
Immunotherapy: Open Access
Open Access
ISSN: 2471-9552

ISSN: 2471-9552
Research Article - (2024)Volume 10, Issue 3
Background: After the failure of Transarterial Chemoembolization (TACE) combined with first-line Tyrosine Kinase Inhibitor (TKI) therapy, the optimal treatment for Hepatocellular Carcinoma (HCC) patients who have progressed is unclear. The study was conducted to investigate the safety and efficacy of subsequent Programmed Death-1 (PD-1) inhibitor combinations compared with switching to the subsequent regorafenib.
Methods: This retrospective study examined the data of patients with HCC who failed TACE combined with first-line TKI therapy. Progression-Free Survival (PFS) and Overall Survival (OS) were assessed as primary study outcomes. An analysis of survival curves using Kaplan-Meier method was conducted and log-rank tests were used to measure differences.
Results: We enrolled a final total of 113 patients, including 73 patients in Group 1 (TACE combined with first-line TKI and PD-1 inhibitors) and 40 patients in group 2 (TACE plus regorafenib). The OS in group 1 (15.0; 95% Confidence Interval (CI), 9.8-20.1 months) was significantly longer compared with group 2 (9.0; 95% CI, 6.6-11.3 months) (log-rank p=0.016). The PFS in group 1 (11.0; 95% CI, 8.4-13.5 months) was also significantly longer compared with group 2 (6.0; 95% CI, 4.6-7.3 months) (log-rank p=0.010). The Overall Response Rate (ORR) (p=0.562) and Disease Control Rate (DCR) (p=0.202) did not differ significantly between groups; however, the percentage of patients with proteinuria in group 1 was significantly lower compared to group 2 (2.73% vs. 20.00%, p=0.006).
Conclusions: After the failure of TACE plus first-line TKI, the subsequent combining PD-1 inhibitors may result in improved OS and PFS compared to switching to subsequent regorafenib.
Transcatheter arterial chemoembolization; First-line tyrosine kinase inhibitors; Regorafenib; Programmed death-1 inhibitors; Unresectable hepatocellular carcinoma
TACE: Transarterial Chemoembolization; TKI: Tyrosine Kinase Inhibitor; PD-1: Programmed Death-1; HCC: Hepatocellular Carcinoma; PFS: Progression-Free Survival; OS: Overall Survival; BCLC: Barcelona Clinic Liver Cancer; ECOG-PS: Eastern Cooperative Oncology Group Performance Status; ALBI: Albumin–Bilirubin; TRAEs: Treatment-Related Adverse Events; CR: Complete Response; PR: Partial Response; ORR: Overall Response Rate; DCR: Disease Control Rate
Hepatocellular Carcinoma (HCC) is the sixth-most common malignancy, as well as the third-leading cause of cancer death, accounting for 8.3% of all cancer deaths worldwide [1]. Further, the majority of new patients with HCC are diagnosed with advanced stages, which renders them ineligible for curative resection [2]. Patients with HCC are usually treated with TACE and systemic therapy [3]. Based on the Barcelona Clinic Liver Cancer (BCLC) staging system, for HCC patients in BCLC stage B, TACE is the standard of care, while for HCC patients with BCLC stage C, systemic treatment including Tyrosine Kinase Inhibitors (TKI) is the standard of care [4,5].
In spite of the fact that TACE and systemic treatment are both beneficial to survival, TACE alone usually causes incomplete necrosis, which ultimately makes TACE less effective [6]. Meanwhile, systemic treatment easily results in intolerance to the medication or a failed treatment response among patients with HCC [7]. Due to these limitations of TACE and systemic treatment alone, TACE combined with systemic treatment has become an important therapy for patients with unresectable hepatocellular carcinoma. Many studies have suggested that this combination treatment for HCC leads to improved outcomes. For example, a study revealed that the prognosis of patients treated with TACE combined with systemic treatment (namely, sorafenib/lenvatinib) was better than patients who were administered repeated TACE alone [8,9].
Unfortunately, resistance to first-line TKI will inevitably arise following a period of PFS in patients receiving first-line TKI in combination with TACE, resulting in an unfavorable outcome [10]. Currently, patients with progressive HCC do not yet have a universally accepted treatment [11]. However, several other treatment options are available [12-14]. For example, the approval of regorafenib was based on the efficacy data in which regorafenib monotherapy was demonstrated to have a survival benefit, extending the OS to 10.3 months [15]. This action is attributed to the fact that compared with first-line TKI such as sorafenib, the molecular target of regorafenib is unique and its pharmacological activity is stronger it can more efficiently impede protein kinase activity required for tumor immunity [16-18]. Additionally, for HCC patients after TACE plus first-line TKI therapy failed, TACE plus regorafenib has been approved for sequential treatment.
As another possible second-line choice of therapy for HCC patients after failure of TACE combined with first-line TKI, PD-1 inhibitors have been shown to improve survival in HCC patients [19]. The data from phase III trials have shown that as second-line therapy, PD-1 inhibitors prolong the OS to 13.9 months and the PFS to three months [20]. It is known that PD-1 inhibitors can modulate the tumor immune response and enhance immunity; thus, they can bring a survival benefit to HCC patients during second-line therapy [21]. However, for these patients, the survival obtained by PD-1 inhibitor monotherapy is still unsatisfactory [19]. Thereby, PD-1 inhibitors in combination with other treatments (such as TACE and TKI) are being considered for these HCC patients during second-line therapy; for instance, a recent study suggested that, as the second-line therapy for HCC patients, PD-1 inhibitors in combination with first-line TKI (e.g., sorafenib) can prolong the survival time (i.e., the median OS can reach 14.1 months and the median PFS can reach 5.3 months) [22].
