Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research - (2024)Volume 15, Issue 6
Background: Xerosis is a common skin manifestation in patients with diabetes and it is associated with an increased risk of severe skin fissuring and then ulcers. Skin foot care for diabetics is an important strategy to prevent further skin damage. Pure Omental Lipids (POL) cream is a commercialized product containing urea and panthenol (able to improve emollience and skin hydration), POL (that improve skin mechanics and strength of fragile skin), and carnosine (able to interfere with the formation of advanced glycated end-products, involved in reduced skin elasticity in diabetic patients).
Study aim: To evaluate the efficacy of POL-Podactive (POL-P) cream in the treatment of foot xerosis in patients with diabetes, in comparison with a glycerol-based emollient cream.
Materials and methods: In a randomized, assessor-blinded, parallel-group, controlled, prospective 4-week trial, twenty-one diabetic subjects (10 women and 11 men, mean age 70 ± 10 years) were enrolled after their informed consent. Fourteen were allocated to the POL-P group (group A) and seven were allocated to the emollient glycerol-based cream (group B). The creams were applied once daily for 4 consecutive weeks on the lower third of the legs and the entire feet. The study outcomes were the clinical evaluation of Dry Area Severity Index (DASI score) assessing xerosis, erythema, scaling and skin fissuration (minimum-maximum score values: 0-20), and the instrumentally evaluation of elasticity (Cutometer®) and hydration (Corneometer®). All outcomes were evaluated at baseline, after 15 days and 4 weeks by an investigator unaware of treatment allocation (active or control groups).
Results: All the enrolled subjects completed the treatment period. At baseline DASI score was 20 for both groups. The DASI Score significantly decreased in both groups (p<0.001): In group A (POL-P cream) the DASI score was reduced to 9.9 ± 3.6 and 5.2 ± 3.0 at T1 and T2, respectively (-50% and -74%); in group B (glycerol-based emollient cream) the DASI score was reduced to 11.7 ± 1.7 and 6.4 ± 2.3 at T1 and T2, respectively (-41% and -68%). After 15 days (T1), more subjects in group A showed a reduction ≥ 10 points in DASI score (57%) than subjects in group B (14%) (p=0.03). The elasticity tends to increase only in group A after 4 weeks of treatment, while a worsening of all parameters related to elasticity was observed in group B. The treatments determined in both groups an increase in skin hydration, however, statistical improvements were observed after 4 weeks in different parts of the feet only in POL-P cream group.
Conclusion: POL-P cream has shown to have a greater emollient and hydrating efficacy in the treatment of xerosis of diabetic feet compared to a glycerol-based emollient cream. This greater effect was supported both clinically and instrumentally. This POL, urea, carnosine and panthenol cream could be considered a useful tool in improving xerosis of the lower limbs in subjects with diabetes.
Xerosis; Pure omental lipids; Diabetic foot; Corneometer®; Cuto
Diabetes mellitus is commonly responsible for several skin changes and complications. Xerosis with pruritus is among the most frequently observed cutaneous manifestations of this disease, especially in the legs and feet [1]. Severe xerosis and fissurations could represent relevant risk factors for the development of ulcers [2].
The skin of diabetic patients generally showed a reduction in the hydration of the stratum corneum and an increase in Transepidermal Water Loss (TEWL) [3]. Different processes could be involved in the changes in the hydration of the stratum corneum, such as the decrease in aquaporin 3 with a consequent proliferation of keratinocytes that is related to the dry skin of people with diabetes [4]. Different non-invasive in vivo techniques are available for the study of skin, regarding different parameters such as skin color, elasticity, stratum corneum water content, and skin barrier function integrity. For example, the Cutometer® (CK electronic GmbH Köln-Germany) is a device able to quantitatively evaluate the distensibility, elasticity, and viscoelasticity of the skin, all parameters related to its composition and structure. The Corneometer® (CK electronic GmbH Köln-Germany) is another non-invasive device for the determination of the stratum corneum water content (skin hydration).
