Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2020)Volume 11, Issue 6
Background: Phototherapy is one of corner stone therapeutic lines in dermatology, and narrowband UVB (NB- UVB) type is one of used treatment in many dermatoses including mycosis fungoides (MF).
Objective: In this study, we analysed the effect of NB-UVB in the treatment of patients with early stage MF.
Methods: The response of 29 patients (8 stage I A, 18 stage I B and 3 stage IIA) with patch stage MF to NB-UVB phototherapy, three times weekly was evaluated. Seventeen patients had skin type III, eleven patients had skin type IV and one patient had skin type II). Clinically the studied MF patients were presented with hypo-hyper pigmentation, poikiloderma and eczematous skin lesions. Mean follow-up period was 3.6 year.
Results: The 29 patients had complete clinical remission; the minimum number sessions for complete remission was 25 sessions and minimum cumulative dose was 18 joule. There are 18(62.1%) patients did not get relapse after remission, while the rest (37.9 %) have relapse after remission. Reported side effects were, itching in 10 (34.5 %) patients, erythema in 9 (31.0%), burning sensation in 4 (14.8%) and 6 (20.7%) patients did not have any side effect.
Conclusion: MF is a cutaneous -T- cell lymphoma, that may be hard to make a diagnosis at early stage, as skin lesions mimic some benign dermatoses, like eczema or psoriasis. Phototherapy is one of tolerated treatment modalities in M.F, especially NB-UVB, can be considered a safe and effective treatment for early stage MF (patch and plaque), even at long term.
Narrowband UVB light; Phototherapy; Cutaneous T-cell lymphoma
Mycosis Fungoides (MF), also known as Alibert-Bazin syndrome, is non-Hodgkin lymphoma, it is the most common lymphoma affecting the skin [1]. MF primarily starts in the skin, it originates mainly from T-cell (75%) and uncommonly from B-cell. MF is a rare T-cell lymphoma variant, as it representing 2% of all lymphomas with 0.3 to 1 per 100 000 incidence of population [2,3].
Many ways for staging MF have been proposed, a simple and widely used system was established by Bunn et al, which combines both clinical and histopathological presentations of MF. It is including Tumor-Node Metastasis (TNM) system [4]. Early stages MF have indolent clinical behaviour, as it is lifelong disorders that recur after discontinuation of therapy, even in cases that do not progress. These stages include involvement of the skin in the form of patches, papules, or plaques (T1–T2) without tumors or erythroderma, clinical lymph node involvement (nodes larger than 1.5 cm), confirmed or not by histology – and if confirmed does not exceed grade 2 of the Dutch histopathology scale (LN0-LN1), and absence of visceral metastases. The diagnosis of early-stage MF is sometimes difficult; but the TNM system makes the diagnosis of early-stage MF more easy (Table 1) [4]. Patches and plaques may affect any area of the skin but they usually start in the lower trunk involving the bathing suit area (hips, buttocks and groin) then axillae and breast in an asymmetrical distribution. The plaques are flat faint erythematous or brown in colour with fine scales associated with pruritus which varies in severity. In dark skin people the patches can appear as hypopigmented lesions. The patches progress to infiltrative plaques with time [3]. The early stages MF has a favourable prognostic outcome for patients, it includes Stages IA, IB, and IIA according to ISCL–EORTC in 2007 [5]. Treatment of early –stages MF, is selective to skin in cases with limited surface area involvement. Topical potent corticosteroids are one of the best options in early stage limited cutaneous MF, as they induce complete remissions of MF patches in 60%–65% [6,7]. Topical Nitrogen mustard or carmustine (metochlorethamine) is another topical agent used in MF, unfortunately the use of these topical agent is limited by their side effects like irritation, skin atrophy and even development of cutaneous malignancy [8]. In addition to side effects, relapse of cutaneous lesions following complete clearance was observed in 67.7% in patients with MF treated with nitrogen mustard [9].
| BL system | Clinical Findings | TNM system staging |
|---|---|---|
| IA | < 10% BSA patch/plaque stage | T1N0 |
| IB | > 10% BSA patch/plaque stage | T2N0 |
| IIA | Patch or plaque stage with palpable nodes Without histological involvement |
T1/2N1 |
| IIB | Cutaneous tumours with or without palpable nodes |
T3N0/1 |
| III | Erythroderma with or without palpable nodes |
T4N0/1 |
| IVA | Non-palpable or palpable nodes with Histological involvement | T-any N2/3 |
| IVB | Visceral involvement | T-any N-M1 |
Table 1: Staging to MF patients done according to Bunn and Lambert system [4].
