Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Research Article - (2017) Volume 8, Issue 11
Objective: To assess the effects of various walking rehabilitation programs on body composition, heart rate variability, aerobic fitness and perceptual responses of overweight and obese young adolescents.
Material and methods: 31 young overweight and obese adolescents (12 boys and 19 girls) were randomly assigned to one of 4 groups: a group walking at 70% of maximal aerobic speed (70% MAS G, n=7), a group walking at 50% of maximal aerobic speed (50% MAS group, n=8), a self-selected walking pace group (SSWP group, n=8), and a control group (C, n=8). Anthropometric, metabolic, and perceptual parameters were measured before and after a 2-month rehabilitation program.
Results: Decreases in body mass, BMI, and body fat were seen in MAS, 70% MAS, and SSWP groups (p<0.01, p<0.01, and p<0.05 respectively), with associated with increases in the distance performed during the 6MWT (all at p<0.01). 50% MAS and 70% MAS groups showed significant increases in MAS, estimated VO2max, and the calculated maximal fat oxidation (all at p<0.01). In addition, we found a decrease in HR values during the recovery period (p<0.05), associated with significant increases in HF (p<0.05 and p<0.05 respectively) and decreases in LF (p<0.05 and p<0.05 respectively) indices of heart rate variability in 50% MAS and 70% MAS groups. In contrast, the ratio LF/HF decreased only in the 50% MAS group (p<0.05). The 2-month rehabilitation program induced significant decreases of RPE values and Hooper scores in 50% MAS (p<0.05, and p<0.01 respectively) and 70% MAS groups (p<0.05, and p<0.01 respectively). The control group showed no changes in the selected parameters.
Conclusions: Rehabilitation programs based on 50% MAS and 70% MAS were more effective strategies to make positive changes on body composition, heart rate variability, aerobic fitness and perceptual responses than the self-selected walking pace, and could be used to ensure an effective intensity of effort in the rehabilitation of overweight and obese adolescents.
Keywords: Obesity; Adolescents; Heart rate variability; Walking rehabilitation; Perceptual; Cardiorespiratory fitness
Obesity among youth has been considered as one of the major world health issues [1,2]. Young obese individuals suffer from a decline of cardiopulmonary function, a poor exercise tolerance and self-esteem and a risk of chronic conditions such as diabetes mellitus. Appropriate tactics are needed to prevent and/or treat obesity in young obese individuals, with the choice of exercise training mode taking due account of initial physical capacities and motivation to assure large changes in body composition.
Regular aerobic training has been proposed as an efficient method to manage overweight or obesity in young subjects [3]. Some studies have used training programs based on the individual's Lipox max [4,5], or high intensity exercises [5,6] such as running or cycling. However, body mass constitutes an important factor that limits running in such individuals. Cycling is a possible alternative, but this mode of physical activity solicits a smaller muscle mass than walking, with a corresponding increase in the risk of fatigue. Walking is thus a very useful mode of physical activity to improve energy balance, physical fitness and perceptual responses in those whom are obese [7]. Experts in sports medicine currently recommend taking at least 10,000 steps a day [8]. Some studies have shown benefits from walking programs in the obese, including reductions in fat mass and blood lipids, improvements in physical fitness, decreases of blood pressure and a greater enjoyment of life. Walking involves most of the body muscles, and can be undertaken almost anywhere, with the individual controlling the intensity of effort. However, to the best of our knowledge, there are no studies assessing the relative impact of different paces of walking on health-related factors in young overweight or obese. Nevertheless, some studies have shown that regular moderate-intensity walking is associated with a reduced risk of cardio-vascular disease in women [9-11] also found improvements in VO2max after walking-based training that was associated with weight loss and reductions in visceral fat stores. Walking pace can indeed be used as an indicator of cardiovascular fitness [12].
Given the dearth of studies looking at the effects of self-selected walking on physical fitness, we have examined relationships between programs with differing pace, on aerobic fitness, heart rate variability, and perceptual parameters in overweight and obese adolescents, with an increased fat mass [13], autonomic nervous system dysfunction [14], poor aerobic fitness, and various perceptual or psychological disorders.
The aim of the study was to assess the effects on overweight and obese adolescents of 2-month rehabilitation programs based on 3 different walking speeds (a self-selected walking pace, walking at the 70% maximal aerobic speed (MAS) and walking at 50% of MAS. Outcome variables included anthropometric parameters, measures of aerobic fitness and heart rate variability, and perceptual responses.
