Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2023)Volume 14, Issue 3
Introduction and objectives: Onychogryphosis (OG) is a common disorder of nail plate growth characterized by an opaque, yellow-brown thickening of the nail plate with hyperkeratosis, elongation, and increased curvature. It is most common in older adults. OG can be an important condition because patients may suffer from pain, secondary complications, and cosmetic concerns. A nail lacquer containing urea 20%, keratinase and hydroxypinacolone retinoate (a retinoid molecule) has recently been demonstrated to be effective and well-tolerated in treating Onychodystrophy/Onychogryphosis (OD/OG) and in improving lichen-planus associated nail alterations. Data on a large population of subjects with OD/OG is lacking, so far.
Materials and methods: In a multicentre, real-life study (The “UKO” trail) conducted in thirty-one Italian out-patient dermatology services, five hundred and nineteen subjects (mean age 59 years, 186 men and 333 women) with nonmycotic OD/OG affecting at least one nail of the feet, participated in the trial, after their written informed consent. The subjects were treated with a nail lacquer containing urea 20%, keratinase and a retinoid molecule (hydroxypinacolobne retinoate), one application daily for eight consecutive weeks. The primary endpoints were the evolution of a 3-item (dystrophy, dyschromia and nail thickness) Onychodystrophy Global score (ODGS) using a 3- point scale (from 0 to 2) (maximum ODGS score: 9) from baseline to week eight, evaluated in a blinded fashion using high-definition colour pictures of the affected-nail and the measurement of nail thickness using a calibrate graduated tool. Secondary endpoints were the global physician’s efficacy and tolerability evaluation performed in an open fashion.
Results: At baseline the ODGS scores (mean ± SD) was: 3.5 ± 0.8. After 8 weeks of treatment, the ODGS score was decreased to 1.5 ± 0.6 (-58% in comparison with baseline; p<0.001). After treatment, a complete normalization of nail appearance (i.e an ODGS score of 0) was observed in 46% of subjects. The nail thickness (mean ± SD) at baseline was 4 ± 4.1 mm. The use of the lacquer reduced significantly nail thickness to 2.1 ± 2.8 (a 48% reduction). The nail lacquer was very well tolerated.
Conclusion: In subjects with onychogryphosis/onychodystrophy of toenail, the use of a urea/keratinase/retinoid lacquer is associated with a significant improvement of nail appearance with a relevant reduction in nail thickness.
Onychogryphosis; Real-life trial; Nail lacquer
Onychogryphosis (OG) is a common disorder of nail plate growth characterized by an opaque, yellow-brown thickening of the nail plate with hyperkeratosis, elongation, and increased curvature [1]. Congenital Onychogryphosis is rare, while acquired onychogryphosis is most common in older adults. In a study conducted on 536 elderly patients (>75 years old), the prevalence of OG was 11.2% [2]. The causes of acquired onychogryphosis could be different: Poor self-care, dermatologic diseases, infections, local causes (e.g. trauma, nail surgery), or medical disorders (e.g. ulcers, thrombophlebitis, varicose veins) could be involve in the development of OG [1,3-6]. OG can be an important condition because patients may suffer from pain, secondary complications, and cosmetic concerns. There are different therapies available for the treatment of OG from conservative treatments (e.g. nail nippers, mechanical bur) to nail avulsion with or without matricectomy or Syme method (one-half of the terminal phalanx is excised together with the nail fold) [1]. A nail lacquer containing urea 20%, keratinase and hydroxypinacolone retinoate (a retinoid molecule) has recently been demonstrated to be effective and well-tolerated in treating onychodystrophy/onychogryphosis (OD/OG) and in improving lichen-planus associated nail alterations [7]. This product contains urea (with hydrating and keratolytic actions), keratinase (a proteolytic enzyme able to digests keratin), and hydroxypinacolone retinoate (a ester of retinoic acid) [8-10]. It is known that retinoids have anti-inflammatory, cell proliferation regulation activity and immune-modulating effects and could have a role in nail disorders [11,12]. Onychogryphosis is an important nail condition however data on a large population of subjects with OD/OG is lacking, so far. The common nail plate growth disorder onychogryphosis is