Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Research Article - (2021)Volume 9, Issue 8
Background: In Multiple Sclerosis (MS) as the demyelination happens, Oligodendrocyte Precursor Cells (OPCs) migrate to the site of injury and start differentiating into mature oligodendrocytes to regenerate the myelin sheaths. This OPC differentiation process can be affected by the epigenetic mechanisms which are mediated by a family of DNA methyltransferases (DNMTs) such as DNMT3A and DNMT3B. However, Dietary factors can alter the DNMTs expressions and even their activities. Therefore, in this study the effect of dietary factors such as vitamin A and D on the expression of DNMT3A and DNMT3B genes was assessed during these cells.
Methods: Rat embryonic stem cells were derived from ganglionic eminence. Then, the stem cells were cultured, differentiated and divided into five groups: control negative, control positive and three treatment modalities consist of vitamin D3, vitamin A, and vitamin A+D. Lastly, the Real-time PCR assay was conducted with total RNA.
Results: The expression of DNMT3B gene was significantly different between the groups, especially in the groups treated with vitamin A.
Conclusion: It seems that vitamin A can affect the expression of DNMT3B which plays a major role in cell differentiation and can be considered as a novel target for treatment of MS disease.
Neural stem cells; Differentiation; Oligodendrocyte precursor cells; DNMT3A; DNMT3B
Multiple Sclerosis (MS) as a chronic inflammatory disease in the Central Nervous System (CNS) could affect about 2.5 million people, especially the women in the world [1]. MS is characterized by demyelination of the nerve sheaths during an inflammatory process [1,2]. This demyelination would slow down the signal transportation and disturb the physical activity of affected people, and even paralysis or death in severe cases [3]. Although MS is a complex disease with poorly distinct etiology, a number of genetic and environmental factors such as infection, climate, stress, and diet can influence its development [4]. It has been reported that there is an association between dietary factors such as vitamins A and D with the relapsing-remitting MS patients [5,6]. These factors can affect the DNA methylation process which is catalyzed by DNA methyltransferases (DNMTs) [7,8]. It has been shown that 1,25-dihydroxy vitamin D3 (1,25(OH)2D3) is able to induce DNA demethylation, however, the mechanism is not clear [9]. The effect of retinoid (Vitamin A (9-cis-RA) and its biologically active metabolites) on gene expression, cell growth and differentiation has also been demonstrated [10,11]. 9-cis-retinoic acid (9-cis-RA) and 1,25 (OH)2D3 have also been reported to improve the differentiation of Neural Stem Cells (NSCs) into oligodendrocytes by suppressing the Notch and Wnt signaling pathways [12]. There are a wide variety of genes responsible for regulation of cellular proliferation and differentiation, modulated by 1,25 (OH)2D3 [13]. This modulation can happen by affecting epigenetic factors. Epigenetic changes occur in early MS lesions and interventions leading to re-myelination in the CNS are of major importance [14]. Epigenetic interventions and oligodendrocyte differentiation are substantial in the axonal re-myelination which is done by oligodendrocytes [15]. As the main involved cells in MS are oligodendrocytes, the induction of NSCs differentiation to oligodendrocytes can be helpful. As a result, the aim of this study is to evaluate the effect of vitamin A and D on DNMT3A and DNMT3B gene expression and differentiation of NSCs to oligodendrocyte.
Isolation of neural stem cell
NSCs were isolated from embryos of pregnant rats on day 14 of gestation. Then, the head of embryos was separated at the cervical spinal cord level. Under a dissecting microscope, the brain was removed from the skull and ganglionic eminences were dissected out [16].
Neurosphere assay
Tissue pieces were collected using 1 ml of NSC medium and transferred to 15 ml centrifuge tubes. The tissue samples were then dissociated thoroughly into single cells by 3-4 times pipetting the suspension up and down. Then, the content of the tubes was centrifuged for 5 minutes at 700 rpm and room temperature. The supernatant was removed and cells were resuspended in 1 mL of complete NSC medium. The medium was gently pipetted up and down to have a homogeneous single cell suspension. Then, 10 μl of the cell suspension was mixed with 90 μl of trypan blue to perform a cell count. Finally, the cells were plated in T25 flasks with the density of 2 × 105 cells/ml in complete NSC medium supplemented with 10 ng/ml basic fibroblast growth factor (bFGF). Neurospheres were formed in 5-7 days culture at 37°C in a humidified incubator with 5% CO2. In 6-7 days, the spheres would be ready for subculture [16].