Participants
The study was approved by the local ethical committee of the Hospital Farhat Hached of Sousse in accord with the Helsinki Declaration. We also obtained the approval of the Regional Administration of Education and the Ministry of Health. During preliminary meeting, participants were accompanied by their parents and were given a detailed copy of the protocol so they could understand the benefits, risks and discomfort related to the training program. The parents then signed a consent form. The participants were 31 young adolescents (12 boys and 19 girls) who suffered from overweight or obesity. They were recruited from local high schools, criteria being age (13-15 years), and a body mass index (BMI) greater than the 97th age-related percentile [15]. Subjects on medication or suffering from disease (metabolic disorders, hypertension, diabetes mellitus, cardio-respiratory and other chronic pathologies) were excluded. Participants were randomly assigned to one of 4 groups: a group walking at the 70% of the maximal aerobic speed (70% MAS G, n=7), a group walking at 50% of the maximal aerobic velocity (50% MAS group, n=8), a self-selected walking pace group (SSWP group, n=8), and a control group (C, n=8). The control group engaged in no formal physical activity program during the study period. Attendance at training sessions was high for all 3 active groups, with a compliance rate of 95%.
Rehabilitation program
Table 1 provides a schematic representation of the rehabilitation program. This program was based on walking for 2 month-duration, 3 sessions per week, for at least one hour per session. All tests and training sessions were carried out under the supervision of medical staff and specialists in physical education. Training was carried out in the laboratory of the Faculty of Medicine on Monday, Wednesday, and Friday afternoons, under controlled environmental conditions (room temperature: 22-24°C, humidity: 65-70%). Each session included a 10 min collective warm-up, based on ball games, followed by 2 × 20 min periods of walking, interspersed by 10 maximal sprints on a cycle ergometer against a braking force equal to 0.75 g/kg body mass. The training protocol aimed to motivate participants to continued adherence even after the end of the program. Training intensities were controlled by measurements of HR, using a polar device (Polar RS810, Kempele, Finland).
| 8-Week rehabilitation programs | |||
| Warm up | 10-min (walk/moderate run, ball games, dynamic stretching) | ||
| Main program | 70% MAS group | 50% MAS group | Self-selected walking pace |
| 2 × 20 min walking at 70% maximal aerobic speed (MAS), | 2 × 20 min walking at 50% maximal aerobic speed (MAS), | 2 × 20 min walking at self-selected pace, | |
| Interspersed by 10 sprints of 6 s on a cycle ergometer. Recovery between sprints 4 s, loading 0.75 g/kg. | Interspersed by 10 sprints of 6 s on a cycle ergometer. | Interspersed by 10 sprints of 6 s on a cycle ergometer. Recovery between sprints 4 s, loading 0.75 g/kg. | |
| Recovery between sprints 4 s, loading 0.75 g/kg). | |||
| Intensity: | |||
| MAS: 70% | Intensity: | Intensity: | |
| HR: 149 ± 10 bpm | MAS: 50% | MAS: <40% MAS | |
| Speed : 6.5 ± 1 km/h | HR:120 ± 10 bpm | HR: 105 ± 10 bpm | |
| Speed: 4.5 ± 1 km/h | Speed: 3 ± 1 km/h | ||
| Cool Down | 5 min of stretching and relaxation exercise |
Table 1: Rehabilitation program designed for experimental groups (walking at 70% MAS, at 50% MAS, and a self-selected pace).
During the study, a nutritionist organized two small-group reunions with the adolescents and their parents, one before the beginning of the study and another a month later. The objective was to correct bad dietary behavior, to define some dietary concepts, and to give advice about dietary equilibrium and healthy foods.
Measurements
Anthropometry and assessments of pubertal development
During the first visit, each subject underwent a medical examination by a physician. Anthropometric measurements were taken by the same investigator. These measurements were repeated after the 2-month training period. Body mass was measured using a digital scale (EKS, Gislaved, Sweden). Height was measured to 1 mm, using a standing stadiometer (Seca, Basel, Switzerland), and body mass index (BMI) was calculated.
Skinfold thickness were measured at 4 sites (biceps, triceps, suprailiac, and subscapular), using the Harpenden Caliper (Lange Instruments, Cambridge, UK), If triplicate values differed by <10%, measurements were repeated. Body density was calculated using the under 17 year’s equations of [16] for subjects
For males, body density (D)=1.1533-(0.0643 × log10 Σ)
For females, body density (D)=1.1369-(0.0598 ×log10Σ
Where Σ is the sum of the 4 skinfold readings.
Body fat was calculated using the formula proposed by [17]
For males: body fat=[(5.07/D–4.64) × 100]
For females: body fat=[(5.10/D–4.66) × 100]
Waist and hip circumferences were measured using a measuring tape. The pubertal development was estimated by an experienced pediatrician using the standard Tanner stages [18].
6-min walking test and related variables
The 6 MWT was carried out before and after the rehabilitation program, in a 40 m hallway, limited by two cones, according to the recommendations of the American Thoracic Society [19]; the floor was marked every meter, Participants were instructed to walk as fast as possible to cover the maximum possible distance during the 6 min; standard verbal encouragement was given every minute. Before and immediately after the test, measurements of heart rate, blood pressure, and dyspnea were taken, using the Borg scale (0-10) to rate breathlessness. Heart rate was measured continuously at rest, during and after the 6 MWT, using the S810 polar monitor (S810, Kempele, Finland).