Psoralen plus ultraviolet A (PUVA) or ultraviolet B (UVB) are used for treatment for wide spread early stage MF. Although the success rate of treatment with PUVA in MF and it is superior to UVB in control of MF as, it showed response in approximately 95% of treated patients comparable to 75%–83% UVB treated patients [10], however, PUVA may be untolerated to some patients because of its serious side effects, especially with long–term use as, nonmelanoma skin cancers like squamous cell carcinoma as well as melanoma may develop [11]. Some PUVA treated patients also, may be uncompliant at starting of treatment due to nausea and phototoxicity as side effect of oral psoralen [12]. UVB in treatment of early stage MF is safe and effective way when it is comparable to PUVA, and even maintenance sessions may not needed to keep the clearance of MF lesions (Figures 1 and 2) [13,14].
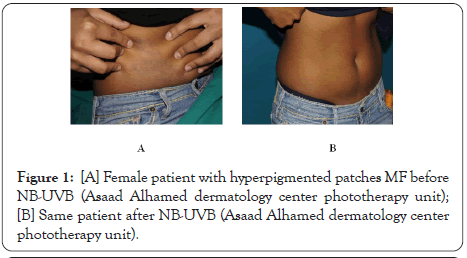
Figure 1: [A] Female patient with hyperpigmented patches MF before NB-UVB (Asaad Alhamed dermatology center phototherapy unit); [B] Same patient after NB-UVB (Asaad Alhamed dermatology center phototherapy unit).
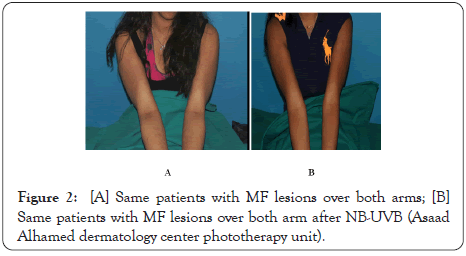
Figure 2: [A] Same patients with MF lesions over both arms; [B] Same patients with MF lesions over both arm after NB-UVB (Asaad Alhamed dermatology center phototherapy unit).
Patient's selection
This study is a retrospective study and emphasizes our experience with MF treated patients with NB-UVB at the phototherapy unit of the Dermatology Department of A'sad Alhamed Center. A'sad Alhamed Center is a tertiary dermatological clinical and academic center in Kuwait. The list of followed up patients in our center from January 2013 to December 2018 was obtained from medical records department. The files were reviewed thoroughly to collect all information needed and detailed history for patients, in a specially designed collection tool. The collection tool includes the followings:
• Demographic data of MF patients e.g. age, sex.
• Clinical presentation of MF was taken and final diagnosis done with skin biopsy for H& E and immune-histochemical study to confirm MF diagnosis. Clinical data are evaluated such as, skin type, co-morbidities, presence of relapse and duration of MF (the date of starting of skin lesions, until they presented to dermatologist).
• Full blood chemistry was done for all patients including, CBC (differential count from peripheral blood smear) admission profile and a referral to ophthalmological examinations. Then the patients sent to KCC (Kuwait cancer center) for U/S to the neck, axillae, abdomen and pelvis for staging of MF and screening for any internal involvement. In cases of any U/S findings, CT would be done and if any lymph node enlargement fine needle aspiration done.
• Past and family history of MF or other malignancies were reported.
• Side effects of phototherapy were reported according to each patient.
• Total number of MF patients
The total number of MF patients in our study was 40 at the first stage, out of whom 11 were excluded. The 11 patients were excluded for the followings reasons: five patients were in stage IIA stage so they started on oral PUVA, due to wide spread surface area of skin involved with palpable lymph nodes, two patients were in IIA stage, they started on oral PUVA and systemic methotrexate, one patient was in stage IB poikiloderma who started on UVA1, one patient started on topical PUVA, while two patients were stage IB who shifted to oral PUVA after 20 sessions of UVB due to lack of response to UVB sessions.