Alamar blue assay
Alamar Blue assay was applied to quantify cellular metabolic activity and determine the concentration of viable cells in the samples. In this regard, neurospheres were harvested from flasks and were dissociated into single cells. Cells were seeded in different densities onto 200 μg/ml Poly-D-lysine-coated coverslips at a density of 5-25 × 103 in 96-well plates in order to assess the optimum cell density. Then, alamer blue 10% (Sigma- Aldrich, MO, USA) was added to each well and the absorbance was measured after 9 minutes at 540-590 nm by microplate reader. Coated coverslips with 15 × 103 cells were treated with different concentrations of 9-cis-RA (0.1, 0.5, 1, 5, 10, 20 μM) and 1,25 (OH)2D3 (12.5, 25, 50, 100, 200, 400 nM) for 7 days. Optimum concentration (EC50) of 9-cis-RA and 1,25 (OH)2D3 were selected according to appropriate curve.
Treatment of the cells with 9-cis-RA and 1, 25(OH) 2D3
Cells with the density of 50 × 103 were coated with Poly-D-lysine (Sigma-Aldrich, MO, USA) in order to differentiate them to oligodendrocyte progenitor cells (OPCs). Cells were allocated in following groups: (i) treated with 10 ng/ml bFGF as negative control, (ii) treated with T3 (Sigma-Aldrich, MO, USA) as positive control, (iii) treated by 1,25 (OH)2D3, (iv) treated with 9-cis-RA and (v) RA+Vitamin D3 group.
Assessment of the expression of DNMT3A and DNMT3B genes
Total RNA was isolated from the following treated cells using Biozol reagent (bioflux). RT-PCR was performed to amplify the target sequence of four genes including MBP, DNMT3A, DNMT3B and GAPDH (as an internal control). The reverse transcription was performed in a 20 μl reaction volume system (Fermentas, Burlington, Canada). The PCR reaction was performed for of 25 μl mixture containing 2 μl cDNA, 1.6 μl forward and reverse primers (10 pmol), 12.5 master mix including 5X PCR buffer, 10 mM dNTP mix, Riblock Ribonuclease inhibitor (20 U/μl), MgCl2 and Taq DNA polymerase (200 U/μl). The primers designed by Allele ID software are displayed by Table 1.
| Target gene | Forward primer | Reverse primer |
|---|---|---|
| DNMT3A | CTGAAGTAGTGATGATAACAA | AAGAGATGATGTCCAACC |
| DNMT3B | CCTGGCTGCTTAGAGTAG | TCCCTTGAGTTTGTTCGT |
| GAPDH | AAGAAGGTGGTGAAGCAGGCATC | CGAAGGTGGAAGAGTGGGAGTTG |
| MBP | CGGCTCACAAGGGATTCAAGG | AGGGCAGGATTCGGGAAGG |
Table 1: Gene specific primers.
First, preheating was done for activation of DNA polymerase at 94°C for 2 min. Then, the reaction continued by 30 cycles of denaturation at 95°C for 30 sec, annealing for 30 sec and 1 min of extension. The PCR products were separated on a 2% (w/v) agarose gel and were visualized by KBC dye. Finally, Gel analyzer software obtained quantitative data from the picture of the gel after the electrophoresis.
Quantitative real-time PCR
Real-time PCR was carried out using ABI real-time PCR 7500 system. The PCR reaction mixture contains 2 μl of cDNA (tenfold diluted), 0.5 μl forward and reverse primers as well as 10 μl of SYBR green DNA PCR Master Mix in a total volume of 20 μl. Real-time PCR amplification was performed with preheating activation of DNA polymerase at 50°C for 2 min, followed by 30 cycles of denaturation at 95°C for 15 sec, annealing at 58°C for 1 min. A melting curve analysis was achieved by performing 70 cycles of 10 sec with a temperature increment of 0.5˚C/cycle starting from 60˚C. The efficiency of the amplification was measured by the slope of a standard curve (correlation coefficients (r2) of>0.9888). The Relative expression level (fold changes) of MBP, DNMT3A and DNMT3B genes was calculated by the 2-ΔΔCT formula. GAPDH was also considered as internal control.