characterised by an opaque, yellow-brown thickening of the nail plate along with hyperkeratosis, elongation, and increased curvature. While acquired onychogryphosis most frequently affects older adults, congenital onychogryphosis is rare. The prevalence of OG was in a study done on 536 elderly patients. Poor self-care, dermatological conditions, infections, local causes such as trauma or nail surgery, or medical conditions such as ulcers, thrombophlebitis, or varicose veins could all play a role in the development of acquired onychogryphosis. Due to the potential for pain, additional complications, and aesthetic issues, OG can be a serious condition. Treatment options for OG range from conservative measures such as the use of nail nippers or mechanical burs to nail avulsion with or without matricectomy or the Syme method, which involves removing half of the terminal phalanx along with the nail fold. Recent research has shown that a nail polish containing urea, keratinase, and hydroxypinacolone retinoate (a retinoid molecule is efficient and well-tolerated in treating onychodystrophy/onychogryphosis and in improving lichen-planus-related nail alterations. Urea, an ingredient that has hydrating and keratolytic properties, keratinase, a proteolytic enzyme that can break down keratin, and hydroxypinacolone retinoate, an ester of retinoic acid, are all present in this product. Retinoids are known to have immune-modulating, anti-inflammatory, and cell proliferationregulating properties, and they may play a part in nail disorders. Onychogryphosis is a significant nail disorder, but there are currently no data on a sizable population of subjects with OD/OG.
Study aim
We evaluated in a real-life, large sample size clinical setting the efficacy and tolerability of this nail lacquer in the treatment of onychogryphosis.
In a multicentre, real-life study (The “UKO” trial) conducted in thirty-one Italian out-patient dermatology services, five hundred and nineteen subjects (mean age 59 years, 186 men and 333 women) with non-mycotic OD/OG affecting at least one nail of the feet, participated in the trial, after their written informed consent. The subjects were treated with a nail lacquer containing urea 20%, keratinase and a retinoid molecule (hydroxypinacolobne retinoate), one application daily for eight consecutive weeks. The primary endpoints were the evolution of a 3-item (dystrophy, dyschromia and nail thickness) Onychodystrophy Global score (ODGS) using a 3-point scale (from 0 to 2) (maximum ODGS score: 9) from baseline to week eight, evaluated in a blinded fashion using high-definition colour pictures of the affected-nail and the measurement of nail thickness using a calibrate graduated tool. Secondary endpoints were the global physician’s efficacy and tolerability evaluation performed in an open fashion.
In this study a total of 519 patients were enrolled. In Table 1 are reported the demographics and baseline characteristics of the population considered. The enrolled patients had a median age of 59.6 ± 15.6 years, the 64.2% were women and the 35.8% were men. Only 107 subjects (20.6%) had diseases that could be associated with OG, principally psoriasis, hallux valgus/rigidus and circulatory failure.
| Characteristics | Total | Male | Female |
|---|---|---|---|
| Patients | 519 (100%) | 186 (35.8%) | 333 (64.2%) |
| Age (years) | |||
| Mean ± SD | 59.6 ± 15.6 | 60.0 ± 15.3 | 59.4 ± 15.7 |
| Median (IQR) | 60 (47-71) | 60 (48-73) | 60 (46-71) |
| Range (min-max) | 30-99 | 30-95 | 30-99 |
| Presence of comorbidities that could promote OG | |||
| No | 412 (79.4%) | 149 (80.1%) | 263 (79.0%) |
| Yes | 107 (20.6%) | 37 (19.9%) | 70 (21.0%) |
| Diseases (each patient could have one or more conditions) | |||
| Psoriasis | 34 (6.6%) | 15 (8.1%) | 19 (5.7%) |
| Hallux valgus/rigidus | 28 (5.4%) | 2 (1.1%) | 26 (7.8%) |
| Circulatory failure | 13 (2.5%) | 2 (1.1%) | 11 (3.3%) |
| Diabetes | 9 (1.7%) | 7 (3.8%) | 2 (0.6%) |
| Obesity | 6 (1.2%) | 2 (1.1%) | 4 (1.2%) |
| Microtraumas | 6 (1.2%) | 4 (2.2%) | 2 (0.6%) |
| Arthrosis | 5 (1.0%) | 1 (0.5%) | 4 (1.2%) |
| Claw fingers | 2 (0.4%) | 1 (0.5%) | 1 (0.3%) |
| Cancer/Lymphoma | 2 (0.4%) | 0 | 2 (0.6%) |
| Chronic renal failure | 1 (0.2%) | 1 (0.5%) | 0 |
| Lichen ruber planus | 1 (0.2%) | 0 | 1 (0.3%) |
| Plantarflexion | 1 (0.2%) | 1 (0.5%) | 0 |
| Finger crossing | 1 (0.2%) | 1 (0.5%) | 0 |
Table 1: Demographics and clinical characteristics of the enrolled population.