Phototherapy unit
Therapy was given in UV phototherapy unit in our center. Patients were exposed to NB-UVB radiation from fluorescent tubes lamps with 120 watt. There are 9 cabinets are available for therapy for NB-UVB, the spectral distribution measurement were performed with a calibrated spectro-radiometer (S.N, 75700-13 and 75702-13 Waldmann UV7002).
Table 2 shows distribution of the studied cases according to some qualitative variables as follow, the total MF 29 patients included in the study 18 (62.1%) patients females and 11 (37.9%) males. only one (3.4%) patient was Skin type II, while 58.6% type III, and 37.9% type IV, 11 patient.
| n | (%) | ||
|---|---|---|---|
| Sex | F | 18 | (62.1) |
| M | 11 | (37.9) | |
| Skin type | II | 1 | (3.4) |
| III | 17 | (58.6) | |
| IV | 11 | (37.9) | |
| TNM | IA | 8 | (27.6) |
| IB | 18 | (62.1) | |
| IIA | 3 | (10.3) | |
| CO | EcP | 1 | (3.4) |
| EPQ | 3 | (10.3) | |
| HM | 2 | (6.9) | |
| HP | 14 | (48.3) | |
| HSM | 1 | (3.4) | |
| hYp PQ | 4 | (13.8) | |
| HypP | 2 | (6.9) | |
| HYPP | 1 | (3.4) | |
| POIL | 1 | (3.4) | |
| CO2 | EcP | 1 | (3.4) |
| EPQ | 3 | (10.3) | |
| HM | 2 | (6.9) | |
| HP | 14 | (48.3) | |
| HSM | 1 | (3.4) | |
| hYp PQ | 4 | (13.8) | |
| HypP | 2 | (6.9) | |
| HYPP | 1 | (3.4) | |
| POIL | 1 | (3.4) | |
| RELAPS | No | 18 | (62.1) |
| Yes | 11 | (37.9) | |
| OTHERM | No | 28 | (96.6) |
| Yes | 1 | (3.4) | |
| FHM | No | 28 | (96.6) |
| Yes | 1 | (3.4) | |
| SE | None | 6 | (20.7) |
| Itching | 10 | (34.5) | |
| Erythema | 9 | (31.0) |
TNM: Tumor-Node Metastasis system, CO: cutaneous presentations, HrP: Hyperpigmented Plaques, HM: Hypopigmented Patches, EcP: Erythematous Eczema like Plaques, HyPq: Hypopigmented Patches with Poikilodermatous changes, Poil: Poikilodermatous, OTHERM: Other Malignancy, FHM: Family History of other Malignancy, SE: Side Effects
Table 2: Distribution of the studied cases according to some qualitative variables.
The cutaneous presentations of the studied cases were five different variants as, 7 (24.1%) patients with hypopigmented patches, 14 (48.3%) patients with hyperpigmented plaques, and 3 (10.3%) patients with erythematous eczema like plaques, and 4 (13.8%) patients with hypopigmented patches with poikildermatous changes, and one (3.4%) patient with limited area of poikilodermatous changes. only Concerning the TNM staging, our cases included 18 (62.1) in stage IB, 8 (27.6 %) IA and 3 (10.3) IIA.
Regarding relapses of cutaneous lesions after complete clearance of MF with NB- UVB, patients did not get any relapse during doing treatment sessions. However, after discontinuation of phototherapy sessions, the patients were under follow up for one year off NB-UVB treatment, there relapse was in only 11 (37.9%) patients, while 18 (62.1%) patients had not any relapse.
Past history of other malignancy in MF patients was found only in one (3.4%) who had uterine carcinoma. Regarding family history of other malignancy, two (6.8%) patients had positive family history, one patient her uncle has lung cancer and her grandmother had breast cancer, and the other her mother had lymphoma, however the 27 (93.2%) did not have any other malignancy, and no family history of MF.
The side effects, some patients got adverse reaction to phototherapy sessions which were, itching in 10 (34.5%) patients, erythema in 9 (31.0%) patients, and burning sensations in 4 (13.8%) patients, while 6 (20.7%) patients did not complaint of any side effects during phototherapy treatment (Table 2).
It was observed that the age of the studied patients ranged between 4 to 86 years, with mean 34.55 years.
