Autism-Open Access
Open Access
ISSN: 2165-7890
ISSN: 2165-7890
Research Article - (2024)Volume 14, Issue 4
This study investigated effects of repetitive Transcranial Magnetic Stimulation (rTMS) therapy on sleep structure and quality in children with Autism Spectrum Disorder (ASD). Sixty children with ASD were selected from Lishui Second People's Hospital from January 2020 to December 2021 and randomly divided into rTMS treatment and pseudo-treatment groups. The rTMS group received bilateral low-frequency (0.5 Hz) stimulation; the pseudo-treatment group received pseudo-stimulation at the same time and location. The Sleep Disturbance Scale for Children (SDSC) and Autism Behavior Checklist (ABC) were used to evaluate the changes before treatment and after 15 and 30 sessions over six weeks. The results showed no significant difference in demographic characteristics or SDSC factors and total scores between the two groups before treatment (p>0.05). After 15 and 30 sessions, the total SDSC and related factor scores in the rTMS group were significantly lower than those in the pseudo-treatment group (p<0.05). Difference t-tests showed that after 30 treatments, the score reduction rate in all dimensions in the rTMS group was significantly higher than that in the pseudo-treatment group (p<0.05). No significant differences were found in ABC scores. rTMS can improve sleep structure and quality in children with significant ASD, particularly after 30 sessions.
Autism spectrum disorder; Transcranial magnetic stimulation therapy; Sleep quality; Dorsolateral prefrontal lobe
Autism Spectrum Disorder (ASD) is a serious neurodevelopmental disorder with various clinical manifestations including social difficulties, deficits in verbal and nonverbal communication and repetitive stereotypical behaviors [1,2]. According to an authoritative report on the Development of Autism Education and Rehabilitation Industry (DAERI) in China in 2015, the total number of patients with ASD in China is estimated to have exceeded 10 million, of which the number of patients in the 0-14 years old age group is particularly significant and may have exceeded 2 million. This highlights the high incidence and severity of the disease in children and adolescents [3]. According to the latest data from the 2016, National Survey of Children's Health in the United States, the incidence of ASD in children aged 3-17 years is as high as 2.79%, whereas the incidence in the corresponding age group in China is approximately 0.265%, further confirming the status of ASD as a global public health challenge [4,5].
In addition to the core symptoms (such as social difficulties and repetitive behaviors), children with ASD often have a complex set of symptoms, particularly sleep problems. These sleep problems include prolonged sleep latency, reduced slow-wave sleep, frequent night awakenings, decreased sleep efficiency and reduced total sleep time, which constitute a vicious circle [6,7]. Lack of sleep not only exacerbates the core symptoms of ASD, but also induces a series of maladaptive behaviors, such as self-injury, aggression, overactivity, non-compliance with instructions, irritability and emotional disorders, which seriously hinder the overall development and quality of life of children [8-15].
Current management strategies for sleep problems in patients with ASD focus on three major areas: medication, behavioral intervention and alternative therapy. Although these approaches have improved sleep outcomes in children with ASD to some extent, pharmacological therapies are often associated with potential side effects and are not universally applicable due to individual differences. Behavioral interventions are limited by multiple factors such as the age of the child, the specific type of sleep problem and the family's sleep habits. Especially in young children, the selection and implementation of an optimal intervention program faces many challenges [16]. Therefore, it is particularly important to explore novel, safe, efficient and welltolerated treatment approaches.
Repetitive Transcranial Magnetic Stimulation (rTMS), as an advanced neuromagnetic stimulation technology, penetrates the skull through a pulsed magnetic field, acts directly on the brain nerve, regulates the membrane potential of the cortical nerve and regulates nerve excitability and inhibition [17]. Studies have shown that rTMS interventions targeting the dorsolateral prefrontal cortex can affect nerve cell excitability in this region, thereby improving anxiety, depression, sleep disorders and ASD-related symptoms [18-22]. Studies have shown that low-frequency rTMS applied to the right dorsolateral prefrontal cortex can effectively reduce cortical excitability and achieve better clinical therapeutic effects [23]. Studies on brain functional connectivity in children with autism have found that functional connectivity in the right dorsolateral region of the superior frontal gyrus and the left middle frontal gyrus is enhanced in children with autism, which is similar to the mechanism of action of rTMS in improving sleep problems [24]. Ni et al., found that comprehensive rehabilitation training combined with repetitive TMS improved autism symptoms and reduced the severity of sleep problems in children with autism [25].