The above contexts suggest that HCC patients with disease progression during TACE combined with first-line TKI, the subsequent combination of PD-1 inhibitors may provide a new approach to second-line therapy. In fact, researchers have documented that, for HCC patients with disease progression during first-line therapy, TACE combined with first-line TKI plus PD-1 inhibitors carries the potential to further improve the efficacy, leading to a significant increase in tumor response and survival benefit [23-25]. Therefore, it is believed that subsequent combining PD-1 inhibitors for HCC patients with disease progression during TACE combined with first-line TKI might result in synergistic anti-tumor activity, leading to improved clinical outcomes. Thus, the purpose of this study was to examine the effectiveness of the subsequent combination of PD-1 inhibitors for these HCC patients, by comparing their outcomes with switching to second‐line TKI therapy (such as regorafenib).
Patients
This retrospective study was endorsed by the Ethics Committee of Fujian Medical University Union Hospital, Fuzhou, China (No: 2022KY217) and was conducted according to the declaration of Helsinki. From July 2019 to August 2022, an initial cohort of 513 HCC patients was screened. The screening process was shown in Figure 1. Finally, 113 HCC patients with disease progression during TACE combined with first-line TKI were enrolled. It was stratified into 73 patients who were administered the subsequent therapy of TACE combined with first-line TKI plus PD-1 inhibitors (group 1) and 40 patients who were administered the subsequent treatment of TACE plus regorafenib (group 2).
The inclusion criteria for enrollment were: (1) Unresectable HCC; (2) Status after the failure of TACE combined with first‐line TKI therapy; (3) Receiving subsequent therapy (TACE plus regorafenib or TACE combined with first-line TKI and PD-1 inhibitors); (4) Child-Pugh class A or B; (5) Eastern Cooperative Oncology Group Performance Status (ECOG-PS) score ≤ 1 point and (6) BCLC stage B or C.
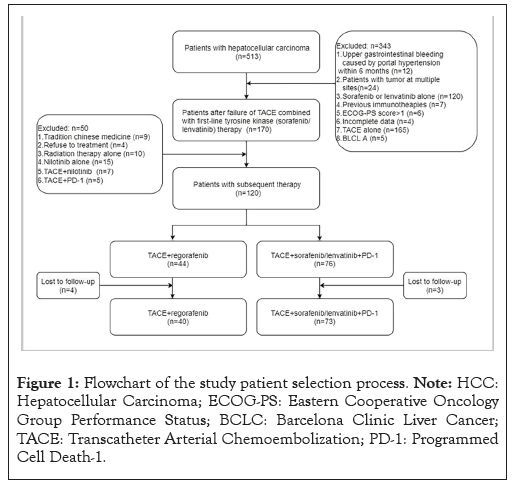
Figure 1: Flowchart of the study patient selection process. Note: HCC: Hepatocellular Carcinoma; ECOG-PS: Eastern Cooperative Oncology Group Performance Status; BCLC: Barcelona Clinic Liver Cancer; TACE: Transcatheter Arterial Chemoembolization; PD-1: Programmed Cell Death-1.
Meanwhile, the exclusion criteria were: (1) Tumors at multiple sites; (2) Immunotherapy alone; (3) Sorafenib or lenvatinib treatment alone; (4) Incomplete data (5) ECOG-PS score >1 point, (6) TACE treatment alone, (7) BCLC; (8) Other subsequent treatments; (9) Patients refusing subsequent treatment and (10) Contraindications for TACE, regorafenib or PD-1 inhibitor treatment.
We recorded patient demographic profiles, biochemistry data and tumor characteristics at baseline and the point of disease progression. The data of interest were: Age, sex, Child–Pugh score, etiology of cirrhosis, cirrhotic level, BCLC stage, extrahepatic metastasis, Albumin-Bilirubin (ALBI) grade, Protein Induced by Vitamin K Absence or Antagonist II (PIVKA-II), Alpha-Fetoprotein (AFP), Carbohydrate Antigen 199 (CA 19-9), Arteriovenous Fistula (AVF), Extrahepatic Collateral Arteries (ECAs), Gamma (γ)-Glutamyl Transferase (GGT), Aspartate Aminotransferase (AST), Prothrombin Time (PT), Alanine Aminotransferase (ALT), Alkaline Phosphatase (ALP). The ALBI grade was calculated and liver cirrhosis was defined as previously described [26,27]. The ALBI grade was proposed for assessing hepatic reserve function calculated using only total bilirubin and albumin. The calculation formula was shown below:
(log10 bilirubin (in µmol/l) × 0.66)+(albumin (in g/l) × -0.085): Grades 1,2,3 = ≤ -2.60, <-2.60 to ≤ -1.39, >-1.39 [26].
TACE plus regorafenib treatment
TACE procedure was conducted by experienced physicians within a 7-day period after diagnosis. TACE was conducted under local anesthesia. Following a successful femoral artery puncture, the tumor-supplying artery was super selected using a micro catheter and chemoembolization was carried out with a combination of lipiodol and epirubicin (30-50 mg/m2). Approximately every 6-8 weeks after TACE treatment, follow-up imaging examinations were performed to assess the outcomes of the treatment. If stable disease or Partial Treatment Response (PTR) were detected, TACE was repeated. One week after TACE, regorafenib (40 mg/pill; Bayer HealthCare AG, Leverkusen, Germany) was administered orally at a dosage of 160 mg daily. Regorafenib was administered for three weeks and was stopped for one week. Each four-week period comprised a treatment cycle.