Statistical analysis
Obtained data were analyzed by GraphPad Prism software (Version 6.01, San Diego, CA, USA) using one-way ANOVA followed by Tukey’s post-hoc test. The values were represented as mean ± standard error of mean, and p<0.05 were statistically considered as significant differences between the groups.
Determining the optimal concentration of 9-cis-RA and 1,25 (OH)2D3 for NSCs treatment
The alamar blue assay was conducted to evaluate the non-toxic concentration of 9-cis-RA and 1,25 (OH)2D3. Based on the findings, the ideal concentration of 9-cis-RA and 1,25 (OH)2D3 for one week treatment of NSCs were determined 10 μM and 100 nM, respectively.
The effect of 9-cis-RA and 1,25 (OH) 2D3 on the expression of myelin basic protein (MBP) gene
In order to determine oligodendroglial differentiation potential of 9-cis-RA and 1,25 (OH)2D3 on treated OPCs, we examined the changes in mRNA expression of MBP gene. The expression of MBP gene was significantly increased in all groups compared to the negative control group (P<0.001) as are shown in Figures 1A and 1B.
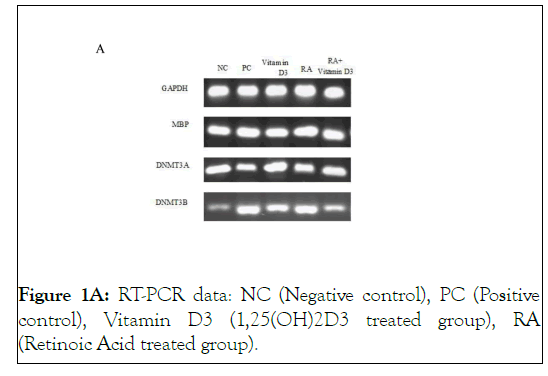
Figure 1A: RT-PCR data: NC (Negative control), PC (Positive control), Vitamin D3 (1,25(OH)2D3 treated group), RA (Retinoic Acid treated group).
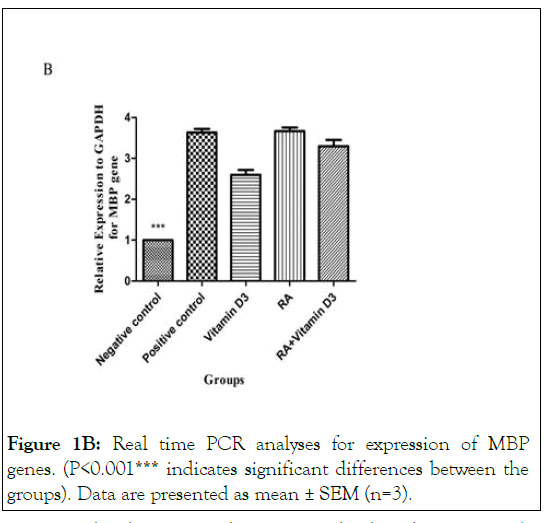
Figure 1B: Real time PCR analyses for expression of MBP genes. (P<0.001*** indicates significant differences between the groups). Data are presented as mean ± SEM (n=3).
Interestingly, the increased expression level, only in 9-cis-RA treated cells was similar to the positive ones. These results reveal that 9-cis-RA and 1,25(OH)2D3 have remarkable effects on OPC maturation however, 9-cis-RA probably plays a more prominent role in differentiation process. Moreover, in 9-cis-RA plus 1,25(OH)2D3 treated cells, a significant reduction in gene expression was observed in comparison with the positive control group (P<0.001). This indicates that the simultaneous use of both vitamins A and D may have an inhibitory effect on the expression of MBP gene (Figures 1A and 1B ).
The effect of 9-cis-RA and 1,25 (OH)2D3 on the expression of DNMT3A gene
As are shown in Figure 1A and Figure 2 , there are not any significant differences in the expression level of DNMT3A gene between the treated groups and negative control ones.
