Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Mini Review - (2021)
Oxidative stress is a phenomenon caused by an imbalance between production and accumulation of oxygen reactive species (ROS) in cells and tissues and the ability of a biological system to detoxify these reactive products. ROS can play, and in fact they do it, several physiological roles (i.e., cell signaling), and they are normally generated as byproducts of oxygen metabolism; despite this, environmental stressors (i.e., UV, ionizing radiations, pollutants, and heavy metals) and xenobiotics (i.e., antiblastic drugs) contribute to greatly increase ROS production, therefore causing the imbalance that leads to cell and tissue damage (oxidative stress).
Nitric oxide; Skin; Cytokeratins; Chemokines; Oxidative stress
Nitric oxide (NO) is a gas with a short half-life (about a few seconds) and has been reported for its diverse biochemical and physiological potentials. This molecule was first discovered in 1978 and nominated as the molecule of the year in 1992 [1,2]. NO is an inner cell and intra cell messenger that plays a key role in the maintenance of body hemostasis [2]. In general, NO accomplishes its function by synthesizing cyclic guanosine monophosphate (cGMP). NO is produced from L-arginine amino acid that synthesis is mediated by nitric oxide synthases (NOS). This enzyme occurs in three major isoforms, nervous, endothelial, and induction [1]. NO cascades result differently in various tissues, e.g., emerging as a vasodilator factor known as endothelium-derived relaxing factor (EDRF) in the cardiovascular system [2]. However, in the nervous system, it is considered as a neurotransmitter. In addition, in some cases, it causes neutrophil-induced cell toxicity, platelet aggregation, blood flow, synaptic transmission, and long-term memory loss [3,4]. Furthermore, NO is synthesized by a group of enzymes known as NOS. Three major isoforms of NOS can be detected, including nervous NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS). Recently, mitochondrial NOS has been discovered, which specifically can be found in the mitochondrion [5]. Also, Immunostaining assessment showed that nNOS is primarily present in the nucleus and cytoplasm of oocytes. However, in the reproductive parts such as the granulosa, theca cells, and the cytoplasm of oocytes, iNOS and eNOS are localized. Most of the apoptotic cascades are developed by producing reactive nitrogen species (RNS) and reactive oxygen species (ROS). Hence, receptors, internal cell proteins such as growth factors, immune system, metabolic pathways, and external chemicals are capable of generating nitrogen and oxygen free radicals.
NO is produced from three sources, which include L-arginine, Snitrosothiol mediator, and nitrite-nitrate mediator [5].
L-arginine
L-arginine codes for three genes related to three major isoforms of NOS (nNOS, eNOS, and iNOS) and catalyzes NO synthesis in association with some cofactors such as tetrahydrobiopterin [also known as sapropterin (THB, BH4)]; riboflavin and adenosine diphosphate, a condensed product of flavin adenosine dinucleotide (FAD); calmodulin (CaM); and protoporphyrin IX [5]. eNOS plays the most important role in controlling cardiac function, and its activity is determined by several stimuli such as acetylcholine, bradykinin, histamine, and 17β-estradiol. Acetylcholine, bradykinin, and histamine act upon specific receptors on the cell membrane of endothelial cells and increase internal Ca2+ concentration, which is attached to calmodulin. This process activates the calmodulin-attached domain of eNOS [2,6,7] (Figure 1).
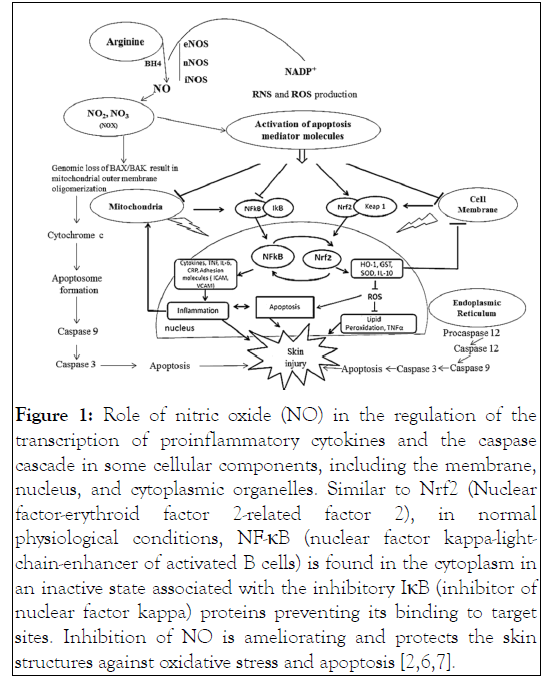
Figure 1: Role of nitric oxide (NO) in the regulation of the transcription of proinflammatory cytokines and the caspase cascade in some cellular components, including the membrane, nucleus, and cytoplasmic organelles. Similar to Nrf2 (Nuclear factor-erythroid factor 2-related factor 2), in normal physiological conditions, NF-κB (nuclear factor kappa-lightchain- enhancer of activated B cells) is found in the cytoplasm in an inactive state associated with the inhibitory IκB (inhibitor of nuclear factor kappa) proteins preventing its binding to target sites. Inhibition of NO is ameliorating and protects the skin structures against oxidative stress and apoptosis [2,6,7].