The dosage of regorafenib was decreased to 80 mg per day in cases of grade 3 or grade 4 Treatment-Related Adverse Events (TRAEs). If the TRAEs did not disappear or decrease within the week after dose adjustment, the patient was counseled to discontinue regorafenib therapy until their symptoms had alleviated or resolved. When the toxicity was below the baseline level (according to the discretion of the investigator), the dosage was recovered to 160 mg daily.
TACE combined with first-line TKI plus PD-1 inhibitor therapy
In a follow-up procedure, the same TACE procedure was performed as mentioned above, followed by first-line TKIs (sorafenib/lenvatinib) and PD-1 inhibitors simultaneously. The dosage of lenvatinib was 12 mg daily (for body weights above 60 kg) or 8 mg daily (for body weights below 60 kg). Approximately 400 mg of sorafenib (200 mg/pill) was administered daily orally. PD-1 inhibitors were administered in the form of camrelizumab (200 mg/bottle) or sintilimab (100 mg/bottle) every three weeks intravenously.
The dose of lenvatinib was reduced in the event of grade 3 or 4 TRAEs to either 8 mg (for body weights over 60 kg) or 4 mg (for body weights beneath 60 kg). A dose reduction of sorafenib to 200 mg daily was performed until the TRAEs was eliminated or alleviated. When severe immune-related TRAEs were associated with PD-1 inhibitor therapy, corticosteroids were considered. Upon adjustment, sorafenib, lenvatinib and PD-1 inhibitor therapy should be discontinued when grade 3 or 4 TRAEs persists, after the toxicity had diminished the dose could be recommenced when the patient was able to tolerate it.
Treatment evaluation and follow-up
The OS and PFS were the primary outcomes of the study. The OS is calculated as the time between the beginning of subsequent treatment and the date of death. While PFS refers to the interval between the start of treatment and the first evidence of PD or death. Based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria, treatment responses were categorized [28]. The ORR refers to the sum of Complete Response (CR) and Partial Response (PR), while DCR refers to the sum of CR, PR and stable disease. Every six to eight weeks, imaging examinations (computed tomography or magnetic resonance imaging) were performed on patients to monitor their disease status. An assessment of TRAEs was conducted using the common terminology criteria for adverse events version 5.0.
Statistical analysis
Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) software version 25.0 (IBM Corporation, Armonk, NY, USA). Continuous variables are displayed as mean ± Standard Deviation (SD) values and categorical variables are expressed using numbers and percentages (n (%)). Continuous variables were compared with an independent-samples t-test and categorical variables were compared utilizing the chi-squared test. An analysis of survival curves using kaplan-meier method was conducted and log-rank tests were used to measure differences. Cox proportional-hazards modeling was used for univariate and multivariate analyses for OS and PFS. Initially, univariate cox model analyses were conducted for each variable. In multivariate analysis, variables with p<0.05 were evaluated as independent predictors. A statistically significant difference was noted at p<0.05.
Baseline characteristics
There was no significant difference at baseline between the two groups as shown in Table 1, (p>0.05). During the follow-up period (range: 8-42 months), the median follow-up duration was 28 months. TACE+sorafenib/lenvatinib+PD-1 inhibitors had a median treatment duration of 8.6 months (ranging from 3.6-19.7), while TACE+regorafenib had a median treatment duration of 8.0 months (ranging from 3.3-18.2 months). Additionally, TACE plus sorafenib/lenvatinib+PD-1 inhibitor had a median treatment duration of 6 (range: 4-13) compared with TACE plus regorafenib, which had a median treatment duration of 5 (range: 3-11). Two types of PD-1 inhibitors including sintilimab (n=28, 38.4%) and camrelizumab (n=45, 61.6%) were applied; while two types of first-line TKI including sorafenib (n=23, 31.5%) and lenvatinib (n=50, 68.5%) were applied. A median of 10 cycles of PD-1 inhibitor treatment were administered, with a range of 2 to 18.
| Characteristics | Overall (n=113) | TACE+sorafenib/lenvatinib+PD-1 (n=73) | TACE+regorafenib (n=40) | p-value |