Patients were evaluated in a blinded fashion at baseline (T0) and after 8 weeks (T1) using the Onychodystrophy Global score (ODGS) based on the evolution of a 3-item (dystrophy, dyschromia and nail thickness) using a 3-point scale (from 0 to 2) using high-definition colour pictures of the affected-nail and the measurement of nail thickness using a calibrate graduated tool (Table 2).
| Onicodistrophy Global Score (ODGS) | T0 | T1 | p-value |
|---|---|---|---|
| Dystrophy | |||
| Absent (0) | 25 (4.8%) | 238 (45.9%) | <0.001 |
| Moderate (1) | 350 (67.4%) | 257 (49.5%) | <0.001 |
| Severe (2) | 144 (27.8%) | 24 (4.6%) | <0.001 |
| Mean (SD) | 1.2 (0.5) | 0.6 (0.6) | <0.001 |
| Dyschromia | |||
| Absent (0) | 84 (16.2%) | 301 (58.0%) | <0.001 |
| Moderate (1) | 316 (60.9%) | 206 (39.7%) | <0.001 |
| Severe (2) | 119 (22.9%) | 12 (2.3%) | <0.001 |
| Mean (SD) | 1.1 (0.6) | 0.4 (0.5) | <0.001 |
| Thickness | |||
| Absent (0) | 43 (8.3%) | 253 (48.7%) | <0.001 |
| Moderate (1) | 320 (61.7%) | 248 (47.8%) | <0.001 |
| Severe (2) | 156 (30.1%) | 18 (3.5%) | <0.001 |
| Mean (SD) | 1.2 (0.6) | 0.5 (0.6) | <0.001 |
| Total OGDS | |||
| Mean (SD) | 3.5 (0.8) | 1.5 (0.6) | <0.001 |
| Nail thickness, mm | |||
| Mean (SD) | 4.0 (4.1) | 2.1 (2.8) | <0.001 |
Table 2: Evaluation of the efficacy of the nail lacquer through ODGS measured at baseline (T0) and after 8 weeks (T1).
At baseline the ODGS scores (mean ± SD) was: 3.5 ± 0.8. After 8 weeks of treatment, the ODGS score was decreased to 1.5 ± 0.6 (-58% in comparison with baseline; p<0.001). After treatment, a complete normalization of nail appearance (i.e an ODGS score of 0) was observed in 46% of subjects. The variation of the score related to dystrophy, dyschromia and thickness is illustrated in Figure 1.
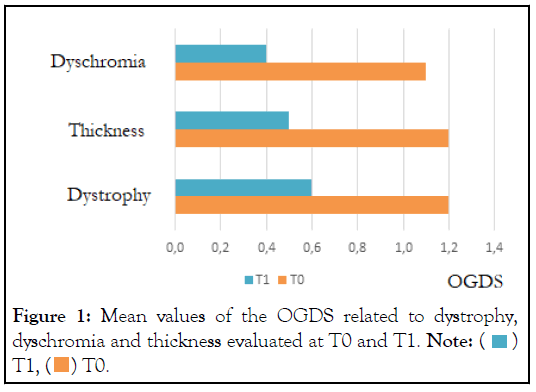
Figure 1: Mean values of the OGDS related to dystrophy,
dyschromia and thickness evaluated at T0 and T1. Note:  T1,
T1,  T0.