The duration of MF (onset of the skin lesions according to patients) ranged between 1 and 18 years, with mean 6.83 years. The minimum follow up period (since the confirmed diagnosis of the MF by histopathology and immune-histochemical study) was one year and the maximum was 11 years with a mean 3.66 years. Our studied patients had CR1(complete remission number of session) after minimum 22 sessions of NB-UVB, and CR2 (complete remission total dose) minimum was 34 joule dose of UVB, and CR1 maximum 119 sessions and CR2 maximum was 324 joule. The mean number of sessions was 62.59 sessions, and the mean dose of complete remission for UVB was 139.71 joule.
The complete remission duration (RMDUR) minimum was 2 months, while the maximum duration for complete remission was 26 months, with a mean 6.72 month.
The partial remission dose (PRDOSE) the minimum was 2.21 joule, the maximum dose for partial remission was 32.40 joule with a mean dose 15.63 joule , while, the partial remission number of session (PRNUM) the minimum was 7 sessions and the maximum number of sessions for partial remission was 51 with mean 20.59 session (Table 3).
| Studied variables | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|
| Age | 4 | 86 | 34.55 | 20.526 |
| Duration | 1 | 18 | 6.83 | 3.992 |
| Follow up | 1 | 11 | 3.66 | 2.468 |
| CR1 | 22 | 119 | 62.59 | 28.89 |
| CR2 | 34 | 324 | 139.71 | 83.40 |
| RMDUR | 2 | 26 | 6.72 | 5.424 |
| PRDOSE | 2.21 | 32.40 | 15.63 | 8.14 |
| PRNUM | 7.00 | 51.00 | 20.59 | 12.47 |
CR1 (complete remission number of session), CR2 (complete remission total dose), RMDUR (complete remission duration), PRDOSE (The partial remission dose), PRNUM (partial remission number of session).
Table 3: Minimum, maximum, mean and standard deviation of some studied quantitative variables.
Table 4 shows comparison of some studied patients according to skin type. There was no statically difference of these variables when skin type was considered, as shown by different p-values of K-W test. The highest median value of complete remission duration was observed in relation to type IV followed by type II then type III, however, these differences were not statistically significant as P=0.340. On the other-hand ,the comparison of other studied variables (age of patients, duration of MF, follow up period, number of sessions for partial or complete remission and UVB doses for partial or complete remission) according to skin type, there were no statically significant difference of theses variables as shown by P-values of K-W test. However a highest median value of RMDU was observed in relation to skin type 4, followed by skin type 2, then skin type 3 came the last, but these differences was not statically significant as P=0.340 (Table 4).
| Studied variables | Skin type 2 n=1 |
Skin type 3 n=17 |
Skin type 4 n=11 |
Chi-Square (2) | p |
|---|---|---|---|---|---|
| Median (Q1-Q3) | Median (Q1-Q3) | Median (Q1-Q3) | |||
| Age | 12 (---) | 31 (18-44.5) | 31 (24-54) | 2.425 | 0.297 |
| Duration (year) | 9 (---) | 6 (4-7.5) | 6 (3-12) | 1.314 | 0.518 |
| Follow up (year) | 8 (---) | 3 (2-5) | 3 (1-4) | 2.198 | 0.333 |
| CR1 | 117 (---) | 54 (29-89) | 67 (48-80) | 2.954 | 0.228 |
| CR2 | 90 (---) | 84.5 (65-205.5) | 117 (105-235) | 0.614 | 0.736 |
| RMDUR (month) | 5 (---) | 4 (4-6) | 6 (4-9) | 2.158 | 0.340 |
| PRDOSE | 26.35 (---) | 12.84 (10.22-15.86) | 15.86 (7.40-26.80) | 1.596 | 0.450 |
CR1 (complete remission number of session), CR2 (complete remission total dose), RMDUR (complete remission duration), PRDOSE (The partial remission dose), PRNUM (partial remission number of session).
Table 4: The relation between skin type and some studied quantitative variables.