|---|---|---|---|---|
| Age (years) mean ± SD | 55.3 ± 11.5 | 54.5 ± 11.8 | 56.8 ± 10.9 | 0.316 |
| Gender n (%) | ||||
| Male | 99 (87.60%) | 67 (91.80%) | 32 (80.00%) | 0.129 |
| Female | 14 (12.40%) | 6 (8.20%) | 8 (20.00%) | |
| Etiology n (%) | ||||
| Hepatitis B | 100 (88.49%) | 64 (87.70%) | 36 (90.00%) | 0.769 |
| Hepatitis C | 2 (1.76%) | 1 (1.40%) | 1 (2.50%) | |
| Non-hepatitis B and C | 11 (9.75%) | 8 (11.00%) | 3 (7.50%) | |
| Child-Pugh score n (%) | ||||
| 5,6 Aug | 96 (84.95%) | 64 (87.70%) | 32 (80.00%) | 0.255 |
| 07 Aug | 17 (15.05%) | 9 (12.30%) | 8 (20.00%) | |
| Cirrhosis n (%) | ||||
| Present | 61 (53.98%) | 38 (52.10%) | 23 (57.50%) | 0.803 |
| Absent | 52 (46.02%) | 35 (47.90%) | 17 (42.50%) | |
| BCLC stage n (%) | ||||
| B | 43 (38.05%) | 30 (41.09%) | 13 (32.50%) | 0.368 |
| C | 70 (61.95%) | 43 (58.91%) | 27 (67.50%) | |
| ALBI grade n (%) | ||||
| 1 | 50 (44.24%) | 28 (38.40%) | 22 (55.00%) | 0.073 |
| 2 | 58 (51.32%) | 43 (58.90%) | 15 (37.50%) | |
| 3 | 5 (4.44%) | 2 (2.70%) | 3 (7.50%) | |
| Largest tumor size (cm, in diameter) | 7.6 ± 3.7 | 8.0 ± 3.1 | 6.9 ± 3.4 | 0.131 |
| Tumor numbers n (%) | ||||
| ≤ 3 | 12 (10.61%) | 7 (9.60%) | 5 (12.50%) | 0.946 |
| >3 | 101 (89.39%) | 66 (90.40%) | 35 (87.50%) | |
| AFP (ng/ml) n (%) | ||||
| <400 | 49 (43.36%) | 32 (43.84%) | 17 (42.50%) | 0.07 |
| ≥ 400 | 64 (56.64%) | 41 (56.16%) | 23 (57.50%) | |
| PIVKA-II (mAU/ml), mean ± SD | 32861.3 ± 83742.1 | 40175.0 ± 99443.9 | 19512.0 ± 39988.1 | 0.122 |
| Vascular invasion n (%) | ||||
| Present | 44 (38.93%) | 30 (41.10%) | 14 (35.00%) | 0.525 |
| Absent | 69 (61.07%) | 43 (58.90%) | 26 (65.00%) | |
| Extrahepatic metastases, n (%) | 50 (44.24%) | 33 (45.20%) | 17 (42.50%) | 0.782 |
| Involved disease sites n (%) | ||||
| Involved disease sites, n (%) lymph node | 7 (6.19%) | 5 (6.84%) | 2(5.00%) | - |
| Lung | 19 (16.81%) | 15 (20.54%) | 8 (20.00%) | |
| Bone | 5 (4.42%) | 4 (5.47%) | 2 (5.00%) | |
| Peritoneum | 3 (2.65%) | 2 (2.73%) | 1 (2.50%) | |
| Others | 8 (7.07%) | 7 (9.58%) | 4 (10.00%) | |
| APFs n (%) | ||||
| Present | 7 (6.19%) | 5 (6.80%) | 2 (2.60%) | 0.16 |
| Absent | 106 (93.81%) | 68 (93.20%) | 38 (97.40%) | |
| ECAs n (%) | ||||
| Present | 27 (23.89%) | 14 (19.20%) | 13 (32.50%) | 0.112 |
| Absent | 86 (76.11%) | 59 (80.80%) | 27 (67.50%) | |
| PT (sec) mean ± SD | 13.3 ± 1.5 | 13.1 ± 1.1 | 13.7 ± 2.2 | 0.056 |
| ALT (IU/L) Mean ± SD | 46.6 ± 38.7 | 49.3 ± 43.7 | 41.8 ± 27.4 | 0.33 |
| AST (IU/L) mean ± SD | 72.0 ± 57.9 | 75.6 ± 60.5 | 65.2 ± 52.0 | 0.363 |
Note: n (%): Presentation of data; mean ± SD: Presentation of data; HCC: Hepatocellular Carcinoma; TACE: Transcatheter Arterial Chemoembolization; HAIC: Hepatic Artery Infusion Chemotherapy; PD-1: Programmed Death-1; HBV: Hepatitis B Virus; BCLC: Barcelona Clinic Liver Cancer; ALBI grade: Albumin-Bilirubin grade; AFP: Alpha-Fetoprotein; PIVKA-II: Protein Induced by Vitamin K Absence or Antagonist II; APFs: Arterioportal Fistulas; ECAs: Extrahepatic Collateral Arteries; PT: Prothrombin Time; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; ALP: Alkaline Phosphatase; GGT: γ-Glutamyl Transferase; CA 19-9: Carbohydrate Antigen 19-9; SD: Standard Deviation.
Table 1: Baseline characteristics of patients after failure of TACE combined with first‐line tyrosine kinase inhibitors therapy.
OS and PFS
There were 59 patients total who died during the follow-up period, including 35 (47.94%) in group 1 and 24 (60.00%) in group 2. The OS in group 1 was significantly higher than that in group 2 (15 months [95% Confidence Interval (CI): 9.8–20.1] vs. 9 months [95% CI, 6.6-11.3]; log-rank p=0.016) (Figure 2).
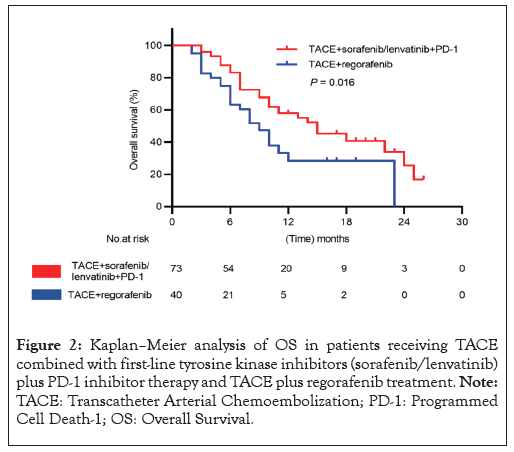
Figure 2: Kaplan–Meier analysis of OS in patients receiving TACE combined with first-line tyrosine kinase inhibitors (sorafenib/lenvatinib) plus PD-1 inhibitor therapy and TACE plus regorafenib treatment. Note: TACE: Transcatheter Arterial Chemoembolization; PD-1: Programmed Cell Death-1; OS: Overall Survival.
The progression of tumors was observed in 42 patients overall with 22 patients (32.87%) in group 1 and 18 patients (45 %) in group 2. The PFS in group 1 was significantly longer compared with that in group 2 (11 months [95% CI: 8.4-13.5] vs. 6 months [95% CI, 4.6-7.3]; log-rank p=0.010) (Figure 3).