Maximal aerobic speed
A progressive shuttle field test was used to measure maximal aerobic speed (MAS) and to estimate VO2max. Participants followed the standard progressive protocol of [20]. The test began at 7.5 km/h and the velocity was increased by 0.5 km/h every min until the participant was unable to follow the rhythm imposed by the beeper on 2 occasions, despite verbal encouragement.
Maximal fat oxidation
Maximal fat oxidation (Lipox max) is intensity of exercise where lipid oxidation is maximal, as measured by indirect calorimetry [21]. We estimated this point, using the formula of based on the distance covered during the 6-min walk test 6 MWD, and waist (WC) and hip (HC) circumferences [22].
For boys: Fat max (mg min¹)=0.17 × 6 MWD (m) × 0.36 × HC (cm) +70.43
For girls: Fat max (mg min¹)=0.13 × 6 MWD (m) × 0.25 × WC (cm) +78.28
Heart rate variability
Cardiac autonomic function was assessed according to heart rate variability (HRV). The variability of the instantaneous R-R intervals gives a good and reproducible estimate of the relative preponderance of sympathetic and parasympathetic nervous system activity. The analysis of HRV has become established as a non–invasive method for evaluation of the autonomic nervous system. R-R intervals represents the time difference between the consecutives R waves derived from electrocardiography (ECG) recording.
Signal processing
Data recording
R-R intervals were also recorded by the Polar monitor (model S-810, Kempele, Finland) at the rest, during the 6-min walking test, and along 10 min of the recovery period (Figure 1). The last 5 min in each 10 min recovery period were used to assess HRV variables similar to [23]. Two recordings were made before and after the 2-month training program. Methodological precautions were followed to have a good ECG signal [24].
RR series extraction
The data for R-R intervals were transferred into Polar trainer Software and expressed in (ms). The aberrant beats (Artifacts, cumulative RR intervals, and extrasystoles) were identified visually and manually replaced with interpolated adjacent RR interval values [25].
Descriptive statistical data analysis
Data analysis was performed by Kubios software (Kubios, Kupio, Finland). The interpolation rate of RR series is 4 Hz (0.25 s) and the windows dimension is 256 points [25].
Time and frequency domain analysis
The mean RR interval and the root-mean square differences of successive RR intervals (RMSSD) were calculated for each sequence.

Where N=number of R-R interval terms.
The instantaneous time frequency components were computed for the low frequency (LF: 0.04-0.15 Hz) and high frequency (HF: 0.15-0.40 Hz) bands of HRV.
Among these parameters, the RMSSD and HF depict vagal activity, LF power reflects predominantly sympathetic and to lesser extent vagal activity [26]. In addition LF/HF was determinated as a measure that may represent sympatho-vagal balance [23].
Furthermore, both components were normalized as follows:
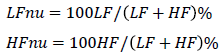
In fact, (RMSSD, LF nu, and HF nu) were logarithmically transformed to normalize data.
Poincare plot analysis
The Poincare plot consists of graphical representation for each RR as a function of the previous RR intervals (Figure 2). The shape of the resulting scatter plot makes it possible to classify HRV visually. The RR interval typically appears as an elongated cloud of points oriented along the line of identity .If the scatter plot is ellipsoidal; the shape can be quantified by calculating the ellipse’s measure [25]. SD1: dispersion (standard deviation) of point’s perpendicular to the axis of line of identity, represents a short-term HR variability indicating parasympathetic activity. SD2: dispersion of points along the axis of line of identity represents long term HR variability.
Assessment of perceptual responses
Perceptual responses were tested before and after the intervention, using the Borg 1-10 RPE scale [27], and the Hooper questionnaire [28]. The latter measures sleep patterns, fatigue, stress, and muscle soreness.
Statistical analysis
Statistical analyses were completed using the SPSS program (SPSS Inc., Chicago, IL, USA, version 16.0). The normality of distribution was checked for all variables using the Kolmogorov-Smirnov test. Data are presented as means and standard deviations (SD). The main effect of each program was assessed with a two-way analysis of variance (ANOVA) with repeated measures. Percentage differences (%Δ) between pre-test and post-test data were calculated for each parameter. One-way ANOVA was used to explore inter-group differences; where appropriate, significant differences between means were assessed using the Bonferroni post-hoc test. The effect size (ES) was calculated using Eta-squared (η²). Threshold values for Cohen ES were >0.2 (small), 0.5 (moderate) and >0.8 (large). Statistical significance was set at p ≤ 0.05 throughout.