T0.
The nail thickness (mean ± SD) at baseline was 4 ± 4.1 mm. The use of the lacquer reduced significantly nail thickness to 2.1 ± 2.8 (48% reduction).
In Table 3 is reported the global physician’s efficacy performed in an open fashion that resulted excellent or good in 91.3% of subjects.
| Global efficacy | |
|---|---|
| Excellent | 245 (47.3%) |
| Good | 229 (44.1%) |
| Scarce | 36 (6.9%) |
| None | 9 (1.7%) |
Table 3: Results on the global physician’s efficacy.
The nail lacquer was very well tolerated as reported by 96.2% of participants (Table 4). Only two patients reported mild adverse events (irritation and slight burning).
Onychogryphosis is a nail disorder characterized by an opaque, yellow-brown thickening of the nail plate associated with gross hyperkeratosis, elongation, and increased curvature. Recently a nail lacquer containing urea 20% and keratinase and hydroxipinacolone retinoate 0.1% (U-KR lacquer) has been available. Urea is a hydrating and keratolytic substance, keratinase is a proteolytic enzyme that digests keratin and hydroxypinacolone retinoate is a cosmetic grade ester of retinoic acid [8-10]. Hydroxypinacolone retinoate, binds directly with retinoid receptors without the need for metabolic modifications to become a more biologically active form [13] and it has been demonstrated to be more stable and cause less skin irritation than retinol [14]. This compound is commonly used in antiageing and anti-acne products [15,16]. Onychogryphosis is commonly observed in clinical practice; however, the optimal treatment of this condition is still matter of debate [17]. Particularly, data on a large population of subjects with OD/OG is lacking, so far. In this context, the aim of this study was to evaluate the efficacy and tolerability of the nail U-KR lacquer in a real-life, clinical setting that involved more than 500 patients.
This product showed a good efficacy in term of reduction in dystrophy, dyschromia and nail thickness. After treatment, a complete normalization of nail appearance was observed in 46% of subjects. The product was judged excellent or good by 91.3% of patients and was very well tolerated.
At baseline the ODGS scores (mean ± SD) was: 3.5 ± 0.8. After 8 weeks of treatment, the ODGS score was decreased to 1.5 ± 0.6 (-58% in comparison with baseline; p<0.001). After treatment, a complete normalization of nail appearance (i.e an ODGS score of 0) was observed in 46% of subjects. The nail thickness (mean ± SD) at baseline was 4 ± 4.1 mm. The use of the lacquer reduced significantly nail thickness to 2.1 ± 2.8 (a 48% reduction). The nail lacquer was very well tolerated. In subjects with onychogryphosis/onychodystrophy of toenail, the use of a urea/keratinase/retinoid lacquer is safe and associated with a significant improvement of nail appearance with a relevant reduction in nail thickness.
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Colombo F , Milani M, Giovannini A, Ciciariello A, Sormani C, Fiorella CS, et al (2023) Efficacy and Tolerability of a Nail Lacquer Formulation with Urea 20%, Keratinase, and Hydroxypinacolone Retinoate in Subjects with Onychogryphosis/Onychodystrophy: A Multicentre, Prospective, Assessor-Blinded, Study on 519 Subjects (the √Ę??uko√Ę?¬Ě Trial). J Clin Exp Dermatol Res. 14:636.
Received: 12-Apr-2023, Manuscript No. JCEDR-23-23490; Editor assigned: 14-Apr-2023, Pre QC No. JCEDR-23-23490; Reviewed: 03-May-2023, QC No. JCEDR-23-23490; Revised: 12-May-2023, Manuscript No. JCEDR-23-23490; Published: 19-May-2023 , DOI: 10.35841/2155-9554.23.14.636
Copyright: © 2023 Colombo F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.