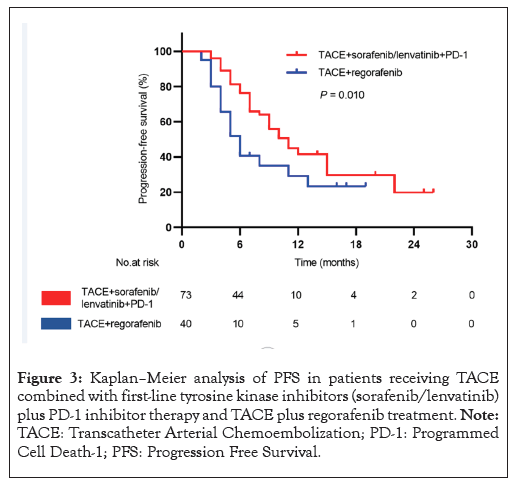
Figure 3: Kaplan–Meier analysis of PFS in patients receiving TACE combined with first-line tyrosine kinase inhibitors (sorafenib/lenvatinib) plus PD-1 inhibitor therapy and TACE plus regorafenib treatment. Note: TACE: Transcatheter Arterial Chemoembolization; PD-1: Programmed Cell Death-1; PFS: Progression Free Survival.
Treatment response
According to the mRECIST criteria, two patients (2.73%) in group 1 but none in group 2 achieved a CR (Figure 4). 20 patients (27.39%) in group 1 and 10 patients (25.00%) in group 2 achieved a PR; 27 patients (36.98%) in group 1 and 12 patients (30.00%) achieved stable disease; 24 patients (32.90%) in group 1 and 18 patients (45.00%) in group 2 had PD; 22 patients (30.12%) in group 1 and 10 patients (25.00%) in group 2 achieved the ORR; 49 patients (67.10%) in group 1 and 22 patients (55.00%) in group 2 achieved the DCR. Neither ORR (p=0.562) nor DCR (p=0.561) had significant between-group differences (Table 2).
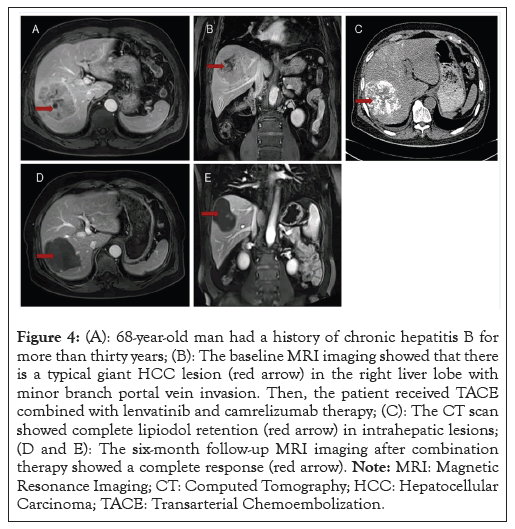
Figure 4: (A): 68-year-old man had a history of chronic hepatitis B for more than thirty years; (B): The baseline MRI imaging showed that there is a typical giant HCC lesion (red arrow) in the right liver lobe with minor branch portal vein invasion. Then, the patient received TACE combined with lenvatinib and camrelizumab therapy; (C): The CT scan showed complete lipiodol retention (red arrow) in intrahepatic lesions; (D and E): The six-month follow-up MRI imaging after combination therapy showed a complete response (red arrow). Note: MRI: Magnetic Resonance Imaging; CT: Computed Tomography; HCC: Hepatocellular Carcinoma; TACE: Transarterial Chemoembolization.
| Curative effect | TACE+sorafenib/lenvatinib+PD-1 | TACE+regorafenib | p-value |
|---|---|---|---|
| Complete Response (CR) | 2 (2.73%) | 0 (0.00%) | 0.756 |
| Partial Response (PR) | 20 (27.39%) | 10 (25.00%) | 0.783 |
| Stable Disease (SD) | 27 (36.98%) | 12 (30.00) | 0.455 |
| Progressive Disease (PD) | 24 (32.90%) | 18 (45.00%) | 0.202 |
| Overall Response Rate (ORR) | 22 (30.12%) | 10 (25.00%) | 0.562 |
| Disease Control Rate (DCR) | 49 (67.10%) | 22 (55.00%) | 0.202 |
Note: mRECIST: Modified Response Evaluation Criteria in Solid Tumors; TACE: Transcatheter Arterial Chemoembolization; PD-1: Programmed Cell Death-1.
Table 2: Treatment response was evaluated according to mRECIST criteria in two groups.
Factors associated with OS and PFS
According to the univariate Cox regression model, subsequent treatment options, sex, liver cirrhosis and ALBI grade all contributed to OS mortality (p<0.05). Multivariate analysis identified subsequent therapy options (Hazard Ratio (HR), 2.145 with 95% CI, 1.183 to 3.889, p=0.012) and ALBI grade (HR, 1.928; 95% CI, 1.253 to 2.966, p=0.003) as significant predictors of overall survival (Table 3).