Considering the lack of clinical studies on the use of rTMS alone in the treatment of ASD accompanied by sleep problems, this study aimed to further explore the specific effects of low-frequency rTMS on the sleep structure and quality of children aged 8-15 years with ASD accompanied by sleep problems. This study is expected to provide a new perspective and means for the treatment of sleep problems in children with ASD and to lay a solid scientific foundation for further optimizing comprehensive rehabilitation programs aiming to improve the quality of life of children with ASD.
Participants
This study was designed as a parallel-controlled intervention trial with a pretrial phase to evaluate the initial effects. Results showed that children in the rTMS treatment group had significantly lower scores on the sleep disorder scale, with a mean reduction of 8.9 ± 11.8 points compared with 6.8 ± 4.9 points in the sham treatment group. To ensure the accuracy and reliability of the study, a confidence level of K=0.9, a unilateral significance level of α=0.05 and a Class II error probability of β=0.10 were set. Based on these parameters, the formula of sample size was adopted.
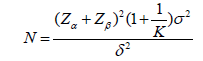
Combined with the variance combination formula, it was concluded that the rTMS treatment group needed at least 17 participants and the pseudo-treatment group needed more than 29 participants, to meet the statistical power requirements. The formula is:
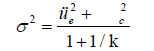
Sample recruitment was completed through the Children and Adolescents Outpatient Department of Lishui No. 2 People's Hospital and online platform advertising. From January 2020 to December 2021, a total of 60 children with ASD aged between 8 and 15 with significant sleep problems were included. The enrollment criteria were strictly set, including age range, righthandedness, no articulatory, hearing or visual impairment, no history of serious physical or neurological disorders, meeting Diagnostic and Statistical Manual of Mental Disorders 5 (DSM-5) criteria for autism, not taking central nervous system medications for at least one week prior to assessment, not receiving systematic rehabilitation training (e.g., sensory integration training, speech therapy) within two years prior to enrollment and exceeding the Sleep Disturbance Scale for Children (SDSC) total score 39 points. Meanwhile, the exclusion criteria excluded patients who were unable to cooperate with the test, had a history of seizures, a history of brain damage or disease and had metal implants in their bodies, among others.
The study protocol was approved by the Ethics Management Committee of Lishui No. 2 People's Hospital (approval number is 2019121803). All patients participating in the study or their legal guardians signed informed consent forms after being fully informed, ensuring ethical compliance of the study and protection of patients’ rights and interests.
Evaluation scale
The SDSC, a standardized assessment tool, has been widely used in the professional assessment of sleep quality in children aged 6-15 years [26]. The scale is well designed and contains 26 items, which are answered by the child or his/her guardian based on their current situation and fully covers difficulty falling asleep and sleep maintenance disorders, sleep disordered breathing, wake disorders, sleep-wake transition disorders, excessive sleepiness and sleep hyperhidrosis (specifically night sweats, six core dimensions. The higher the SDSC score, the more serious the sleep problems. Based on Bruni et al., a total score of 39 was set as the critical threshold, which not only matched the upper quartile of the normal control group but also ensured the high specificity (0.74) and sensitivity (0.89) of the scale; that is, a total SDSC score above 39 was clearly identified as indicating significant sleep problem [26].