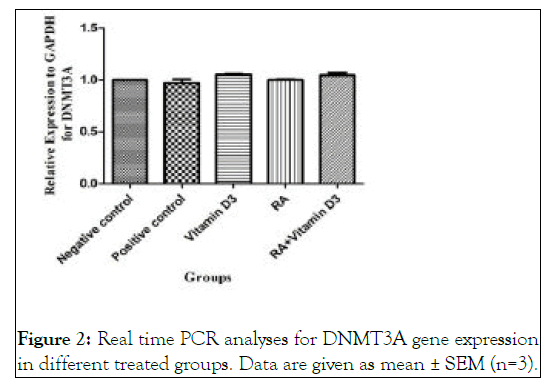
Figure 2: Real time PCR analyses for DNMT3A gene expression in different treated groups. Data are given as mean ± SEM (n=3).
The effect of 9-cis-RA and 1,25 (OH)2D3 on the expression of DNMT3B gene
Treatment with 9-cis-RA caused a significant reduction in the expression of DNMT3B compared to the negative control, 1,25 (OH)2D3 and RA+vitamin D3 groups (p<0.001) ( Figures 1A and Figure 3 ).
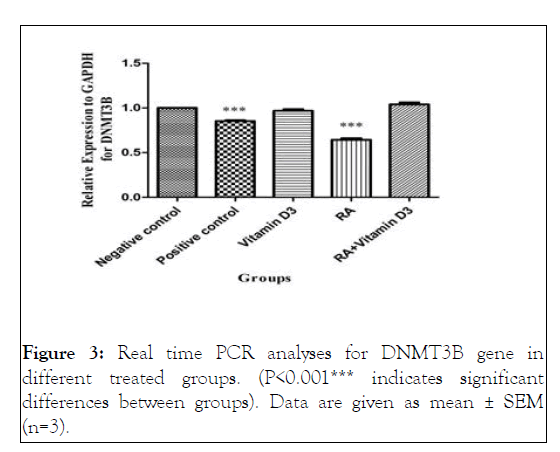
Figure 3: Real time PCR analyses for DNMT3B gene in different treated groups. (P<0.001*** indicates significant differences between groups). Data are given as mean ± SEM (n=3).
However, there was no significant difference between positive control and RA groups. These data suggested that 9-cis-RA might be able to increase the differentiation of oligodendrocytes through suppression of DNMT3B expression [17-40].
As the demyelination happens, OPCs migrate to the site of injury and start differentiating into mature oligodendrocytes with the ability of renewing myelin sheaths around axons to enhance the number of oligodendrocytes. Myelin sheaths are required for the propagation of fast impulse along the myelinated neurons which are damaged during MS. However, remyelination, the regenerative process following demyelination, is often insufficient or ineffective due to the failure in the differentiation of precursor cells. The OPC differentiation process is regulated by different signaling molecules which can affect the epigenetic mechanism. DNA methylation as a known epigenetic regulation process mediated by a family of DNMTs such as de novo DNMT3A and DNMT3B, can alter the gene expression patterns. Accordingly, several studies have announced that epigenetic modification by DNMTs is essential for OPC differentiation process. In a way that simultaneous lack of these two enzymes in embryonic stem cells can reduce cell differentiation. Besides, dissimilar patterns in the expression of DNMT3A and DNMT3B enzymes in CNS precursor cells, astrocytes, oligodendrocytes, and post-mitotic neuronal cells have been verified. It is offered that dietary factors may influence DNA methylation through impacting on the available methyl group supplies as well as modification in DNMTs expression and activity. The impacts of biologically active metabolites of vitamin A and D on cellular differentiation and proliferation of stem cells via affecting the epigenetic regulators such as DNA methylation have also been approved in several studies. Moreover, retinoic acid and vitamin D signaling pathways are connected directly. Therefore, in this study, we RXRtried to differentiate NSCs of rat embryos to oligodendrocytes in vitro , to evaluate the effect of vitamin A and D on the DNMT3A and DNMT3B expression during the differentiation process. Our findings revealed that there are no significant differences in DNMT3A gene expression among groups. Nevertheless, DNMT3B gene expression showed meaningful alterations. Based on our results, treatment with vitamin A led to DNMT3B down regulation. In consistent with our study, the consequence of treatment with homocysteine in NSCs as another dietary factor has revealed a decrease in protein expression of DNMT3A and DNMT3B as well as their enzymatic activity. This reduction led to a decline in the level of DNA methylation and suppression in NSCs proliferation. Conversely, evidence from treatment with ethanol has shown an increase in the total DNMT activity resulting in the alteration in cell cycle progression. However, results of a recent report demonstrated that chronic exposure to ethanol in NSCs induces the overall DNA hypo-methylation and alters DNA methylation correlated genes’ expression and differentiation in sex/strain specific manner. In addition, another finding has investigated that folic acid as another dietary factor enhances DNMT activity which in turn increases NSCs differentiation into neurons.