| Characteristics | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | |
| Subsequent therapy options | 1.877 (1.096-3.214) | 0.022 | 2.145 (1.183-3.889) | 0.012 |
| Age (years) | 1.000 (0.979-1.022) | 0.976 | - | - |
| Gender male (vs. female) | 0.421 (0.215-0.821) | 0.011 | 0.446 (0.217-0.918) | 0.088 |
| Etiology of HCC, HBV (vs. others) | 0.703 (0.431-1.147) | 0.158 | - | - |
| Child-Pugh score, 5-6 (vs. 7-9) | 1.814 (0.957-3.439) | 0.068 | - | - |
| Cirrhosis (yes vs. no) | 0.701 (0.529-0.928) | 0.013 | 0.746 (0.561-0.991) | 0.112 |
| BCLC stage C (vs.) | 1.096 (0.642-1.871) | 0.737 | - | - |
| ALBI grade 1(vs. 2-3) | 1.854 (1.208-2.846) | 0.005 | 1.928 (0.253-2.966) | 0.003 |
| Tumor numbers ≤ 3 (vs.>3) | 0.827 (0.608-1.126) | 0.227 | - | - |
| Largest tumor diameter, (per cm) | 1.032 (0.964-1.105) | 0.361 | - | - |
| AFP(ng/ml) ≤ 400( vs.>400) | 1.459 (0.869-2.450) | 0.154 | - | - |
| Vascular invasion (yes vs. no) | 1.048 (0.908-1.210) | 0.521 | - | - |
| Extrahepatic metastases (yes vs. no) | 0.916 (0.533-1.576) | 0.752 | - | - |
| APFs (yes vs. no) | 0.778 (0.284-2.136) | 0.626 | - | - |
Note: CI: Confidence Interval; HR: Hazards Ratio; BCLC: Barcelona Clinic Liver Cancer; HBV: Hepatitis B Virus; ALBI grade: Albumin–Bilirubin grade; PIVKA-II: Protein Induced By Vitamin K Absence Or Antagonist II; AFP: Alpha Fetoprotein; APFs: Arterioportal Fistulas; OS: Overall Survival.
Table 3: Results of univariable and multivariable cox regression analyses for OS.
According to the univariate Cox regression model, subsequent therapy options, sex, Child–Pugh score, liver cirrhosis, ALBI grade, AFP level were risk factors associated with PFS (p<0.05) (Table 4). Multivariate analysis identified subsequent therapy options (HR, 2.096; 95% CI, 1.126-3.905, p=0.020), cirrhosis (HR, 0.656; 95% CI, 0.490-0.879, p=0.005), ALBI grade (HR, 1.915; 95% CI, 1.096-3.345, p=0.022) as significant predictors of PFS (Table 4).
| Characteristics | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95%Cl) | p-value | HR (95%Cl) | p-value | |
| Subsequent therapy options | 1.948 (1.140-3.329) | 0.015 | 2.096 (1.126-3.905) | 0.02 |
| Age (years) | 0.999 (0.978-1.021) | 0.924 | - | - |
| Gender male, (vs. female) | 0.433 (0.221-0.848) | 0.015 | 0.502 (0.236-1.069) | 0.074 |
| Etiology of HCC, HBV (vs. others) | 0.697 (0.430-1.131) | 0.144 | - | - |
| Child-Pugh score, 5-6 (vs.7-9) | 1.937 (1.025-3.661) | 0.042 | 0.666 (0.281-1.583) | 0.358 |
| Cirrhosis (yes vs. no) | 0.658 (0.496-0.873) | 0.004 | 0.656 (0.490-0.879) | 0.005 |
| BCLC stage, C (vs. B) | 1.153 (0.677-1.964) | 0.601 | - | - |
| ALBI grade 1 (vs. 2-3) | 1.763 (1.134-2.742) | 0.012 | 1.915 (1.096-3.345) | 0.022 |
| Tumor numbers ≤ 3 (vs.>3) | 0.872 (0.639-1.190) | 0.388 | - | - |
| Largest tumor size (per cm) | 1.033 (0.964-1.107) | 0.363 | - | - |
| AFP(ng/ml) ≤ 400(vs.>400) | 1.778 (1.056-2.992) | 0.03 | 1.466 (0.851-2.525) | 0.168 |
| Vascular invasion (yes vs.no) | 1.080 (0.934-1.249) | 0.297 | - | - |
| Extrahepatic metastases (yes vs. no) | 0.784 (0.458-1.345) | 0.377 | - | - |
| APFs (yes vs. no) | 0.778 (0.284-2.136) | 0.626 | - | - |
Note: CI: Confidence Interval; HR: Hazards Ratio; BCLC: Barcelona Clinic Liver Cancer; HBV: Hepatitis B Virus; ALBI grade: Albumin–Bilirubin grade; PIVKA-II: Protein Induced By Vitamin K Absence Or Antagonist II; AFP: Alphafetoprotein; APFs: Arterioportal Fistulas.
Table 4: Results of univariable and multivariable cox regression analyses for time to progression.
Treatment safety
In group 1, 60 patients (84.91%) had treatment-related TRAEs. An incidence of 46.57% of patients experienced Hand-to-Foot Skin Reactions (HFSR), which is the most frequent TRAE; other TRAEs with an occurrence of >15% included hypertension (31.50%), thrombocytopenia (31.50%), fatigue (23.28%) hypothyroidism (23.28%), anorexia (19.17%), skin rash (19.17%) and diarrhea (17.80%). In group 2, 35 patients (87.50%) experienced TRAEs. An incidence of 47.50% of patients experienced thrombocytopenia, which is the most frequent TRAE, other TRAEs with an occurrence of >15% were HFSR (30.00%), hypertension (20%) and proteinuria (20%).