Since the Autism Behavior Checklist (ABC) was proposed by Krug et al., in the United States in 1978, it has become an important auxiliary tool for diagnosing ASD [27]. The scale contains 57 items carefully designed around the five key dimensions of sensation, social interaction, physical movement, language ability and selfcare. Each item is assigned 1-4 points according to the severity of symptoms and is completed by the parents or main caregivers of children with ASD. The assessment process of the ABC scale is not only safe, fast, economical and convenient but also shows high specificity and sensitivity, which is of great significance for early screening and diagnosis of ASD. Specifically, a total score of less than 31 can effectively rule out the possibility of autism, while a total score of 53-66 indicates suspected autism and a total score of 67 or more confirms autism. In addition, the reliability of each component scale ranges from 0.57 to 0.81 and the internal consistency reliability of the total scale was as high as 0.86, which guarantees the stability and reliability of the assessment results [28].
Quality control measures
Before the start of the experiment, the researchers were trained in the systematic questionnaire filling technique and operational specifications of rTMS. The aim was to ensure the consistency and standardization of procedures throughout the treatment process, thereby effectively reducing measurement bias due to differences in procedures and improving the accuracy and reliability of the study data. During the stimulation process, each subject wore a special electrode cap to precisely locate the stimulation area. The researchers adopted a dual data entry mechanism to ensure the integrity and accuracy of data collection.
Statistical analysis
In this study, Statistical Package for Social Sciences (SPSS) 25.0 was used for data analysis. For continuous variables, we described them in the form of Mean ± Standard Deviation (SD). To compare the data between the two groups, we used the independent sample t-test; the difference t-test was used to analyze the changes before and after intervention within the same group. The significance level of all statistical analyses was set at p<0.05.
Procedure
rTMS intervention scheme design: Children with autism were randomly divided into two groups such as the pseudo-treatment group and rTMS treatment group, with 30 children in each group. In the grouping process, it was ensured that there were no statistically significant differences in sex, age, course of disease, level of education and other general characteristics among the groups to ensure objectivity and comparability of the study results. To strictly control the experimental conditions and avoid interference from the placebo effect, the children in the two groups received the following interventions.
(a) rTMS pseudo-treatment group: Received pseudo-stimulation intervention, according to the treatment frequency recommended by domestic and foreign literature (about 5% MT, frequency of 0.5 Hz, 300 pulses each time), once a day for 6 weeks, 5 times a week.
(b) rTMS treatment group: Received true stimulation intervention, the treatment frequency was set to 100% MT, 0.5 Hz and the same 300 pulses were given each time and the treatment frequency was the same as that of the pseudo-treatment group.
Therapeutic effect evaluation system: To comprehensively evaluate the effect of the intervention, key indicators, including the SDSC and ABC, were evaluated after 15 and 30 treatments, respectively. Through regular and systematic evaluation, we aimed to scientifically and objectively assess the improvement in sleep status and behavioral characteristics of children with autism after rTMS treatment.
Demographic characteristics of participants
Through rigorous screening, 60 children were recruited and randomly assigned to either a treatment (n=30) or a sham treatment group (n=30). The mean age of the children in the treatment group was 11.1 ± 2.3 years old, including 19 males (63.3%) and 11 females (36.7%). The sex distribution of the two groups was balanced. The mean age of children in the pseudo-treatment group was 11.3 ± 2.4 years, including 12 males (40%) and 18 females (60%). Data were collected using questionnaires. Sixty valid questionnaires were distributed and recovered, with a recovery rate of 100%. Statistical analysis showed that the differences in demographic variables such as gender distribution (χ²=3.27, p=0.071) and age (t=0.22, p=0.826) between the two groups were not significant, which ensured the effectiveness and comparability of subsequent analysis (Table 1).
| Demographics | Treatment group (n=30) | Pseudo-treatment group (n=30) | F/squared | p-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 19(63.3%) | 12(40%) | 3.27 | 0.071 |
| Female | 11(36.7%) | 18(60%) | ||
| Age | 11.1 ± 2.3 | 11.3 ± 2.4 | 0.22 | 0.0826 |
Table 1: Comparison of demographic variables between repetitive Transcranial Magnetic Stimulation (rTMS) and sham treatment groups.