Changes in anthropometric and body composition parameters
The physical characteristics of our participants are summarized in Table 2. There were no significant inter-group differences in terms of anthropometric parameters at baseline (Table 2, all at p>0.05). However, there were significant decreases in body mass, BMI, and body fat in the 50% MAS, 70% MAS, and self-selected walking pace groups (p<0.01, p<0.01, and p
| 50% MAS group | 70% MAS group | Self-selected walking pace group | Control group | |||||
|---|---|---|---|---|---|---|---|---|
| N=8 (5Girls/3Boys) | N=7 (4Girls/3Boys) | N=8 (5Girls/3Boys) | N=8 (5Girls/3Boys) | |||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Age (yr) | 13.8 ± 0.5 | 14.5 ± 1.0 | 14.2 ± 0.8 | 14.5 ± 0.9 | ||||
| Tanner stage (III/IV/V) | ||||||||
| 2/5/2001 | 0/5/2 | 0/5/3 | 1/5/2002 | |||||
| Height (m) | 1.60 ± 0.05 | 1.61 ± 0.09 | 1.63 ± 0.05 | 1.64 ± 0.06 | 1.58 ± 0.07 | 1.59 ± 0.07 | 1.62 ± 0.04 | 1.65 ± 0.10 |
| Body mass (kg) | 80.7 ± 13.24 | 71.7 ± 11.3** | 76.9 ± 6.9 | 69 ± 6.21** | 75.8 ± 10.2 | 73.6 ± 10.3* | 81.1 ± 18.2 | 80.5 ± 18.7 |
| BMI (kg·m²) | 30.7 ± 4.1 | 28.57 ± 2.9** | 29.7 ± 3.0 | 27.5 ± 2.5** | 28.8 ± 3.5 | 28.01 ± 3.5 | 31.2 ± 3.01 | 31.5 ± 3.1 |
| BMI | 0.36 ± 1.18 | -0.29 ± 0.86 | 0.06 ± 0.96 | -057 ± 0.74 | 0.22 ± 1.0.3 | 0.40 ± 1.05 | 0.48 ± 0.88 | 0.50 ± 0.90 |
| Z-score | ||||||||
| WC (cm) | 90.6 ± 6.1 | 86.1 ± 6.5 | 86.6 ± 13.4 | 75.9 ± 11.7** | 90.6 ± 9.2 | 88.5 ± 7.4 | 91.1 ± 7.7 | 91.6 ± 8.6 |
| HC (cm) | 102.0 ± 7.8 | 100.0 ± 8.5 | 100.5 ± 8.8 | 99.9 ± 8.2 | 101.5 ± 9.5 | 100.8 ± 8.8 | 103.0 ± 10.1 | 104.3 ± 9.3 |
| W/H Ratio | 0.88 ± 0.18 | 0.86 ± 0.15 | 0.84 ± 0.11 | 0.82 ± 0.12* | 0.89 ± 0.26 | 0.87 ± 0.19 | 0.88 ± 0.25 | 0.89 ± 0.31 |
| Body Fat (%) | 26.1 ± 4.4 | 23.4 ± 3.6** | 26.5 ± 6.4 | 21.3 ± 4.8** | 30.9 ± 7.7 | 27.1 ± 7.0* | 27.6 ± 6.2 | 27.4 ± 6.7 |
Table 2: Physical characteristics of the participants before and after rehabilitation; Data are expressed as means ± SDs; WC: Waist Circumference; HC: Hip Circumference.
Changes in heart rate and heart rate variability (HRV) indices
Heart rate data did not differ among groups at baseline (Table 3). After rehabilitation, the experimental groups showed significant decreases in recovery HR (50% MAS, F=9.2, p=0.02, η²= 0.6; 70% MAS: F=39.9, p=0.001, η²=0.87; G self-selected walking pace, F=16.7, p=0.006 η²=0.73). There were no significant changes in HRV for the time domain (R-R, and RMSSD), but there were significant increases in high frequency (HF) activity in 50% MAS and 70% MAS groups (F=18.7, p=0.005, η²=0.75; and F=5.78, p=0.05, η²=0.49 respectively) associated with reductions in the low frequency component (LF) (F=18.4, p=0.005, η²=0.75; and F=9.01, p= 0.02 ; η²=0.6 respectively). The LF/HF ratio was increased significantly only in the 50% MAS group (F=6.2, p=0.047, η²=0.51). In addition, we observed an increase in the geometric parameter of the Poincare plot [SD1] in 50% MAS and 70% MAS groups (F=6.7, p=0.04, η²=0.53; and F=6.9, p=0.03, η²=0.54 respectively). The control group showed no changes in any of the HR values or HRV indices.