A comparison of some studied cases according to TNM grading, the highest CR2 (complete remission cumulative dose) median (139 joule) was observed in relation to TNM3 followed by TNM1 (121.50 joule) and then the last was TNM2 as CR2 median (115 joule). These were not statistically significant difference observed (P=0.880) although P value near to be significant which is the same for PRDOSE as P value was 0.885. Otherwise all studied variables were not statistically significant in relation to TNM grading system (Table 5).
| Studied variables | TNM1 n=8 |
TNM2 n=18 |
TNM3 n=3 |
Chi-Square (2) | p |
|---|---|---|---|---|---|
| Median (Q1-Q3) | Median (Q1-Q3) | Median (Q1-Q3) | |||
| Age | 36.5 (24.75-51.75) | 31 (18-42.5) | 18 (10- ---) | 1.032 | 0.597 |
| Duration | 6 (3.5-7.75) | 6.5 (4.5-9.25) | 4 (3- ---) | 1.558 | 0.459 |
| Follow up | 3.5 (1.25-4.75) | 3 (2-5) | 3(1- ---) | 0.454 | 0.797 |
| CR1 | 71.00 (38.25-79.50) | 55.50 (35.25-90.50) | 58.00 (28.00- ---) | 0.786 | 0.675 |
| CR2 | 121.50 (53.75-249.25) | 115.00 (71.50-191.75) | 139.00 (105.00- ---) | 0.256 | 0.880 |
| RMDUR | 5 (4-20.75) | 6 (4-6) | 6 (4- ---) | 0.473 | 0.789 |
| PRDOSE | 12.22 (8.20-24.07) | 13.03 (10.75-23.80) | 15.86 (2.21- ---) | 0.245 | 0.885 |
| PRNUM | 15.50 (10.50-41.25) | 18.00 (10.75-25.75) | 12.00 (10.00- ---) | 0.685 | 0.710 |
CR1 (complete remission number of session), CR2 (complete remission total dose),
RMDUR (complete remission duration), PRDOSE (The partial remission dose),
PRNUM (partial remission number of session), TNM (tumour staging).
Table 5: The relation between TNM grading and some studied quantitative variables.
Spearman's correlation matrix among some studied quantitative variables was done (Table 6).
| Follow up | Age | Duration | CR1 | CR2 | RMDUR | PRDOSE | ||
|---|---|---|---|---|---|---|---|---|
| Age | r | -0.054 | - | - | - | - | - | - |
| P | 0.781 | - | - | - | - | - | - | |
| Duration | r | 0.530** | -0.341 | - | - | - | - | - |
| P | 0.003 | 0.070 | - | - | - | - | - | |
| CR1 | r | 0.228 | -0.216 | 0.108 | - | - | - | - |
| P | 0.234 | 0.260 | 0.579 | - | - | - | - | |
| CR2 | r | 0.099 | 0.053 | -0.115 | 0.212 | - | - | - |
| P | 0.609 | 0.785 | 0.553 | 0.270 | - | - | - | |
| RMDUR | r | 0.277 | -0.119 | 0.321 | 0.165 | -0.165 | - | - |
| P | 0.146 | 0.539 | 0.089 | 0.392 | 0.393 | - | - | |
| PRDOSE | r | -0.090 | 0.013 | 0.006 | 0.238 | 0.177 | 0.236 | - |
| P | 0.642 | 0.948 | 0.976 | 0.214 | 0.359 | 0.217 | - | |
| PRNUM | r | 0.102 | -0.190 | 0.137 | 0.640 | -0.111 | 0.578 | 0.335 |
| P | 0.598 | 0.324 | 0.480 | 0.000 | 0.565 | 0.001 | 0.076 |
CR1 (complete remission number of session), CR2 (complete remission total dose),
RMDUR (complete remission duration), PRDOSE (The partial remission dose),
PRNUM (partial remission number of session), TNM (tumour staging).
Table 6: Spearman's correlation matrix among some studied quantitative variables.
It was found that there were statistically significant positive (or direct) correlation between RMDUR and PRNUM (r=0.578, p=0.001), between CR1 and PRNUM (r=0.640, p<0.001), and lastly between Duration and Follow up (r=0.530, p=0.003).
There were no other statistically significant correlations between each two variables as shown by the different p-values of the Spearman's rho correlation coefficients in the table.
Ultraviolet-based therapy (phototherapy) is one of successful ways in control of itchy skin dermatoses [15]. Phototherapy also is an effective method in other chronic dermatoses like psoriasis, vitiligo, cutaneous T-cell lymphoma, parapsoriasis, and eczemas, in which phototherapy provided a good outcome [16] NB -UVB may have a bigger role in treatment of different skin diseases and at same time it appears to be equally effective or even more effective than broadband UVB [17]. NB-UVB is safe therapy with tolerated adverse effects and even it accepted treatment for pregnant women and children with generalized vitiligo [18]. Many previous studies showed that NB-UVB therapy is an effective and safe treatment with the effect lasting for months for patients with early-stage MF [19-21].