Baseline comparison of sleep and behavior problems between the pseudo-treatment and rTMS treatment groups before treatment
All participating children successfully completed the pretreatment assessment and none dropped out because of adverse reactions. An independent samples t-test was used to analyze the baseline assessment of sleep problems (SDSC scale) and behavioral problems (ABC scale) before treatment. The results showed no significant differences between the two groups in the total SDSC scores, the scores of each sub-item (difficulty falling asleep and sleep maintenance disorder, sleep disordered breathing, awakening disorder, sleep-wake transition disorder, excessive sleepiness and nighttime hyperhidrosis) and the ABC scale scores (p>0.05), which verified the comparability of baseline data and provided a solid foundation for the evaluation of follow-up intervention effects (Table 2).
| Dimensions | Pseudo-treatment group (n=30) |
rTMS treatment group (n=30) | t-value | p-value |
|---|---|---|---|---|
| Difficulty falling asleep and sleep maintenance disorders | 26.33 ± 3.25 | 26.20 ± 3.93 | 0.14 | 0.887 |
| Sleep-disordered breathing | 3.37 ± 0.62 | 3.33 ± 0.66 | 0.20 | 0.840 |
| Arousal disorder | 3.93 ± 1.02 | 3.87 ± 0.86 | 0.27 | 0.785 |
| Sleep-wake transition disorder | 13.80 ± 2.83 | 13.93 ± 2.92 | 0.18 | 0.858 |
| Excessive sleepiness | 11.27 ± 2.33 | 11.03 ± 2.40 | 0.38 | 0.704 |
| Excessive night sweating | 4.20 ± 1.27 | 4.33 ± 1.09 | 0.44 | 0.665 |
| Total SDSC score | 62.90 ± 8.79 | 62.70 ± 9.21 | 0.09 | 0.887 |
| ABC rating | 81.03 ± 8.39 | 78.53 ± 7.23 | 1.24 | 0.932 |
Note: SDSC: Sleep Disturbance Scale for Children; ABC: Autism Behavior Checklist; rTMS: repetitive Transcranial Magnetic Stimulation.
Table 2: Comparison of Sleep Disturbance Scale for Children (SDSC) and Autism Behavior Checklist (ABC) scores between pseudo-treatment and repetitive Transcranial Magnetic Stimulation (rTMS) treatment groups before treatment.
Improvement of sleep and behavior problems in the pseudo-treatment and rTMS treatment groups after treatment
The SDSC and ABC scores of children in both groups were assessed again after completing 15 and 30 rTMS treatments. After 15 sessions of rTMS treatment, the total SDSC score and sub-items (sleep difficulty and sleep maintenance disorder, sleep-wake transition disorder, excessive sleepiness and other dimensions) in the RTMS treatment group were significantly lower than those in the pseudo-treatment group (p<0.05). This trend was more significant after 30 treatments, further confirming the effectiveness of rTMS therapy for improving sleep problems in children. However, no significant difference was observed in behavioral problems (ABC scale score) between the two groups (p>0.05), suggesting that the direct effect of rTMS treatment on behavioral problems may be limited (Table 3).
