Fisheries and Aquaculture Journal
Open Access
ISSN: 2150-3508
ISSN: 2150-3508
Research Article - (2018) Volume 9, Issue 2
An experiment was conducted to determine the effects of Moringa oleifera leaves, Lannea barteri bark and antibiotic additives on haematological parameters of C. gariepinus fingerlings. Moringa and Lannea were subjected to three (3) treatments (whole, aqueous and ethanol). Four hundred and eighty (480) C. gariepinus fingerlings of 4.60 g ± 0.02 g mean weight were acclimated for two weeks and distributed randomly into 24 re-circulatory tanks of 50 litres volume capacity representing 8 experimental diets. The experimental diets comprised the same inclusion levels. A commercial reference diet (CRD) was used as control. Moringa whole (MWL), Moringa aqueous extract (MAE) and Moringa ethanol extract (MEE) represented diet 2, 3 and 4 respectively while Lannea whole (LWL), Lannea aqueous extract (LAE) and Lannea ethanol extract (LEE) representing diet 5, 6 and 7 while diet 8 was antibiotic (ANTB). After feeding trial, the haematological parameters revealed that, white blood cells (WBC) count was significantly higher (P<0.05) in the group of fish fed LWL (247.40 × 103 mm-3), LAE (235.50 × 103 mm-3) and LEE (234.15 × 103 mm-3) based diets and significantly lower (P<0.05) was obtained in group of Antibiotic based diet (1.65 × 103 mm-3). There significant difference (P<0.05) across the red blood cells (RBC) of the initial fishes and experimented fishes were recorded. The Packed Cell Volume (PCV) ranges between LWL (46.05%) and MWL (10.70%). Haemoglobin was significantly lower (P<0.05) in the Initial (4.87 g/dl) and CRD (5.13 g/dl) based diets. The platelets count were significantly different (P<0.05) in all the treatments. There were no significant differences (P>0.05) in all mean corpuscular haemoglobin and mean corpuscular haemoglobin concentration in all the treatments. The findings have positive impact on the haematological parameters of C. gariepinus fingerlings compared with antibiotic (ANTB).
Keywords: Natural; Additive; Catfish; Haematology
Feed additives are substances such that they are added in traces amount which provides a mechanism by which such dietary deficiencies can be addressed as it benefits both nutrition and growth of animal concerned. Most of some growth promoting feed additives includes hormones, antibiotics, ionospheres and probiotics [1,2]. The culture of African Catfish (C. gariepinus) is widely practiced in many tropical and subtropical regions of the world which constitutes one of the largest groups of freshwater fish farmed in Nigeria [3].
Nutritional requirements of an animal are a fundamental aspect that depends solely on species, habitat and live cycle stage [4]. The use of antibiotics and other chemotherapeutics for controlling diseases has been criticized for their negative impacts. The inclusion of herbal feed supplements often provides cooperative action to various physiological functions. The synergistic effect of herbs has been reported in many fishes, including Japanese flounder and C. gariepinus [5,6].
However, in most cases, the knowledge of haematological characteristics of the fish is important in toxicological studies and its implication on final consumers which is man. In culture fisheries these studies are usually associated with the feed input. The White blood cell count (WBC), Red blood cells (RBC) count, haematocrit (PCV) and Haemoglobin (Hb) concentration vary with diet and strain as well as temperature, season of the year and nutritional status of the fish [7,8]. Chemical and Biological analysis of the blood is of considerable value in confirming the diagnosis and response to treatment in variety of diseases. Cells naturally contain enzymes for their functions such that damages to cellular membrane lead to their escape into the blood where their presence or activities can be measured as an index of cell integrity [9].
Therefore, Fish haematology is gaining importance in fish culture because of its importance in monitoring the health status of fish [8,10]. Hrubec et al. [10] reported that, haematological characteristics of most fish have been studied with the aim of establishing normal value range and deviation from it may indicate a disturbance in the physiological process. The aim of this work is to determine the haematological parameters of C. gariepinus fingerlings fed differently processed M. oleifera (leaves), L. barteri (bark) and Antibiotic (oxytetracycline) additives.