| Group walking at 50% MAS | Group walking at 70%MAS | Group walking at self-selected pace | Control group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | %Δ | Pre | Post | %Δ | Pre | Post | %Δ | Pre | Post | %Δ | 1 way-ANOVA | |
| HR (bpm) | 90 ± 2 | 87 ± 1* | -3.1 | 89 ± 1 | 85.7 ± 2.0** | -3.5 | 88.9 ± 3.24 | 87.9 ± 3.2* | -1.2 | 89.0 ± 3 | 89.8 ± 3.3 | 0.87 | F(3.27)=9.93 |
| $ | $ | P=000 | |||||||||||
| R-R | 646.9 ± 50.5 | 667.1 ± 67.2 | 3.3 | 644 ± 40.6 | 670.4 ± 64.1 | 3.9 | 618.3 ± 24.3 | 659.4 ± 59.0 | 6.6 | 611 ± 30.5 | 621.7 ± 42.01 | 1.58 | F(3.27)=1.1 |
| (ms) | P=0.39 | ||||||||||||
| lnRMSSD | 1.59 ± 0.23 | 1.71 ± 0.12 | 9.5 | 1.58 ± 0.2 | 1.78 ± 0.2 | 13.8 | 1.58 ± 0.2 | 1.69 ± 0.1 | 8.6 | 1.53 ± 0.19 | 1.62 ± 0.33 | 7.1 | F(3.27)=0.12 |
| (ms) | P=0.94 | ||||||||||||
| HF (nu) | 26.2 ± 12.3 | 35.6 ± 15.4** | 38.9 | 24.6 ± 14.4 | 34.5 ± 15.1* | 53.3 | 25.9 ± 7.2 | 28.3 ± 11.6 | 9.3 | 27.02 ± 8.98 | 28.1 ± 6.46 | 15.9 | F(3.27)=1.05 |
| P=0.38 | |||||||||||||
| LnHF (ms²) | 2.5 ± 0.4 | 2.6 ± 0.5 | 7.6 | 2.5 ± 0.5 | 2.6 ± 0.5 | 6.9 | 2.4 ± 0.4 | 2.57 ± 0.5 | 7 | 2.3 ± 0.51 | 2.37 ± 0.81 | 1.89 | F(3.27)=0.09 |
| P=0.96 | |||||||||||||
| LF | 71.7 ± 13.1 | 45.6 ± 13.4** | -35.5 | 72.2 ± 12.8 | 48.1 ± 23.9* | -33.9 | 70.1 ± 12.9 | 57.1 ± 12.3 | -17.8 | 71.81 ± 22.34 | 71.17 ± 19.56 | 0.05 | F(3.27)=3.8 |
| (nu) | $ | $ | P=0.02 | ||||||||||
| LnLF | 2.9 ± 0.5 | 2.5 ± 1.2 | -5.9 | 2.9 ± 0.5 | 2.5 ± 1.3 | -7.6 | 2.8 ± 0.48 | 2.5 ± 0.8 | -4.2 | 2.59 ± 0.35 | 2.83 ± 0.55 | 10.12 | F(3.27)=0.27 |
| (ms²) | P=0.84 | ||||||||||||
| LF/HF | 3.7 ± 1.9 | 2.0 ± 0.8* | -26.6 | 4.0 ± 2.3 | 2.6 ± 1.6 | -20.3 | 3.4 ± 1.8 | 2.1 ± 0.7 | -15.8 | 2.81 ± 0.9 | 2.91 ± 1.28 | 24.05 | F(3.27)=0.81 |
| P=0.49 | |||||||||||||
| SD1 (ms) | 33.1 ± 9.5 | 42.6 ± 14.3* | 30.8 | 34.8 ± 10.5 | 45.7 ± 15.6* | 34.2$ | 34.8 ± 7.1 | 40.0 ± 16.4 | 13.3 | 34.5 ± 8.16 | 35.29 ± 17.5 | 3.69 | F(3.27)=1.09 |
| P=0.36 | |||||||||||||
| SD2 (ms) | 66.44 ± 21.31 | 89.07 ± 22.5 | 30.69 | 75.5 ± 32.1 | 98.21 ± 23.59 | 30.9 | 65.3 ± 21.1 | 90.7 ± 22.4 | 16.47 | 69.3 ± 10.5 | 77.57 ± 12.8 | 14.2 | F(3.27)=0.18 |
| P=0.9 |
Table 3: Heart rate variability indices measured during the recovery period following the 6-min walking test (6 MWT) before (pre) and after (post) rehabilitation; Values are means ± SDs; HR: Heart Rate; RMSSD square root of the difference in the sum of squares between R-R interval; HF: High Frequency; LF: Low Frequency; SD1 standard deviation of instantaneous R-R interval variability; SD2 standard deviation of long term continuous R-R interval variability; 6 MWT: 6-min walk test; Significant change from baseline * (P<0.05); ** (p<0.01); significantly different from control $ (p<0.05).
The one-way ANOVA showed significant differences between groups in HR values (F (3.27)=9.93, P=0.01), and the LF component (F(3.27)=3.8 P=0.02) measured during the recovery period after the 6MWT. Post-hoc analysis showed statistical differences between the control group (GC) and 50% MAS, and 70% MAS groups for HR values and LF (both at p<0.05).
Changes in 6MWT and in aerobic fitness
There were improvements of 6MWT performance after rehabilitation program in all 3 experimental groups (Table 4, 50% MAS group: F=7.2, p=0.01, η²=0.51; 70% MAS group: F=78.15, p=0.05, η²=0.9; and self-selected walking pace group: F=8.64, p=0.05, η²=0.55).