| Dimensions | Number of interventions | Pseudo-treatment group (n=30) | rTMS treatment group (n=30) | t-value | p-value |
|---|---|---|---|---|---|
| Difficulty falling asleep and sleep maintenance disorders | 15 treatments | 25.37 ± 3.62 | 21.53 ± 4.00 | 3.89 | <0.00 |
| 30 treatments | 24.63 ± 3.07 | 18.23 ± 3.29 | 7.80 | <0.001 | |
| Sleep disordered breathing | 15 treatments | 3.23 ± 0.50 | 3.27 ± 0.52 | 0.25 | 0.802 |
| 30 treatments | 3.30 ± 0.47 | 3.27 ± 0.52 | 0.26 | 0.795 | |
| Arousal disorder | 15 treatments | 3.77 ± 0.68 | 3.63 ± 0.85 | 0.67 | 0.505 |
| 30 treatments | 3.63 ± 0.62 | 3.50 ± 0.63 | 0.83 | 0.410 | |
| Sleep-wake transition disorder | 15 treatments | 13.20 ± 2.68 | 11.83 ± 2.21 | 2.15 | 0.036 |
| 30 treatments | 13.03 ± 2.33 | 11.37 ± 2.24 | 2.83 | 0.006 | |
| Excessive sleepiness | 15 treatments | 10.93 ± 2.08 | 9.77 ± 1.83 | 2.30 | 0.025 |
| 30 treatments | 10.87 ± 2.30 | 9.20 ± 1.69 | 3.20 | 0.002 | |
| Sweating at night | 15 treatments | 4.00 ± 1.15 | 3.93 ± 0.83 | 0.26 | 0.797 |
| 30 treatments | 3.90 ± 0.99 | 3.83 ± 0.70 | 0.30 | 0.765 | |
| Total SDSC score | 15 treatments | 60.50 ± 7.88 | 53.97 ± 7.38 | 3.31 | <0.00 |
| 30 treatments | 59.37 ± 6.74 | 49.40 ± 5.72 | 6.17 | <0.00 | |
| ABC score | 15 treatments | 79.73 ± 8.50 | 77.10 ± 7.20 | 1.295 | 0.201 |
| 30 treatments | 79.93 ± 8.44 | 77.03 ± 7.21 | 1.43 | 0.158 |
Note: SDSC: Sleep Disturbance Scale for Children; ABC: Autism Behavior Checklist; rTMS: repetitive Transcranial Magnetic Stimulation.
Table 3: Comparison of Sleep Disturbance Scale for Children (SDSC) and Autism Behavior Checklist (ABC) scores between pseudo-treatment and repetitive Transcranial Magnetic Stimulation (rTMS) treatment groups after treatment.
Comparison of improvement in sleep and behavior problems between the pseudo-treatment and rTMS treatment groups after 30 sessions of treatment
To reflect the treatment effect more directly, a difference t-test was used to analyze the improvement amplitude of the two groups of children in various dimensions after 30 treatment sessions. The results showed that the improvement range of SDSC total score and all dimensions (except sleep-disordered breathing and night-time hyperhidrosis) in the rTMS treatment group was significantly higher than that in the pseudo-treatment group (p<0.05), indicating that rTMS treatment had a significant effect on improving children’s sleep problems. At the same time, although the difference of ABC scale reduction between groups was not significant (p>0.05), the rTMS treatment group still showed a certain downward trend, and future studies can further explore its potential impact (Table 4).
| Dimensions | Pseudo-treatment group (n=30) |
rTMS treatment group (n=30) | t-value | p-value |
|---|---|---|---|---|
| Difficulty falling asleep and sleep maintenance disorders | 1.70 ± 2.20 | 7.97 ± 2.75 | 15.88 | <0.001 |
| Sleep-disordered breathing | 0.07 ± 0.79 | 0.07 ± 0.37 | 1.00 | 0.321 |
| Arousal disorder | 0.30 ± 0.88 | 0.37 ± 0.72 | 2.80 | 0.007 |
| Sleep-wake transition disorder | 0.77 ± 1.07 | 2.57 ± 1.89 | 7.45 | <0.001 |
| Excessive sleepiness | 0.40 ± 0.97 | 1.83 ± 1.34 | 7.49 | <0.001 |
| Excessive night sweating | 0.30 ± 0.88 | 0.50 ± 0.94 | 2.92 | 0.005 |
| ABC rating | 2.70 ± 1.54 | 8.70 ± 2.49 | 11.22 | <0.001 |
| Total SDSC score | 3.53 ± 3.28 | 13.30 ± 5.01 | 14.54 | <0.001 |
| ABC rating | 1.10 ± 0.76 | 1.5 ± 1.10 | 1.16 | 0.109 |
Note: SDSC: Sleep Disturbance Scale for Children; ABC: Autism Behavior Checklist; rTMS: repetitive Transcranial Magnetic Stimulation.