Plants preparations
M. oleifera : M. oleifera leaves were harvested from horticultural garden, Department of Crop Production Federal University of Technology, Minna. The leaves were taken to Water Resources, Aquaculture and Fisheries Technology (WAFT) Departmental laboratory, air dried at room temperature for 5 days, the dry leaves were ground to powdery form following [11] procedures sieved with 1 mm mesh size, packed in polythene leather and preserved in a deep freezer at 4°C.
L. bateri : Bark of L. bateri was also harvested from Gupa-Miffi village via Lapai Local Government Area of Niger State, cut into smaller sizes, air dried at room temperature for 5 days, pounded into smaller particles and fed to hammer mill at animal departmental laboratory, FUT Minna ground into powdery form [12,13], sieved with 1mm mesh size, packed in polythene leather and preserved in a deep freezer at 4ºC.
Extract preparation procedures
Fifty (50) grams of the plants (M. oleifera leaf and L. bateri bark) were measured each and soaked into 500 ml of ethanol (C2H5OH) of 99/100% concentration following the [14] procedures for 72 hours in 1000 ml bottle. The bottles were tightly covered to prevent evaporation of the ethanol. The bottles were agitated thoroughly at intervals and the soaked materials were doubled filtered using muslin cloth. The concentrated liquids were fed to 1 litre flask and clipped to a rotary evaporator (RE300) rotating on a water bath filled with water to a capacity which was boiling steadily at 100°C and the ethanol solvents were separated from the extract. The extracts were poured into 100 ml bottle, exposed to air until ethanol solvent was finally removed. The bottles were labelled as ethanol extracts covered and stored at room temperature in the laboratory deep freezer at 4°C until used.
For the aqueous extract 50 gms of M. oleifera leaf and L. bateri bark were measured soaked with distilled water of 500 ml in 1000 ml bottles and procedure for extraction was as the same as that of ethanol extraction. The aqueous extracts were in the laboratory deep freezer at 4°C until used.
Diet formulation and level of inclusion
Eight diets were formulated for the experiment. Each of the experimental diet was formulated to contain 45% Crude protein (CP) as compare with the label of the package in the Commercial reference diet (CRD) used as control. The proximate analyses of the major ingredients were determined before formulating the diets (Table 1). Maize meal (14% CP) was used as energy source while fish meal (72.4% CP) was used as protein source. Other ingredients used were vitamin and mineral premix, fats and oil representing 3% inclusion levels each while Moringa, Lannea and Oxytetracycline representing 2% inclusion levels each (Table 1). The experimental diets were formulated using Pearson square method and compounded into 2 mm size pellet using hand pelletizer. The diets were prepared by individually weighing of each component while the ingredients were thoroughly mixed to ensure homogeneity. The feeds were oven dried at 60°C and chemical compositions of each feed was carried out as in Table 1. The feeds were packed in polythene, labelled and stored in the departmental laboratory deep freezer prior to the commencement of the experiment.
| Feed ingredients (g) | Diet 1 (CRD) | Diet 2 (MWL) | Diet 3 (MAE) | Diet 4 (MEE) | Diet 5 (LWL) | Diet 6 (LAE) | Diet 7 (LEE) | Diet 8 (ANTB) |
|---|---|---|---|---|---|---|---|---|
| FM | - | 49.4 | 49.4 | 49.4 | 49.4 | 49.4 | 49.4 | 49.4 |
| MM | - | 43.6 | 43.6 | 43.3 | 43.6 | 43.6 | 43.6 | 43.6 |
| VMP | - | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Fat | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Additive | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total | - | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Moisture | 4.12 | 2.48 | 2.84 | 2.22 | 2.55 | 2.82 | 2.38 | 2.41 |
| Crude protein | 46.6 | 44.86 | 44.89 | 44.1 | 44.5 | 44.5 | 44.75 | 45.43 |
| Ether extract | 6 | 8.8 | 9.7 | 9 | 8.4 | 9.9 | 9.2 | 9.3 |
| Crude fibre | 2.86 | 2.6 | 2.67 | 2.58 | 2.4 | 2.67 | 2.58 | 2.58 |
| Ash | 17.4 | 18.6 | 17.01 | 15.57 | 17.4 | 16.8 | 13.58 | 14.9 |
| NFE | 23.02 | 22.66 | 22.89 | 26.3 | 24.75 | 23.25 | 27.51 | 25.38 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Table 1: Diets formulation and chemical compositions of experimental diets fed C. gariepinus fingerlings for 56 Days of feeding trial.