As mentioned before, several studies previously have declared that DNMTs activities are essential for epigenetic modification during OPC differentiation process. However, the result of a novel study demonstrated that DNMT3A contribute in proliferation of OPCs in a way that knockdown of DNMT3A by siRNA leads to reduction in the number of OPCs, conversely DNMT3B is not widely expressed in OPCs. Cell localization of DNMT3 also alters during differentiation processes. In undifferentiated NSCs, DNMT3A is found in the nucleus, however translocate into the cytoplasm at the start of differentiation. However, it is also declared that DNMT3A is mostly expressed in the late stage of all neural cells development including NPS and oligodendrocytes, as a result may play a role in development of the nervous system. Besides, DNMT3B mediates the early stage of embryonic and neural progenitor cells differentiation, because it is primarily expressed early in these cells during neurogenesis. Therefore, it appears that DNMT3A/B complement each other’s expressions and differently cooperate in the development of NSCs.
All-trans RA Following binding to retinoic acid receptors (RARs) can alter their interactions with multiple transcriptional complexes and then exert its epigenetic modifications and potent impacts on NSCs proliferation, differentiation and maturation. It is especially suggested that RXR-γ can positively regulate the differentiation and development of oligodendrocytes. The relation between myelination and vitamin A deficiency and the impact of exogenous RA on OPCs differentiation has been previously proposed. Next, the supportive role of RA via RXR-γ signaling in differentiation of OPCs and re-myelination in spinal cord injury has been proved. Lately, Morrison and his colleague have found that RA is essential for OPCs production and differentiation in the postnatal mouse corpus callosum. On the other hand, the accelerative role of 1,25(OH)2D3 on NSCs proliferation and differentiation into neurons and oligodendrocytes has been suggested. In addition, Gomez‐Pined and his coworkers has promoting the proliferation and differentiation of NSCs in the subventricular zone and even, their migration to the site of injury in the corpus callosum. Regarding the effects of 9-cis-RA and 1,25 (OH)2D3, it has been verified in our previous report that these dietary factors induce the differentiation of NSCs into oligodendrocytes through the inhibition of the Notch and Wnt signaling pathways. For detecting the effect of 9-cis-RA and 1,25(OH)2D3 treatment on oligodendrocyte differentiation, the mRNA level of MBP as an oligodendrocyte marker was measured. The results show that the MBP expression was elevated through treatment, however this enhancement for 9-cis- RA was more and for 1,25(OH)2D3 was less. It shows that besides treatment, OPCs have well differentiated into MBPexpressing oligodendrocytes.
Overall, it appears that 9-cis-RA biologically as an active metabolite of vitamin A could influence the expression of DNMT3B which is dominantly expressed in early embryonic and neural progenitor cells, therefore can be considered as a target for well differentiation of OPCs during MS disease. Of course, more studies are needed to investigate the mechanisms underlying this relationship.
Citation: Mokarram P, Alizadeh P, Saeb S, Rahmani-Kukia N, Siri M, Babazadeh M (2021) Effects of Vitamin A and D on DNA Methyltransferases 3A and 3B Expression and Neural Stem Cell Differentiation. J Leuk. 9(8): 270.
Received: 31-Aug-2021 Accepted: 14-Sep-2021 Published: 21-Sep-2021 , DOI: 10.35248/2329-6917.21.9.270
Copyright: © 2021 Mokarram P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.