S-nitrosothiol
This factor not only acts as an NO expression regulator agent, but it is also considered as an NO resource. Moreover, there is an S-nitrosoalbumin reservoir circulating in the plasma, and its level directly corresponds with NOS activity. Therefore, reduction of NOS levels leads to reduction of S-nitrosoalbumin production [8].
Nitrite-nitrate mediator
Nitrite and nitrate are metabolites of NO metabolism, which are also considered as NO resources [9,10]. In some circumstances, various enzymes are able to reduce nitrite and nitrate from NO [2]. Hemoglobin is a Fe-containing protein in the red blood cells, playing a significant role in oxygen transportation. This oxygen-sensitive protein administers NO production from nitrite in hypoxic situations. Whenever hemoglobin is occupied by oxygen (40%–60%) at pH 6.4, maximum NO production occurs from nitrite sources. Myoglobin is also a Fe-containing protein in the muscle cells that binds with oxygen [11]. Oxygen sensors (hemoglobin and myoglobin) produce NO in the hypoxic situation instead of reducing NO. It leads to NO dispersion, vasodilation, and increased blood flow to hypoxic tissues. In the anoxic tissue, NO is synthesized from nitrite or nitrate by xanthine oxidoreductase, which acts as a protective shield against ischemia. Equivalently, cytochrome c oxidase implements NO production from nitrite in the mitochondrion, and the synthesis flux increases as pH declines [2].
The proliferative compartment of the epidermis is located in the innermost basal layer, where stem cells and transiently amplifying cells attach to an underlying basement membrane of the extracellular matrix [12]. In a homeostatic and self-renewing tissue, transiently amplifying basal cells periodically withdraw from the cell cycle, lose their contact with the basement membrane, and activate a program of terminal differentiation [12,13]. As the cells move upward toward the surface, they progress through the following three distinct stages: spinous, granular, and stratum corneum. After a short lag period of several hours after injury, the opening phase of reepithelialization is marked by the first keratinocytes of the cut edges that start to migrate. Keratinocytes account for about 90%–95% of the cells in the epidermis [14]. Keratinocytes are guided into the wound by fibronectin and fibrin bundles in the provisional clot, which are sensed by the cells by the expression of highly controlled integrin receptor subsets [15]. Migrating keratinocytes do not differentiate, and the leading edge keratinocytes express the constituents of very active fibrinolytic machinery such as plasminogen activators and matrix metalloproteinases (MMPs) [16]. Keratinocytes at the wound margins start to proliferate 1 or 2 days after injury. However, unexpected mediators such as the cytokine leptin [17] and the small gaseous molecule NO [18], as reviewed below, crucially contribute to cutaneous reepithelialization as well. The major cell types driving the generation of new stroma are the macrophages, fibroblasts, and endothelial cells [15,17].
Expression of both constitutive and inducible isoforms has been shown in several cells resident in the skin, including keratinocytes and fibroblasts [19]. In the normal human epidermis as well as during wound healing processes, cytokeratins 6 (K6) and K16 are expressed transiently. These cytokeratins are constitutively expressed at low levels. On the other hand, an increasing number of investigations have shown local cytotoxic effects of NO when generated at higher concentrations via the activation of iNOS. Moreover, it has been convincingly demonstrated local organ destruction in autoimmune diseases due to NO [20].