Experimental procedures
Four hundred and eighty (480) C. gariepinus fingerlings with an average body mass of 4.60 g ± 0.02 g were acclimated for two weeks at water resources; aquaculture and fisheries (WAFT) departmental recycling tanks. During acclimation, they were fed commercial diet (Coppens) at 5% of their body weight [3] before being randomly distributed into 24 recycling plastic tanks of 50 litres capacity filled with 20 litres volume of water each. Each of the tanks contains 20 fish, 3 replicate representing 8 dietary treatments. Fish in the group of treatment 1 represent control diet (CRD) while the rest were fed the tested experimental diets. Water flow rate was maintained at 1.5 litres per minute [2] and water quality parameters were measured weekly. Temperature and dissolved oxygen was measured using hand held DO meter (AZ8403), PH was measured using pocket size PH meter (HI96107), total conductivity was measured using pen type digital conductivity meter (CT-3030) and total alkalinity was determined chemically in the laboratory. All the parameters measured were within international recommended ranges. Fish were fed 5% of their body weight per day in two equal meals between 10 am and 4 pm for a period of 56 days.
Blood sample collection
Fishes were randomly selected and their blood samples were collected and labelled as initial sample in triplicate for haematology analysis before the commencement of the research. At the end of the feeding trial, haematology analysis was also conducted on the fishes from each treatment in triplicate. The fishes used were thoroughly washed to avoid contamination before their blood samples were collected [14]. They were bled from caudal artery by severing the caudal fin and the blood were collected into anticoagulant bottle (EDTA bottle) labelled to represent each treatment. All the samples collected were taken to Haematology Laboratory, Federal Medical Centre, Bida, Niger State for Haematological Parameters. Sysmex (KS-21N) digital was used for the haematology determination. Parameters determined were; direct measurement of erythrocyte otherwise known Red Blood Cells (RBCs) values, White Blood Cells (WBCs), Packed Cell Volume (PCV) and Platelets. Haemoglobin (Hb), Mean Corpuscular Haemoglobin Concentration (MCHC), Mean Corpuscular Haemoglobin (MCH) and Mean Corpuscular volume (MCV) were calculated according to the formulae described by Dienye et al. [8,15-17].
Haemoglobin (Hb) values were determined using;
Mean Corpuscular Haemoglobin Concentration (MCHC) was determined using;
Mean Corpuscular Haemoglobin (MCH) was determined using;
Mean Corpuscular Volume (MCV) was determined using;
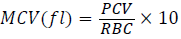
Statistical analysis
The haematological results were subjected to one-way analysis of variance (ANOVA) using P>0.05 significance level to test for significant difference. The parameters of mean comparison were applied according to Duncan Multiple Range Test [18]. All the statistics were computerised using stat graphics (version 3.0) and Minitab (version 9.2) packages.
