suggested that the probability of no change in OSA increases with an increase in preoperative AI and a decrease in preoperative %SpO2 mean (Table 3). Based on this result, the classification accuracy rates for improvement and no change were 89.5% and 76.4%, respectively. The rate of improvement in OSA was calculated from the AHI recorded in the weeks when improvement was achieved, and the AHI recorded at the time of the last PSG that confirmed no change. A total of 36 of 83 patients achieved an AHI <15 events/h, and the improvement rate was 43.4% (Figure 2C). One-way ANOVA was performed using the data obtained in the weeks when “improvement” was achieved and at the time of the last PSG that confirmed “no change” to calculate δ AHI, δ AI, δ HI, δ BMI, and δ visceral adipose tissue mass and to determine to what extent AHI, AI, and HI would decrease if BMI decreases by 10 kg/m2 and visceral adipose tissue mass by 10 cm2. Analysis of all the patients revealed that when BMI decreased by 10 kg/m2, AHI decreased by 25.9 events/h, AI by 9.3 events/h, and HI by 16.8 events/h. Analysis of the male and female patients separately showed that the AHI of male patients decreased by 21.8 events/h with a 10 kg/m2 decrease in BMI. For female patients, AHI decreased by 39.9 events/h and AI by 40.7 events/h when BMI decreased by 10 kg/m2. These results suggested that the effects of the BMI on the AHI, AI, and HI differ between male and female patients (Table 4).
| Characteristics | Improvement (n=36) | No change (n=47) | p-value |
|---|---|---|---|
| Age (year) | 42.9 ± 12.0 | 45.7 ± 11.2 | NS |
| BMI (kg/m2) | 41.1 ± 5.4 | 42.1 ± 4.8 | NS |
| SAT (cm2) | 514.8 ± 128.7 | 513.0 ± 131.9 | NS |
| VAT (cm2) | 266.8 ± 80.7 | 268.4 ± 84.4 | NS |
| TST (mins) | 438.2 ± 86.1 | 444.4 ± 71.9 | NS |
| SE (%) | 79.0 ± 13.0 | 81.6 ± 10.3 | NS |
| N1 (%) | 18.9 ± 9.9 | 31.4 ± 19.4 | <0.001 |
| N2 (%) | 51.8 ± 11.7 | 46.9 ± 15.6 | NS |
| N3 (%) | 12.4 ± 10.1 | 7.8 ± 8.4 | <0.05 |
| REM (%) | 12.4 ± 10.1 | 7.8 ± 8.4 | NS |
| AHI (events/h) | 43.9 ± 20.4 | 68.4 ± 29.4 | NS |
| AI (events/h) | 8.2 ± 10.1 | 35.0 ± 23.9 | <0.001 |
| HI (events/h) | 35.6 ± 16.9 | 33.4 ± 27.2 | NS |
| REM-AHI (events/h) | 57.0 ± 19.5 | 62.4 ± 30.7 | NS |
| NREM-AHI (events/h) | 37.8 ± 20.3 | 65.9 ± 30.5 | <0.001 |
| ArI (events/h) | 20.2 ± 12.4 | 43.0 ± 27.1 | <0.001 |
| 3%ODI (events/h) | 43.9 ± 20.6 | 68.3 ± 32.3 | <0.001 |
| Mean SpO2 (%) | 92.2 ± 1.9 | 90.8 ± 4.3 | NS |
| Minimum SpO2 (%) | 75.3 ± 9.6 | 69.2 ± 12.8 | <0.05 |
Note: p value of ≤ 5% was considered statistically significant; The data are presented as mean ± Standard Deviation (SD); BMI: Body Mass Index; SAT: Subcutaneous Adipose Tissue; VAT: Visceral Adipose Tissue; %EWL: %Excess Weight Loss; TST: Total Sleep Time; SE: Sleep Efficiency; ArI: Arousal Index; AHI: Apnea Hypopnea Index; AI: Apnea Index; HI: Hypopnea Index; REM: Rapid Eye Movement; NREM: Non-Rapid Eye Movement; 3% ODI: 3% Oxygen Desaturation Index; SpO2: Oxygen Saturation of peripheral artery; NS: Not Significant.
Table 2: Comparison of the baseline characteristics and polysomnography results and the improvement and no change groups.
| Variable (preoperative) | Partial Regression coefficient | OR | Significance test for partial regression coefficients | |
|---|---|---|---|---|
| Wald | p-value | |||
| AI | 0.1507 | 1.1626 | 16.3897 | p<0.001 |
| NREM-AHI | 0.0192 | 1.0194 | 1.6378 | 0.2006 |
| SpO2 mean | 0.3502 | 1.4193 | 4.9453 | <0.05 |
Note: AI: Apnea Index; NREM: Non-Rapid Eye Movement; SpO2: Oxygen Saturation of peripheral artery; p value of ≤ 5% was considered statistically significant; Akaike Information Criterion=68.3678; Nagelkerke’s R2=0.6228; likelihood ratio=50.9063 (p<0.001).