Oxidative stress, NO, and cellular signalling
In acute wounds, a temporary increase in the level of oxidants has been observed. Antioxidant defence mechanisms are based on gradual detoxification of oxidants and on a gradual return of cells to the state of redox homeostasis. However, in chronic wounds, the detoxification process is hindered due to persistent and uncontrolled production of ROS and RNS during the inflammatory phase. Antioxidative response, though elevated in chronic wounds, is not able to quench the oxidants because of a reduction in the antioxidative activity caused by excess oxidants [21]. The cellular response to increase intracellular NO concentrations appears to depend to a significant extent on the redox potential of the cell, which is itself influenced by the resting levels of NO [22]. Nitroxidative stress also increases matrix degradation and apoptosis in ulcers. Arginase is responsible for increased matrix deposition. However, due to a high nitroxidative stress and subsequent proteolytic activity, defective matrix deposition in ulcers occurs. High levels of NO produced by iNOS interact with oxygen free radicals derived from polymorphonuclear (PMN) cells and macrophages to produce peroxynitrite (Figure 1). Peroxynitrite induces apoptosis or necrosis depending on its concentration in the ulcer site [23]. Oxidative stress has been linked to causing stress-induced premature senescence in human diploid fibroblasts. This suggests that high oxidative stress in chronic wounds might be a causative factor for inducing senescence in wound fibroblasts [24]. Senescent fibroblasts are unable to replicate but remain metabolically active with altered cell functions. They are less motile, accumulate in tissue due to their resistance to apoptosis, and produce a different array of proteins, including elevated levels of MMPs and proinflammatory cytokines. The proinflammatory cytokines affect tissue integrity and normal healing. Distinct cytokines plays prominent rolle in various degradative pathways. Transforming growth factor beta (TGF-β) and interleukin 4 (IL-4) increase arginase and inhibit iNOS activity, whereas interferon gamma (IFN-γ) and IL-1 work inversely [24,25]. High oxidative stress can inhibit the migration and proliferation of keratinocytes, especially hydrogen peroxide when given in micromolar concentrations, which has been shown to inhibit these processes [26]. One of the key functions of NO in wound healing appears to be its permissive influence on keratinocyte and fibroblast proliferation, which helps promote wound reepithelialization [27].
The likely importance of NO-modulated cytokine signaling in the wound healing process has been noted by others [28] and of particular interest appears to be the NO-induced activation of TGF-β1 and the enhancement of IL-1 and IL-8 production. Furthermore, NO is known to stimulate epithelial cells to produce and release chemokines [29] and other growth mediators such as vascular endothelial growth factor (VEGF). Although the in-vitro signals of iNOS induction have been well investigated, little is known about the in-vivo signals during wound healing. Of the numerous cytokines and growth factors secreted and released into the wound environment, interleukin-1 and TNF-α are the most likely inducers of iNOS. Wound fluid, as a biological reflection of the wound environment, induces NO synthesis in a variety of cells [30]. A definitive role of NO in wound healing has still not been established. One study even showed NO inhibits collagen synthesis in wounds [31]. On the other hand, several studies have implicated that NO might play a vital role in all the phases of wound healing [28]. All three NOS isoforms are present in the skin and expressed during wound healing. nNOS is expressed in keratinocytes and melanocytes, whereas eNOS is present in keratinocytes of basal epidermal layer, dermal fibroblasts, endothelial cells, and eccrine glands. iNOS is induced in keratinocytes, fibroblasts, Langerhans cells, and endothelial cells [32]. NO has a vital role in the inflammatory process as it acts as a vasodilator and antimicrobial, prevents platelet aggregation, and induces vascular permeability. NO is responsible for both the upregulation and downregulation of the inflammatory phase of wound healing. NO acts as a proinflammatory molecule by acting as a chemo attractant for various cytokines such as IL-1 and TGF-β1, monocytes, and neutrophils. High levels of NO may also be anti-inflammatory during the later phase of inflammation [28,33].
NO is a lipophilic molecule with a short half-life and is synthesized by several organs in the body. Moreover, it is introduced as an inner cell and intra cell messenger directing most of the physiological cascades. Among other functions, NO is associated with cell growth, apoptosis, and reproduction signal transduction, as well as in the regulation of blood flow, regulation of vascular tonicity, epidermal formation, and defense mechanisms. The process of cutaneous wound repair is characterized by inflammation, reepithelialization, granulation tissue formation, and the late remodeling phase of repair. Wound healing is characterized by an organized secretion of growth factors. This represents a potential target for regulating wound healing. Little is known whether NO can directly affect growth factors or cytokine secretion, activation, or time of action. Arginine is known to downregulate TNF-α after trauma, thereby affecting the outcome. Although iNOS expression is high during the early phases of wound healing, little is known about the downregulation of iNOS activity at the wound site during the later phases of healing. Presumably, iNOS activity can be downregulated by the resolution of the inflammatory response or by cytokine signaling.
The authors declare no conflict of interest.
Citation: Nilforoushzadeh MA, Razzaghi M, Rustamzadeh A, Alimohammadi A, Zare S, Farshi S, et al (2021) Effects of Nitric Oxide Activity on the Induction of Oxidative Stress in Skin Cell Signalling, Migration and Apoptosis. J Clin Exp Dermatol Res. S9:572.
Received: 28-Jun-2021 Accepted: 12-Jul-2021 Published: 19-Jul-2021 , DOI: 10.35248/2155-9554.21.s9.572
Copyright: © 2021 Nilforoushzadeh MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.