The haematological evaluations of fingerlings of C. gariepinus fed supplemented diets of plant additives from M. oleifera leaves (Powder), L. barteri bark (Powder) and antibiotic based diets are shown in Table 2. The total white blood cells count result revealed in fish fed Lannea whole (247.40 × 103 mm-3), Lannea aqueous extract (235.50 × 103 mm-3), Lannea ethanol extract (234.15 × 103 mm-3), Moringa ethanol Extract (229.40 × 103 mm-3) and Moringa aqueous extract (227.40 × 103 mm-3) based diets were significantly higher than Initial (42.60 ± 3.54 × 103 mm-3) haematology value recorded while control reference diet (40.30 × 103 mm-3) have little significant variation from the initial value and Antibiotic (1.65 × 103 mm-3) based Diets revealed the lowest significant value among all the treatments in terms of white blood cells count. There was no significant difference in red blood cells amongst Fish of LWL (2.75 × 106 mm-3), LAE (2.55 × 106 mm-3), LEE (2.50 × 106 mm-3) and MEE (2.45 × 106 mm-3) based diet. There was significant difference (P<0.05) between the haematology values of Initial (1.30 × 106 mm-3)and the fish fed experimental based diets belonging to MAE (2.30 × 106 mm-3), ANTB (1.60 × 106 mm-3), MWL (0.30 × 106 mm-3) and CRD (1.20 × 106 mm-3) in which MWL (0.30 × 106 mm-3) based diet recorded the lowest performance in terms of RBCs counts.
The parked cell volume results evaluated revealed that, Lannea whole based diet was significantly higher (46.05%) while Moringa whole based diet (10.70%) was significantly lower than the rest of the results from other experimental based fishes. The haemoglobin results evaluated shows that, the Initial (4.87 g/dl), commercial reference diet (5.13 g/dl) antibiotic (7.08 g/dl) were the least performed group as compared to other groups of the experimental fishes. The mean corpuscular volume of the experimental fish showed that the fish from the group of Lannea whole (167.25 fl) based diet was significantly higher in terms of values recorded while that of Moringa whole (43.99 fl) was significantly lower. The mean corpuscular haemoglobin and mean corpuscular haemoglobin concentrations showed no significant different in all the parameters evaluated (Table 2). Platelets showed significance across all the experimental fishes while initial fish and that commercial reference diet were not significantly different.
| Parameters | Initial | CRD | MWL | MAE | MEE | LWL | LAE | LEE | ANTB |
|---|---|---|---|---|---|---|---|---|---|
| WBC (103 mm-3) | 42.60d ± 3.54 | 40.30e ± 0.00 | 5.75e ± 6.72 | 227.40c ± 7.07 | 229.40bc ± 0.71 | 247.40a ± 1.13 | 235.50b ± 0.14 | 234.15bc ± 0.21 | 1.65e ± 0.92 |
| RBC (106 mm-3) | 1.30cd ± 0.28 | 1.20d ± 0.14 | 0.30e ± 0.29 | 2.30b ± 0.00 | 2.45ab ± 0.07 | 2.75a ± 0.07 | 2.55ab ± 0.07 | 2.50ab ± 0.07 | 1.60c ± 0.28 |
| Hb (g/dl) | 4.87c ± 0.23 | 5.13c ± 0.52 | 3.50c ± 4.43 | 10.9b ± 0.71 | 12.82ab ± 0.69 | 15.34a ± 1.93 | 12.67ab ± 0.47 | 11.62ab ± 0.12 | 7.08c ± 0.45 |
| PCV (%) | 14.60d ± 0.71 | 15.40d ±1.56 | 10.70e ± 13.29 | 32.70b ± 2.12 | 38.50ab ± 2.12 | 46.05a ± 5.87 | 38.00ab ± 1.41 | 34.90ab ± 0.42 | 21.3c ± 1.14 |
| MCV (fl) | 113.65d ± 18.30 | 128.44cd ± 2.17 | 43.99e ± 5.36 | 142.19bc ± 9.21 | 157.10ab ± 4.10 | 167.25a ± 17.05 | 149.01abc ± 1.40 | 139.61bc ± 1.68 | 134.46bcd ± 14.96 |
| MCH (pg) | 38.04a ± 6.63 | 42.79a ± 0.69 | 83.54a ± 71.36 | 47.41a ± 3.10 | 52.29a ± 1.29 | 55.67a ± 5.56 | 49.66a ± 0.48 | 46.46a ± 0.48 | 44.73a ± 5.13 |
| MCHC (%) | 33.33a ± 0.00 | 33.31a ± 0.02 | 33.21a ± 0.18 | 33.33a ± 0.00 | 33.33a ± 0.07 | 33.34a ± 0.07 | 33.26a ± 0.08 | 33.26a ± 0.08 | 33.27a ± 0.10 |
| Platelets | 198.00a ± 6.86 | 200.90a ± 6.93 | 7.18f ± 0.25 | 45.15d ± 1.68 | 27.68e ± 0.95 | 47.15d ± 1.63 | 62.53c ± 2.15 | 118.90b ± 4.10 | 51.25d ± 1.77 |
Table 2: Haematology parameters of C. gariepinus fed experimental diets for a period of 56 days.