Table 4: Comparison of Sleep Disturbance Scale for Children (SDSC) and Autism Behavior Checklist (ABC) subtractive values between pseudo-treatment and rTMS treatment groups after 30 sessions.
During the critical period of children's development, adequate and high-quality sleep plays an irreplaceable role in the maturation of the brain structure, improvement of cognitive function, memory consolidation and improvement of learning abilities [29]. Studies have shown that children with ASD are at a higher risk of developing sleep problems than the general population. Approximately 44%- 86% of children and adolescents with ASD have significant sleep disorders, which is significantly higher than 25%-40% of normal children and adolescents, highlighting the severe challenge of sleep health in children with ASD [30-35]. This was further confirmed by the research of Li et al., in China [28]. A comparative survey of children with ASD and healthy children found that children with ASD had more serious problems in terms of difficulty falling asleep, sleep maintenance, total sleep duration, sleep quality and night-wake frequency.
As an innovative neuromodulation technology, rTMS acts directly on the cerebral cortex through pulsed magnetic fields to regulate neuronal excitability, thus showing potential for treating various neurological diseases. This study focused on the effects of rTMS on sleep problems in children with ASD. We systematically evaluated the effect of low-frequency (0.5 Hz) rTMS on bilateral dorsolateral prefrontal cortex stimulation for six weeks (five times per week) by setting up a true stimulation versus a pseudo-stimulation control group. The results showed that the treatment group was significantly better than the pseudo-treatment group in improving sleep (p<0.05), which was reflected in the positive changes in the scores and total scores on the SDSC, suggesting that this therapy had a clear therapeutic effect on sleep problems in children with ASD.
The results of this study are consistent with previous literature, suggesting that low-frequency rTMS may improve sleep quality in children with ASD by regulating the excitation–inhibition balance of the cerebral cortex, increasing the levels of gamma-aminobutyric acid and brain-derived neurotrophic factor and decreasing motorevoked potentials [36-38]. Compared with traditional behavioral interventions and medications, rTMS, a noninvasive, painless treatment with few side effects, provides a new and more feasible option for the management of sleep problems in children with ASD. Especially in the context of behavioral rigidity and social disorders unique to children with ASD, rTMS therapy avoids the difficulty of understanding and implementing behavioral interventions and reduces the potential risks and limitations of drug therapy.
Although this study makes some contributions, it still has limitations such as limited sample size, large age span and single intervention area. Future studies should further expand the sample size, refine the age stratification and explore the intervention effect of rTMS on different brain regions (such as the parietal cortex and deep brain regions) to comprehensively evaluate its therapeutic potential. At the same time, considering the influence of depression, anxiety and other emotional factors on sleep, future studies should also strengthen the evaluation and analysis of the psychological state of children with ASD, to develop a more comprehensive and personalized treatment plan.
In summary, this study confirmed that low-frequency rTMS stimulation of the bilateral dorsolateral prefrontal cortex can significantly improve the sleep status of children with ASD, especially after a 6-week (30 sessions) treatment. Given its safe, effective and non-invasive advantages, rTMS is expected to be an important alternative therapy for the treatment of sleep problems in children with ASD.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Zhang Y, Yan J, Xing H, Wang J, Fang K (2024). Effects of Repeated Transcranial Magnetic Stimulation on Sleep Structure and Improvement of Sleep Quality in Children with Autism. Autism-Open Access. 14:411
Received: 05-Sep-2024, Manuscript No. AUO-24-31970; Editor assigned: 09-Sep-2024, Pre QC No. AUO-24-31970 (PQ); Reviewed: 23-Sep-2024, QC No. AUO-24-31970; Revised: 30-Sep-2024, Manuscript No. AUO-24-31970 (R); Published: 07-Oct-2024 , DOI: 10.35248/2165-7890.24.14.411
Copyright: © 2024 Zhang Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.