The two groups did not show any significant differences between any grades of TRAEs except for proteinuria. Meanwhile, the percentage of patients with proteinuria in group 1 was significantly lower compared to group 2 (2.73% vs. 20.00%, p=0.006). When comparing the TRAEs with grade >3 severity, no significant differences were found between the two groups with regard to HFSR (p=0.898), hypertension (p=0.940), skin rash (p=0.485) and gastrointestinal bleeding (p=0.457). Furthermore, the percentage of patients with severe TRAEs (grade>3) in group 1 was insignificantly different than that in group 2 (13.69% vs. 17.50%, p=0.589) (Table 5).
| All grades of TRAE | TRAE (more than grade 3) | |||||
|---|---|---|---|---|---|---|
| TACE+sorafenib /lenvatinib+PD-1 (n=73) | TACE+regorafenib (n=40) | p-value | TACE+sorafenib /lenvatinib+PD-1 (n=73) | TACE+regorafenib (n=40) | p-value | |
| Total patients with TRAE, n (%) | 60 (84.91%) | 35 (87.5%) | 0.461 | 10 (13.69%) | 7 (17.5%) | 0.589 |
| Cholecystitis, n (%) | 1 (1.36%) | 1 (2.50%) | 0.664 | - | - | - |
| Liver abscess, n (%) | 1 (1.36%) | 0 (0.00%) | 0.457 | - | - | - |
| Hypertension, n (%) | 23 (31.50%) | 8 (20.00%) | 0.19 | 2 (2.73%) | 1 (2.50%) | 0.94 |
| Hand-foot skin reaction, n (%) | 34 (46.57%) | 12 (30.00%) | 0.086 | 5 (6.84%) | 3 (7.50%) | 0.898 |
| Diarrhea, n (%) | 13 (17.80%) | 7 (17.50%) | 0.967 | - | - | - |
| Skin rash, n (%) | 14 (19.17%) | 3 (7.50%) | 0.097 | 2 (2.73%) | 3 (7.50%) | 0.485 |
| Proteinuria, n (%) | 2 (2.73%) | 8 (20.00%) | 0.006 | - | - | - |
| Fatigue, n (%) | 17 (23.28%) | 6 (15.00%) | 0.295 | - | - | - |
| Bleeding (gingiva), n (%) | 4 (5.47%) | 1 (2.50%) | 0.796 | - | - | - |
| Hoarseness, n (%) | 1 (1.36%) | 0 (0.00%) | 0.459 | - | - | - |
| Anorexia, n (%) | 14 (19.17%) | 6 (15.00%) | 0.578 | - | - | - |
| Hypothyroidism, n (%) | 17 (23.28%) | 4 (10.00%) | 0.082 | - | - | - |
| Elevated serum AST or ALT, n (%) | 5 (6.84%) | 3 (7.50%) | 0.897 | - | - | - |
| Thrombocytopenia, n (%) | 23 (31.50%) | 19 (47.50%) | 0.093 | - | - | - |
| Pruritus, n (%) | 0 (0.00%) | 1 (2.50%) | 0.759 | - | - | - |
| Oral ulcer, n (%) | 2 (2.73%) | 0 (0.00%) | 0.756 | - | - | - |
| Paresthesia, n (%) | 1 (1.36%) | 0 (0.00%) | 0.457 | - | - | - |
| Alopecia, n (%) | 1 (1.36%) | 1 (1.30%) | 0.664 | - | - | - |
| Gastrointestinal bleeding, n (%) | 1 (1.36%) | 0 (0.00%) | 0.457 | 1 (1.36%) | 0 (0.00%) | 0.457 |
Note: AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; TRAE: Treatment-Related Adverse Event; TACE: Transarterial Chemoembolization; PD-1: Programmed Cell Death-1 n (%): Presentation of data.
Table 5: TRAEs in the study population.
There were 19 (26.0%) dose reductions in the TACE+sorafenib/lenvatinib+PD-1 inhibitor group and 3 (4.1%) discontinuations of sorafenib/lenvatinib and PD-1 inhibitor because of HFSR (n=2) and skin rash (n=1), respectively, There were 12 (30.0%) dose reductions and 2 (5.0%) discontinuations of regorafenib in the TACE+regorafenib group due to HFSR (n=1) and hypertension (n=1).
Our study investigated the efficacy and safety of subsequent therapy for HCC patients with disease progression after the failure of TACE combined with first-line TKI therapy. Our major findings were as follows:
• The subsequent combination of PD-1 inhibitors (TACE combined with first-line TKI and PD-1 inhibitors) led to a better survival benefit compared to switching to regorafenib (TACE plus regorafenib).
• An additional therapy option significantly predicted OS and PFS.
• The percentage of patients with proteinuria in the TACE plus TKI with PD-1 inhibitors treatment group was significantly lower than that in the TACE plus regorafenib treatment group and other TARE aspects did not show significant differences between groups.
The median OS and PFS of patients treated with TACE plus regorafenib were 9.0 and 6.0 months in our study, which were lower than those observed in another study, which reported 11.7 months for OS and 6.7 months for PFS [29]. Greater OS and PFS have also been documented in prior studies [30,31]. Further, the DCR and ORR observed in patients who received TACE plus regorafenib in the present study were 55.0% and 25.0%, respectively, which were lower than those observed in another study in which ORR reached 42.3% and DCR reached 66.1% [32]. Possibly, differences in baseline patient characteristics contributed to the differences in oncological outcomes between our study and these previously mentioned trials. For example previous trials enrolled only small proportions of patients with high tumor burdens [33], while we enrolled HCC patients with a more severe tumor burden (the average largest median tumor diameter >6.9 cm and 87.5% of patients with more than three tumors). Poor survival outcomes are associated with both hepatic dysfunction and high tumor burdens [34,35].