Our study evaluates the efficacy of NB-UVB in 29 patients, regarding complete remission (CR1) the minimum duration to achieve complete remission was 2 months, and the maximum duration was 26 months with average 6.7 months, when we compare this result with previous study which showed that, the average duration needed with NB-UVB treatment for complete clearance was 32 weeks (about 8 months), both results are comparable [22]. Another study showed the percentage of patients achieved complete clearance, as sixtyeight patients stage IA (84%) had achieved complete response (CR) after treatment with NB-UVB [23]. In our study 8 patients stage IA (27.5% of total pt.) had achieved complete remission (CR1) within 5 months (mean duration), and eighteen patients stage IB (62% of total pt.) had achieved complete remission within 6 months (mean duration), and 3 patients stage IIA (10.3 %) had achieve CR1 also within 6 months. Our MF patients with different stages whatever IA, IB, or IIA had achieved 100% complete remission which is higher than previous study, that showed 84% only had achieved complete response [23], this explained, as we already had excluded some cases of early stage MF, (as our recorded cases were 40 patients) that already had started on NB–UVB and then discontinued the treatment after lack of response to NB-UVB, and shifted to PUVA alone or with systemic treatment depending on staging. There was no statistical difference between NB-UVB and PUVA treatment in early stage MF (stage IA, IB) after 48 sessions at both clinical and histopathological side, and UVB-NB was as beneficial as PUVA in the treatment of early stages of MF (stages IA and IB) ted [24]. Relapse rate is very important, as an indicator for effectivity of any treatment, in MF patients treated with NB-UVB , the relapse rate was high, as of 6 patients with complete response,3 (50%) relapsed within 14 months as average after discontinuation of NB-UVB [25,26]. In our study, 11 patients (37.9%), had relapse, while 18 patients (62.1%) had not any relapse after complete remission, and discontinuation of treatment within 18 months of follow up. So our patients had showed better response to UV-UVB regard relapse rate, this may due that our patients exposed to sun rise as a natural source of ultraviolet light. In our study there were no statistically difference between the complete remission sessions number, and complete remission cumulative dose (CR1, CR2) with TNM grades of MF and the studied quantitative variables for our patients, except with TNM3 (IIA) group with CR2 as which showed highest cumulative dose and p-value=0.013, although that the patients number at TNM3 are few in number three patients only which cannot be reliable for comparison. An objective finding in our study, which is a positive statistically significant relation between PRNUM and PRDOSE, RMDUR and PRNUM, as well as between CR1 and CR2.
The side effects reported in our studied patients to NB-UVB were minimal and tolerated, as 6 (20.7%) patients had no side effects, and others had minimal side effects as erythema, itching and burning sensations, these side effects are not comparable with those reported in patients treated with PUVA therapy, which have increased the risk of Non-Melanoma Skin Cancers (NMSC) treatment, specially increase in the incidence of cutaneous SCC.
MF is a cutaneous -T- cell lymphoma, it has indolent course as, the skin changes develop slowly, often over many years, and it may be hard to make a diagnosis at early stage, as skin lesions mimic some benign dermatoses, like eczema or psoriasis, later once the patches stage diagnosed or thickened to plaque stage, early stage MF diagnosis become well settled. Mycosis fungoides patients may need follow up for many years, if not decades, even with adequate treatment, so it is essential to manage it with suitable protocol and safe treatment. Phototherapy is one of tolerated treatment modalities in M.F, especially NB-UVB, can be considered a safe and effective treatment for early stage MF (patch and plaque), even at long term.
Citation: Almasry MI, Albathali AA, Alajmi H, Al-Lafi A, Sadek A, Lazarevic V, et al (2020) Efficacy and Safety of Narrowband-UVB in Early Stage Cutaneous T-Cell Lymphoma in Kuwaiti Patients. J Clin Exp Dermatol Res. 11:536.
Received: 21-Oct-2020 Accepted: 04-Nov-2020 Published: 13-Oct-2020 , DOI: 10.35248/2155-9554.20.11.536
Copyright: © 2020 Almasry MI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.