The basis of haematological parameters is to reveal the components which are valuable in monitoring toxicity constituents in fish diets as it affects the formation of blood in fish culture. The increase in white blood cell (WBC) count in Lannea whole (LWL), Lannea aqueous extract (LAE), Lannea ethanol extract (LEE) and Moringa aqueous extract (MAE) based diets may be a protective response of the fish to improve its immunity while low WBC count in antibiotic (ANTB) might be an indication of weakening of the immune system due to stress effect of antibiotic. This findings were in accordance with the report of Adedeji et al. [3,17,19]. The authors observed that, increase WBC count improves defensive cells of the body which are involved in protecting the body against stress which lead to infectious disease and foreign invaders. According to Akinwande et al. [20] a measurable increase in WBC count of fish or any animal is a function of immunity or resistance to disease. Douglas et al. [21] also revealed that, low amount of WBC count has implication in immune responses and the ability of the species to fight against infection. The decrease values of parked cell volume (PCV) ranges obtained from the fishes of initial, CRD and MWL were contrary to the range of 20% to 50% as reported by Dienye et al. [8,22]. The decrease in PCV could be as a result of the presence of anti-nutritional factor such as phytic acid, saponin and tannins in raw Moringa leaves incorporated into the diet fed. Similar trend was also reported by Dienye et al. [8,23] that decreasing in PCV blood concentration in C. gariepinus fed raw M. oleifera leaf meal is attributed to the presence of anti-metabolites such as tannin and phenol in Moringa leaf incorporated into the diet. An increase in PCV observed in the fish fed plant additive diets might be an indication of high immunity on the fish [23].
Therefore, the fish species with higher values of leucocyte will be able to fight infection. However, lower values recorded in ANTB based diet is in agreement with the findings of Hentschel et al. [24-26] who reported that long term use of antibiotic on fish can induce nephrotoxicity, liver damage and residual deposit in fish tissues and fish products from prolong application. The low red blood cell count in MWL based fed diet may be attributed to effect of raw incorporation of Moringa into the diet while the high values recorded for extracted based diets were in agreement with that of Fagbenro et al. [17] who fed raw sunflower and sesame seed to C. gariepinus. Furthermore, Dienye et al. [8] also reported that, erythrocyte (red blood cells (RBC) count greater than 1.00 × 106 mm-3 is considered high and is an indication of high oxygen carrying capacity of the blood which is the characteristics of fish capable of aerial respiration and high metabolic activity [27-29]. The high values obtained for haemoglobin (Hb) for fishes fed plants additives compared with other diets were in agreement with the findings of Adedeji et al. [3,8,17] which is a measure of anaemic condition of the fishes. The mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) ranges, mean corpuscular haemoglobin concentrations (MCHC) were in agreement with the findings of Dienye et al. [8] who fed dietary Moringa leaf meal at varying inclusion levels to C. gariepinus species and Fagbenro et al. [17] report of feeding C. gariepinus species with sunflower and sesame meal-based diet.
It can be concluded that haematological parameters of C. gariepinus was significantly affected by plant additive especially the extracted form of Moringa and Lannea plants.
It can be recommended that ethanol extract form of Moringa and Lannea can be incorporated in the diets of C. gariepinus instead of antibiotic.