In this study, combination of PD-1 inhibitor with TKI and TACE therapy after the original first-line (sorafenib, lenvatinib) resulted in an ORR of 30.12% and a DCR of 67.10%. The results of checkmate-040 and keynote-240 showed that the ORR and DCR of nivolumab and pembrolizumab in the second-line treatment of progressed HCC was 15-20% and 55-60%, respectively [20,36]. Both ORR and DCR were slightly higher in the combination therapy of PD-1 inhibitors with TKI and TACE compared to navulizumab and pembrolizumab monotherapy. Additionally, the median PFS in this study was 11.0 months, which is superior to the 4.0 to 4.9 months reported in second-line clinical trials of navulizumab and pembrolizumab. It is worth noting that all patients in the aforementioned clinical trials who had failed first-line systemic therapy had a Child-Pugh grade A, whereas 20.0% of patients with Child-Pugh grade B were included in this study. These findings suggest that the combination therapy may enhance the efficacy of PD-1 inhibitors monotherapy in progressed HCC.
PD-1 medications block Programmed Death-Ligand 1 (PD-L1) from reaching its receptor on T-cells to inhibit tumor growth. When used alone, PD-1 antibodies are insufficient to intensify anticancer immunity in patients with HCC. However, TACE has the potential to enhance clinical effectiveness by increasing the release of antigens further, sorafenib or lenvatinib, enhances PD-L1 expression in tumors and promotes the infiltration of immune cells. PD-1 inhibitors combined with first-line TKIs offer unique immunomodulatory effects that can overcome TKI resistance and low response rates [36-42]. Thereby, TACE combined with first-line TKI plus PD-1 inhibitor therapy was expected to enhance tumor response rates and improved survival rates in our study patients.
As we found in our study, cirrhosis, ALBI grade and subsequent therapy options were independently associated with risk for PFS; whereas ALBI grade and subsequent therapy options were independently associated with risk for OS. Better PFS was observed in patients without liver cirrhosis before the second-line treatment. This result was similar to previous research [43,44]. Several independent research groups had validated the ALBI score, which was based solely on bilirubin levels and serum albumin, was an objective measure of liver function in HCC [26,45]. ALBI grade may be applied to screen patients who may benefit from second-line treatment. It has been reported ALBI grade 2~3 were correlated with the poor survival of patients with HCC, which is similar to the results of our study [46].
In the present study, after TACE plus first-line TKI therapy failed, TRAEs with the subsequent combination of PD-1 inhibitors or switching to subsequent regorafenib were manageable and consistent with previous data [23,47-49]. TRAEs in the TACE+sorafenib/lenvatinib+PD-1 inhibitor group occurred with similar rates and severity as for the TACE+regorafenib group. According to these results TACE+sorafenib/lenvatinib+PD-1 inhibitors showed good tolerability. Subsequent combining of PD-1 inhibitors did not significantly increase additional TRAEs risk, indicating an acceptable safety profile. However, the group receiving TACE plus regorafenib had a higher incidence of proteinuria. It may be due to the regorafenib application. By inhibition of vascular endothelial growth factor receptors signaling, proteinuria is frequently observed during regorafenib treatment as previous study reported [50]. These results suggested that subsequent combination PD-1 inhibitor therapy was feasible and acceptable for these patients.
This present study involved a cohort of patients with extra hepatic metastasis and portal vein cancer thrombus invasion, which are not suitable for TACE treatment in the BCLC guidelines. However, in the latest 2024 China Liver Cancer (CNLC) guidelines, TACE is indicated for patients with CNLC IIIa (portal vein thrombus invasion) and CNLC IIIb (extrahepatic metastasis). In the latest national multicenter study [51], it was clearly stated that advanced hepatocellular carcinoma cannot be treated without TACE and these patients can still benefit from it.
There were a few limitations. Due to the fact that it was a single-center study, we were unable to draw general conclusions from it. Moreover, due to the local medical insurance policy, both of the PD-1 inhibitors (camrelizumab and sintilimab) we used, whose effectiveness and safety have been confirmed for HCC [52-54]. Additionally, camrelizumab, a selective, humanized, high-affinity IgG4 PD-1 monoclonal antibody, has been approved as a second-line treatment in patients with advanced HCC in China [55]. These drugs will be increasingly widely used in the treatment of advanced HCC. Thereby, we only included data related to these two inhibitors rather than data for pembrolizumab and nivolumab, which were widely used worldwide as second-line therapy, limiting the application of our findings globally [56]. Finally, a selection bias could also arise from the fact that options for subsequent treatment were determined by the preferences of the physicians and the patients.
In conclusion, compared to subsequent regorafenib therapy, the subsequent combining of PD-1 inhibitors after the failure of TACE plus first-line TKI showed significantly better OS and PFS with manageable toxicity in advanced HCC patients and was a safe and effective therapeutic approach. This approach deserves consideration as a prioritized option during subsequent therapy. Our findings should be verified by randomized controlled trials and large-sample.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Yang WZ, Lin LW, Nian YX, Chen R, Ke K, Lin JQ, et al. (2024). Efficacy and Safety of Transarterial Chemoembolization Combined with First-Line Tyrosine Kinase Inhibitors and Programmed Death-1 Inhibitors for Progressed Hepatocellular Carcinoma. Immunotherapy (Los Angel). 10:259.
Received: 19-Sep-2024, Manuscript No. IMT-24-34131; Editor assigned: 23-Sep-2024, Pre QC No. IMT-24-34131 (PQ); Reviewed: 07-Oct-2024, QC No. IMT-24-34131 (QC); Revised: 14-Oct-2024, Manuscript No. IMT-24-34131 (R); Published: 21-Oct-2024 , DOI: 10.35248/2471-9552.24.10.259
Copyright: © 2024 Yang WZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.