Table 3: Evaluation of preoperative factors associated with the effects of bariatric/metabolic surgery on obstructive sleep apnea.
| Participants | Variables | Univariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Dependent variable | Independent variable | Regression coefficient | Constant term | SE | t-ratio | p-value | |
| All participants | δ AHI (postoperative to preoperative change) | δ BMI (postoperative to preoperative change) | 2.59 | -7.20 | 0.487 | 5.00 | <0.0001 |
| δ AI (postoperative to preoperative change) | 0.93 | -8.11 | 0.487 | 2.09 | <0.05 | ||
| δ HI (Postoperative to preoperative change) | 1.68 | 0.85 | 0.487 | 2.96 | <0.005 | ||
| Male participants | δ AHI (Postoperative to preoperative change) | δ BMI (Postoperative to preoperative change) | 2.18 | -9.40 | 0.771 | 3.41 | <0.001 |
| δ AI (Postoperative to preoperative change) | 1.04 | -11.35 | 0.771 | 1.67 | NS | ||
| δ HI (Postoperative to preoperative change) | 1.23 | 3.27 | 0.771 | 1.96 | NS | ||
| Female participants | δ AHI (Postoperative to preoperative change) | δ BMI (Postoperative to preoperative change) | 3.99 | 1.45 | 0.534 | 4.144 | <0.0001 |
| δ AI (Postoperative to preoperative change) | -0.14 | -11.35 | 0.534 | 0.254 | NS | ||
| δ HI (Postoperative to preoperative change) | 4.07 | 11.40 | 0.534 | 3.944 | <0.0001 | ||
Note: p value of ≤ 5% was considered statistically significant; ArI: Arousal Index; AHI: Apnea-Hypopnea Index; AI: Apnea Index; HI: Hypopnea Index.
Table 4: Changes in apnea-hypopnea index, apnea index, and hypopnea index due to decreased body mass index.
Rates of improvement in OSA with and without considering rebound cases
PSG data obtained in the weeks when the patients in the improvement group achieved an AHI<15 events/h and in the last week when the AHI of the patients in the no change group was ≥ 15 events/h were used for this analysis. The rate of improvement in the patients, excluding those who had rebound OSA (those who were classified into the improvement group but were later moved to the no change group), was 43.4% (36 out of 83 patients).
When the patients who had rebound OSA were included, the improvement rate was 31.3% (26 out of 83 patients) (Figure 3).
Figure 3: Rate of improvement in OSA without taking rebound cases into consideration.
In the present study, MS substantially improved BMI, subcutaneous and visceral adipose tissue masses, and PSG parameters associated with OSA. However, not all patients achieved improvement. Five of the eight patients who rebounded were observed 96 to 144 weeks after surgery. In addition, only 31.3% of the patients showed AHI improvement to <15 events/h.
Using a cutoff AHI of 15 events/h, at which the prevalence of fatal vascular disorders starts increasing, we defined “improvement” as AHI of <15 events/h and no change as AHI of >15 events/h. Analysis of the preoperative data to identify the prognostic factors that contribute to improvement in OSA revealed that the probability of no change increases with higher in preoperative AI and lower in preoperative %SpO2 mean. To the best of our knowledge, the present study is the first study on the evaluation of preoperative prognostic factors for OSA in Japanese patients.
Poor sleep quality and sleep fragmentation may compromise efforts to lose weight and maintain weight loss [20]. When the amount of sleep is insufficient and the quality is poor, the secretion of and responses to mediators (ghrelin and leptin) involved in appetite stimulation and satiety change. Changes in these hormones increase appetite (particularly for high-calorie foods) which leads to weight gain [20]. In OSA, sleep is fragmented by episodes of apnea and hypopnea caused by upper airway obstruction during sleep. Consequently, daytime sleepiness, non-restorative sleep, and a decrease in sleep quality occur. Obesity is a risk factor for OSA, and OSA may be a risk factor for obesity. Complications associated with metabolic syndrome create a vicious cycle wherein obesity and OSA mutually aggravate each other.
MS is an effective therapeutic approach to weight control in patients with severe obesity who have failed to change their lifestyle habits. MS is widely accepted as the mainstay of the treatment of morbid obesity and is the most successful strategy for achieving sustained weight loss and markedly reducing the risks of obesity-related morbidity and mortality [12,13].