| Group walking at 50% MAS | Group walking at 70% MAS | Group walking at self-selected walking pace | Control group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pré | Post | %Δ | Pré | Post | %Δ | Pré | Post | %Δ | Pré | Post | %Δ | 1way ANOVA | |
| 6 MWT parameters | |||||||||||||
| HRrest (bpm)) | 77.8 ± 3.7 | 74.2 ± 3. 7 | -4.5 | 78.2 ± 3.7 | 70.4 ± 2.1 | -11 | 77.4 ± 3.3 | 76 ± 3.18 | -1.75 | 78.25 ± 2 | 79.5 ± 1 | 0.87 | F (3.27)=18.2 |
| $ | $ | P=000 | |||||||||||
| Mean HR 6MWT (bpm) | 144.1 ± 13.6 | 131.3 ± 5.8 | -8.2 | 148.9 ± 13.7 | 139.4 ± 9.3** | -6.12 | 137.5 ± 8.1 | 144.1 ± 6.9 | 5.28 | 137.1 ± 16.9 | 146 ± 7.9 | 7.55 | F (3.27)=5.7 |
| $ | $ | P=0.03 | |||||||||||
| 6MWT distance (m) | 537 ± 52.25 | 592.75 ± 39.3** | 11.2 | 546.7 ± 18.22 | 619.8 ± 27.1** | 13.4 | 540.25 ± 50.7 | 591.26 ± 35.6* | 10.1 | 567.37 ± 86.2 | 543.75 ± 55.35 | -2.9 | F (3.27=3.91 |
| $ | P=0.019 | ||||||||||||
| Dyspnée (AU) | 2.3 ± 1.4 | 1.06 ± 0.6 | -21.8 | 2.8 ± 1.06 | 0.9 ± 0.18* | -59.5 | 2.25 ± 1.03 | 1.06 ± 0.41 | -31.2 | 1.75 ± 1.16 | 1.4 ± 1.2 | -8.3 | F (3.27)=0.8 |
| P=0.5 | |||||||||||||
| So2 (final)(%) | 96.5 ± 1.5 | 96.1 ± 0.9 | -0.3 | 96.4 ± 1.3 | 95.5 ± 2.5 | -0.8 | 94.8 ± 4.5 | 96 ± 4.5 | 1.2 | 95.8 ± 2.8 | 94.8 ± 4.6 | -0.8 | F (3.27)=0.4 |
| P=0.47 | |||||||||||||
| SBP (mmHg) | 121.6 ± 7.06 | 118.6 ± 5.7* | -3.2 | 119.4 ± 6.3 | 116.4 ± 7.1* | -3 | 123.6 ± 5.7 | 121.8 ± 6. 2 | -2.3 | 124.8 ± 12. 5 | 125.5 ± 11.2 | 0.6 | F (3.27)=0.85 |
| P=0.61 | |||||||||||||
| DBP (mmHg) | 63.4 ± 5.9 | 60.1 ± 6.3* | -5.21 | 68.2 ± 5.2 | 64 ± 3.9** | -6.1 | 70 ± 7.8 | 68.3 ± 5.3 | -2.4 | 69.3 ± 7.9 | 68.7 ± 6.9 | -0.5 | F (3.27)=0.9 |
| P=0.5 | |||||||||||||
| Aerobic fitness | |||||||||||||
| Lipox max (mg min) | 127.9 ± 7.5 | 156.1 ± 5.2** | 22.19 | 128.03 ± 3.6 | 172.4 ± 5.65** | 34.7 | 135.6 ± 14.6 | 145.11 ± 9.8 | 7.5 | 130.75 ± 9.7 | 131.5 ± 6.7 | 0.49 | F (3.27)=45.5 |
| $ | $ | P=0.00 | |||||||||||
| MAS (km/h) | 10.25 ± 0.59 | 11.12 ± 0.35* | 8.7 | 9.71 ± 0.39 | 10.71 ± 0.4** | 10.4 | 10.3 ± 0.51 | 10.6 ± 0.37 | 3.1 | 9.62 ± 0.51 | 9.81 ± 0.37 | 2 | F (3.27)=7.8 |
| $ | $ | P=0.001 | |||||||||||
| VO2max | 39.11 ± 3.31 | 44.51 ± 2.65** | 14.1 | 36.14 ± 2.18 | 41.68 ± 2.17** | 15.1 | 39.8 ± 2.85 | 41.53 ± 2.06 | 4.6 | 36.33 ± 2.1 | 36.68 ± 2.06 | 0.99 | F (3.27)=11.9 |
| (ml-min kg) | $ | $ | P=0.006 |
Table 4: Main variables measured during 6-min walking test (6 MWT) and Aerobic fitness parameters measured before and after the rehabilitation programs; Values are mean ± SD; 6MWTD: 6 Min Walk Test Distance; SO2: Oxygen saturation; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; MAS: Maximal Aerobic Speed; VO2max: Maximal Oxygen Consumption; *Significantly different from baseline at p<0.05; ** Significantly different from baseline at p<0.01; $ significantly different from control (P < 0.05).
The one-way ANOVA showed significant inter-group differences in 6MWD (F (3.27)=3.91, p=0.019) HR at rest (F(3.27)=18.2 ,P=000) and mean HR during the 6MWT (F(3.27)=5.7, p=0.03).