In candidates of bariatric surgery with BMI of ≥ 35 kg/m2, the prevalence of OSA may range from 60% to 83% [13]. In a Mexican study on the prevalence of OSA in 52 patients, 51 patients (98%) had an AHI of ≥ 5 events/h [21]. It has been reported that when the severity of obesity in Asians and Caucasians is comparable, Asians are more likely to develop severe OSA than Caucasians and are more likely to develop OSA even if they have a lower BMI [16- 18]. One of the reasons for these tendencies is micrognathia (the anatomical features of the maxillofacial region) in Asians, which may contribute to the increased prevalence of OSA among them [16-18]. In other words, some Asians have both micrognathia and obesity, which may cause them to develop severe OSA.
Regarding the effects of MS on OSA, not all patients are cured of OSA after achieving surgical weight loss. Indeed, the effects of weight loss on OSA vary across individuals [9,13-15]. This is associated with the pathogenesis of OSA which is affected by different ratios of the four pathological components of OSA among patients, as explained by Wellman et al., [22,23].
These components are pathophysiology, which involves narrowing of the upper airway caused by obesity and collapse of the maxillofacial morphology during sleep; the instability of the respiratory center (hypersensitive ventilator control: High loop gain); decreased compensatory (response) ability of the pharyngeal muscles for negative pressure in the airway; and decreased arousal threshold (ease of arousal). Given that diverse phenotypes are involved in OSA, improvement in obesity alone does not cure OSA in all patients with concomitant severe obesity.
In the present study, we continuously performed PSG and observed the clinical courses of the patients over a long period. Patients with OSA whose AHI decreased to <15 events/h accounted for 43.4% of the study cohort. The AHI of some patients decreased to <15 events/h once but subsequently increased to >15 events/h over the follow-up period. When these rebound cases were taken into consideration, the rate of improvement in OSA was 31.3%. In the long-term follow-up observation, we found that AHI and HI started to increase at 96 and 144 weeks as BMI and visceral adipose tissue mass increased. Another study conducted using follow-up PSG data showed that although the AHI of the participants substantially decreased, moderate OSA persisted in many individuals [10,15]. In the present study, residual moderate to severe OSA or rebound was observed in 79.5%, 62.7%, 55.2%, 47.4%, 63.3%, 61.9%, and 75% of the patients with moderate or severe OSA at 4, 24, 48, 96, 144, 192, and 240 weeks after surgery, respectively. A meta- analysis of 12 studies showed that most of the patients analyzed (62%) had residual moderate to severe OSA after surgery [10,15].
Continuation of diet therapy (very low-calorie diets [24] and Mediterranean diets [25]) and exercise therapy, as well as addition of drug therapy, may need to be considered as countermeasures against rebound OSA after MS. Notably, a substantial number of patients may have high AHI owing to residual moderate to severe and rebound OSA after MS. This supports the reports indicating the need for continuation of CPAP therapy after MS [26-28].
There are few studies on the effects of MS on sleep architecture. In one of such studies conducted by Dixon JB et al., PSG was used to examine the effects of MS on sleep in 25 patients with OSA complicating severe obesity; the authors reported that the participants showed substantial increase in their proportions of REM, stage 3, and stage 4 sleep [29]. These changes in sleep architecture are similar to those described by Charuzi, et al., [30]. In the present study, performing PSG before surgery and at 4, 24, 48, 96, 144, 192, and 240 weeks after surgery allowed us to investigate changes in sleep architecture over a long period. The results showed that TST peaked at 48 weeks after surgery and did not significantly change thereafter. In addition, SE peaked at 24 weeks after surgery and remained almost unchanged thereafter. %stage N1 significantly decreased from 4 weeks after surgery, whereas %stage N2 significantly increased. These changes were mostly maintained over the course of 240 weeks. However, in contrast to the results reported by Dixon JB et al., %stage N3 did not change in the present study. %stage REM significantly increased from 24 weeks after surgery and remained almost unchanged thereafter. Studies of post-MS patients often suffer from the weakness of relatively high rates of withdrawal of consent from repeat PSG examination and the difficulty of examining individually matched pre- operative and post-operative data. In the present study, only 16 patients underwent PSG at all the specified weeks. Bivariate logistic analysis performed to identify the preoperative physical factors and PSG parameters associated with the effects of MS on OSA revealed that the probability of no change increases with higher in preoperative AI and lower in preoperative SpO2 mean.