There were significant improvements of maximal aerobic speed in 50% MAS and 70% MAS groups (Table 4) (F=28.1, p=0.02, η²=0.8; and F=49.2, p=0.01, η²=0.84 respectively), associated with increases in estimated VO2max (F=28.25, p=0.01, η²=0.9; and F=28.1, p=0.002, η²=0.8 respectively). The calculated maximal fat oxidation (Lipox max) was also increased after rehabilitation in these same 2 groups (F=23.5, p=0.01, η²=0.97, and F=24.7, p=0.01, η²=0.97 respectively).
Changes in perceptual data
Changes in RPE and Hooper scores are summarized in Table 5. Perceptions did not differ among groups at baseline. However, the 2-month rehabilitation program induced significant decrease in RPE values in the 50% MAS and 70% MAS groups (50% MAS: F=10.8, p=0.01, η²=0.64; 70% MAS group: F=6.2, p=0.047, η²=0.5), with parallel changes in Hooper scores (F=23.8, p=0.003, η²=0.79; and F=13.8, p=0.010, η²=0.6 respectively).
| Group walking at 50% MAS | Group walking at 70% MAS | Group walking at self-selected walking pace | Control group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | %Δ | Pre | Post | %Δ | Pre | Post | %Δ | Pre | Post | %Δ | 1way | |
| ANOVA | |||||||||||||
| RPE | 3.7 ± 0.75 | 3.14 ± 0.37* | -12.8 | 3.8 ± 0.37 | 3.28 ± 0.75* | -13 | 3.9 ± 0.89 | 3.7 ± 0.95 | -5.4 | 3.8± 0.35 | 3.9 ± 0.53 | 2 | F=0.14 |
| P=0.93 | |||||||||||||
| Hooper | 14.57 ± 2.99 | 11.28 ± 1.1** | -29.2 | 15.57 ± 5.09 | 10.57 ± 1.81** | -28.5 | 14.14 ± 2.7 | 13.7 ± 4.9 | -13 | 16 ± 1.69 | 14.75 ± 2.25 | -6 | F=3.9 |
| Index | $ | $ | P=0.02 | ||||||||||
| Rating of sleep | 4.57 ± 1.46 | 2.6 ± 0.71 ** | -32.3 | 3.85 ± 1.46 | 2.42 ± 1.13** | -32.3 | 4.14 ± 0.89 | 2.4 ± 0.5** | -40.2 | 3.25 ± 1.28 | 3.75 ± 1.28 | 30 | F=5.04 |
| $ | $ | $ | P=0.007 | ||||||||||
| Rating of stress | 3.66 ± 0.86 | 1.85 ± 0.37** | -47.8 | 4.85 ± 1.46 | 2.42 ± 0.78** | -43 | 4.85 ± 0.69 | 3 ± 1.15 | -36.6 | 4.12 ± 1.64 | 3.75 ± 0.88 | -19 | F=4.06 |
| $ | $ | P=0.018 | |||||||||||
| Rating of fatigue | 3.6 ± 1.5 | 3 ± 0.75 | -8.7 | 3.14 ± 1.04 | 2.85 ± 0.37 | -2 | 4.8 ± 0.3 | 3 ± 1 | -38 | 3.25 ± 1.16 | 3.37 ± 0.74 | 22.5 | F=2.37 |
| P=0.09 | |||||||||||||
| Rating of muscle soreness | 4 ± 1.51 | 3.12 ± 0.64 | -12.2 | 3.85 ± 1.95 | 2.57 ± 0.78 | -10 | 4.42 ± 1.27 | 3.57± 1.26 | -15.2 | 3.37 ± 1.4 | 3.6 ± 0.74 | 29.7 | F=1.2 |
| P=0.32 |
Table 5: Perceptual and psychological data for participants before (pre) and after (post) rehabilitation; Values are means ± SDs; RPE: Rating of perceived exertion on Borg CR 1-10 scale; *significantly different with pre-test at p<0.05; **significantly different with pre-test at p<0.01; $ significantly different from control (P < 0.05).
The main finding from the present study was that either walking at 50% MAS or at 70% MAS was an effective short-term tactic for improving body composition, heart rate variability, aerobic fitness, and perceptual scores in over-weight and obese adolescents. However, the slower self-selected walking induced only changes in body composition and 6 MWT performances.
The four groups did not differ in baseline characteristics. However, over the 2-month intervention, the three experimental groups all showed decreases in body mass, BMI, and body fat, whereas the control group showed no changes in anthropometric parameters. The 70% MAS group also showed reductions in Waist circumference and W/H ratio. The intensity of effort corresponding to maximal fat oxidation was predicted from the equation of Makni et al. A previous study highlighted the importance of training at the Lipox when seeking to enhance body composition in obese children [29]. Some have suggested that the self-selected walking pace is an effective approach to preventing and correcting obesity in youth [30,31] found that this mode of locomotion was inversely associated with BMI z-score and skin folds. [32] also found beneficial changes in body composition and lipids profile in children after 12-month aerobic training based on walking and jogging. Exercise training can modify walking kinematics, with reductions in the energy cost of exercise in obese adolescents [33]. In a recent study, [34] reported a reduction of body fat, and an increase of fat-free mass, associated with reductions in c-reactive protein and insulin resistance in overweight girls after 12-months of aerobic (walking/running) and resistance exercises. In our study, changes in body composition after the intervention were associated with increases in the distance covered during the 6 MWT in all 3 experimental groups. The 50% MAS and 70% MAS groups also showed significant gains in MAS, estimated VO2max, and calculated Maximal fat oxidation. In the same context, Lopes et al (2016) found an increase in peak VO2, 1 RM for leg and bench press after combined training in adolescents.