In addition, the classification accuracy rates were 89.5% for improvement and 76.4% for no change. Currie et al., reported that the factors associated with remission of OSA are a younger age, a lower BMI, a higher %EWL, and LSG or laparoscopic Roux-en-Y gastric bypass. Bae Ek et al., reported that patients with low preoperative minimum SpO2 and high preoperative supine AHI, measured using PSG, may have residual OSA after bariatric surgery and require close follow-up. Moreover, the authors indicated that it is difficult to predict OSA improvement, which 70% of patients achieve after MS [31,32].
There is no consensus on the prediction of improvement of OSA in the existing literature. However, when we separately analyzed AI and HI instead of AHI in the present study, the results suggested that the probability of no change increased with higher in preoperative AI. When we examined to what extent AHI, AI, and HI would decrease after a 10 kg/m2 decrease in BMI, we found that weight loss was less effective for improving AI than for improving HI. In male patients, AHI decreased as BMI decreased. However, when AI and HI were evaluated separately, a decrease in BMI was less effective for improving the indices. In female patients, a decrease in BMI greatly contributed to a decrease in AI. These results suggest that the effects of a decrease in BMI differ between male and female patients. Regarding reports of the effects of MS, studies in which cases of rebound OSA were taken into consideration are rare. In the present study, the OSA improvement rate without taking rebound cases into consideration was 43.3%, whereas it was 31.3% when considering rebound cases. Prior to the present study, there were no reports of studies in which rebound OSA was evaluated during long- term follow-up using PSG. The authors of the abovementioned short-term studies reported that because a substantial number of patients may have moderate to severe residual lesions after surgical weight loss, CPAP therapy is still necessary [24-26]. In the present study, the AHI of 68.7% of the patients rebounded to 15 events/h or higher after MS. Thus, long-term follow-up using PSG is necessary. In addition, we recommend careful consideration of CPAP therapy discontinuation.
To examine the effects of MS on OSA complicating severe obesity, we performed PSG a total of seven times, from the period before surgery to 240 weeks after surgery. Given that only 16 patients completed all the PSG sessions, it was difficult to perform paired comparisons. Thus, further studies with a large sample of patients who are continuously evaluated using PSG are needed. All the patients in the present study underwent CPAP therapy at the time of preoperative diagnosis; however, several patients discontinued the therapy and could not be evaluated. Considering that we recommend the continuation of CPAP therapy based on our results, further comparative studies are necessary to evaluate postoperative adherence to CPAP therapy and changes in pressure settings, sleep architecture, and sleep- disordered breathing indices measured using PSG with and without CPAP after surgery.
In the present study, the prevalence of OSA in Japanese patients with severe obesity was 98.5%. The prevalence of OSA among the female and male patients was 97% and 100%, respectively. These results are comparable to the highest rates reported in previous studies. This suggests that factors such as micrognathia and obesity further increase the prevalence of OSA in Asians with severe obesity. Furthermore, the preoperative predictive factors suggested that the probability of no change in OSA increases with higher in preoperative AI and lower in preoperative %SpO2 mean. We found that although surgical weight loss substantially improved obesity-related OSA, sleep-disordered breathing indices, such as BMI and visceral adipose tissue mass, increased 96-144 weeks after surgery. We recommend long-term postoperative follow-up using PSG and careful consideration of CPAP therapy discontinuation.
The data that supported the findings of this study are available upon request from the correspondent author.
TM and TN were responsible for study design. TM, TN, KH, RH, ST, AA, MS, and SR were responsible for diagnosis, sample collection, and data collection of laboratory tests. ST, RC, CT, and KA analyzed the PSG data. TM, TY and TN were responsible for data analysis. TN and AS participated in conducting the study, including data interpretation and critical review of the report. All authors contributed to preparation of the report and approved the final version.
Financial support for the present study was provided by a grant from the Project for Development of Innovative Medical Devices. No other financial support was received. The authors declare no conflicts of interest that may have influenced the results of the study or their interpretation.
All data generated or analyzed during this study are included in this published article.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Mineta T, Hosokawa K, Shiraishi T, Shirahama R, Yamanguchi T, hosokawa R, et al. (2024). Effects of Metabolic Surgery on Obstructive Sleep Apnea Complicating Severe Obesity. J Sleep Disord Ther. 13:583.
Received: 07-Sep-2024, Manuscript No. JSDT-24-33910; Editor assigned: 09-Sep-2024, Pre QC No. JSDT-24-33910 (PQ); Reviewed: 23-Sep-2024, QC No. JSDT-24-33910; Revised: 30-Sep-2024, Manuscript No. JSDT-24-33910 (R); Published: 07-Oct-2024 , DOI: 10.35248/2167-0277.24.13.583
Copyright: © 2024 Mineta T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.