Obesity is often linked with cardiac dysfunction [35], characterized by decreases in heart rate variability. After training, we found a decrease in HR values during the recovery period, with significant increases in HF and decreases in LF indices in both 50% MAS and 70% MAS groups. In contrast, the LF/HF ratio decreased only in the 50% MAS group. Moreover, we observed an increase in SD1, as a geometric parameter of the Poincare plot. However, the control group showed no changes in HR values or HRV indices.
In agreement with the literature, we found a decrease in resting HR and in recovery HR data after the 6 MWT in response to 50% MAS or 70% MAS rehabilitation. In addition, post-hoc analyses showed statistical differences between HR values for the control and intervention groups after the rehabilitation. During the post-6 MWT recovery period, there was also a tendency to an increase in R-R intervals after the intervention, but this did not reach statistical significance. There was a significant decrease in the LF/HF ratio for the 50% MAS group only, a decrease of LF and an increase in HF power in both 50% MAS and 70% MAS groups after rehabilitation, indicating an increase of cardiac parasympathetic activity. Little is known about how various walking pace exercise programs modify HRV in adolescents, although some studies have shown changes in autonomic balance after endurance training [26,36]. However, the present findings are walking programs can be effective in this regard, if pursued at 50 % MAS or 70 % MAS intensities.
The 2-month rehabilitation program also induced significant decreases in RPE and in Hooper scores for the 50% MAS and 70% MAS groups, probably reflecting improvements of aerobic fitness [23]. It has underlined relationships between the improvement in metabolic profile, cardio respiratory fitness and parasympathetic nervous system activity, and these adaptations could affect perceptual responses of the subjects.
We can assert that rehabilitation programs based on exercise at 50% MAS and 70% MAS intensities were effective tactics to induce positive changes in body composition, heart rate variability, aerobic fitness and perceptual responses. The self-selected walking pace also produced some positive changes, including a reduction of body fat and an increase of 6 MWT walking distance, but was not intense enough to induce significant changes in other anthropometric parameters, HRV, or aerobic fitness. However, self-paced effort could be used in the initial phases of rehabilitation to habituate obese adolescents who are interested in a rehabilitation program. Walking is a natural activity that they could practice at any time. Subsequent use of higher intensity effort, such as 50% MAS or 70% MAS walking, offers larger healthrelated fitness benefits, with a likely impact on a subject’s motivation to continue rehabilitation. The program adopted in the present study was quite varied, including a warm-up based on walk/moderate run, ball games, and stretching; the main phase of rehabilitation comprised 2 × 20 min walks interspersed with maximal sprints. The injection of sprints is efficient not only in the view of short term motivation and long-term adherence, but also metabolically [37], stimulating the quasi-totality of the muscle fibers. In addition, the intensity of 70% MAS adopted in the present study was quiet close to the intensity that favorites maximal fat oxidation when using the predictive equation proposed by Makni et al. [22] on young obese group similar to the present sample.
Conclusions from the present study face several obvious limitations. Firstly, the sample size was relatively small, limiting the statistical power of inter-group comparisons. Secondly, respiratory frequency can affect HRV modulation, and this parameter was not controlled in the present study. Thirdly, although dietary counseling was offered, there was no deliberate attempt to reduce energy intake; given that obesity reflects an imbalance between dietary intake and energy expenditure, it will be interesting to combine future walking programs with specific dietary interventions to see whether the changes that we observed are enhanced by caloric restriction. These various limitations highlight a need for further studies with a larger sample size, although the reductions of HR at rest and post-exercise seem in line with enhancement of the activity of the parasympathetic system and a decrease of the activity of the sympathetic one after training.
In summary, 50% MAS and 70% MAS rehabilitation programs had similar effects. Both modes of exercise induced significant improvements in body composition, autonomic cardiac control, and aerobic fitness, with decreases in RPE and Hooper scores. Given that rapid walking (close to the estimated maximal fat oxidation rate) is effective, it seems a good tactic to prevent and/or reduce obesity in adolescents.
The authors thank the Ministry of Higher Education and Scientist Research for the financial support of the study. We also thank the participants and parents for their efforts.
Conflict of interest
All the authors declare that he has no conflict of interest.
Ethical approval
This study was approved by the local ethical committee of the Hospital Farhat Hached of Sousse in accordance with the 1964 Helsinki Declaration. We also obtained the approval of the Regional Administration of Education and the Ministry of Health of Tunisia.
Informed consent
Informed consent was obtained from all participants and their parents in this study.