Skin foot care in diabetics represents a key strategy to prevent future skin damage [5]. Skin xerosis is usually treated using emollient and moisturizer (such as urea) for the long-term [6]. In a recent trial, the efficacy of a cream (POL-P cream) containing, urea, POL, carnosine, and panthenol was demonstrated: In a group of diabetic subjects, the POL-P cream, applied on the lower third of the legs and feet, showed to have emollient and hydrating efficacy, with a reduction of DASI of 74% [7]. POL extract is considered an effective topical product with emollient and moisturizing actions, and thanks to its richness in Vascular Endothelial Growth Factor (VEGF) exerts also angiogenic properties [8]. Carnosine could interfere with the formation of Advanced Glycated End-products (AGE) that seem to be responsible for the reduced elasticity of the skin in diabetic patients [7]. Finally, panthenol is a potent emollient, able to improve skin barrier function, decreasing TEWL [9].
Study design
We adopted a randomized, assessor-blinded, parallel-group, controlled, prospective 4-week trial study design.
The study was carried out between September 2023 and May 2024. The trial was conducted according to the Good Clinical Practice guidelines (GCP) and Helsinki Declaration, consistent with the GCP regulatory requirements. All subjects provided a signed informed consent. A total of twenty-one subjects (10 women and 11 men, mean age 70 ± 10 years) were enrolled and randomized in a 2:1 allocation ratio and instructed to apply the POL-P cream once a day (group A, n=14) or to apply the glycerol- based emollient cream once a day (group B, n=7) on the lower third of the legs and the entire feet. Both products were commercially available, in particular, POL-P was commercialized by Cantabria Labs Difa Cooper POL-P, Caronno Pertusella, Italy). POL-P is a moisturizing gel containing 15% urea, pure omental lipids technology, carnosine, and panthenol representing an adjuvant treatment for dryness and thickening of fragile feet and for the maintenance of the skin integrity of the feet of diabetic patients. The main inclusion criteria were: Male or female subjects with age>30 years; with a diagnosis of type 2 diabetes mellitus and a clinically relevant xerosis of the feet. The main exclusion criteria were: the presence of ulcerative lesions in the lower limb ("diabetic foot"); the presence of significant peripheral vascular disease and acute dermatitis in the lower limb. The efficacy of the treatments was evaluated through the DASI assessing scaling, roughness, redness, cracks, and itching (minimal-maximum score values: 0-20). Additional trial outcomes were the instrumental evaluation of elasticity and hydration. The Cutometer® MPA 580 (CK electronic GmbH, Köln, Germany) was used to evaluate the skin elasticity. These devices apply a specified amount of negative pressure on the surface of the skin to draw skin into the circular aperture of the measurement probe (2 mm-diameter probe, 450 mbar of vacuum followed by a 3-second relaxation period). This allows for optical assessment of skin displacement using a laser. The amount of tissue elevation and speed of return when negative pressure is released represents the amount of elasticity of the skin drawn into the probe [10]. Different parameters were considered: R0 (Uf), R2 (Ua/Uf, gross elasticity), R5 (Ur/Ue, net-elasticity), R7 (Ur/Uf, biologic elasticity) (Figure 1).
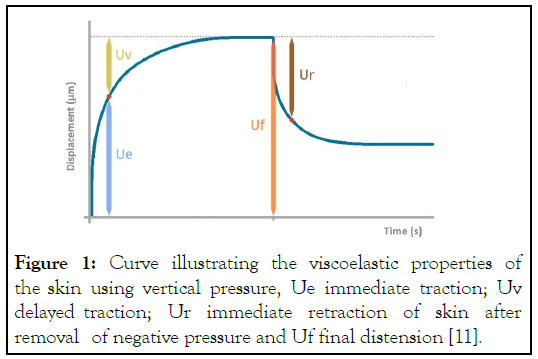
Figure 1: Curve illustrating the viscoelastic properties of the skin using vertical pressure, Ue immediate traction; Uv delayed traction; Ur immediate retraction of skin after removal of negative pressure and Uf final distension [11].
The hydration level of the skin surface was determined using Corneometer® (CK electronic GmbH, Köln, Germany), the results were expressed in arbitrary units. All outcomes were evaluated at baseline (T0), after 15 days (T1) and 4 weeks (T2) by an investigator unaware of treatment allocation (active or control groups).
The instrumental measurements were taken on the left and right midfoot for the Cutometer®, and on the left and right midfoot, heel and forefoot for the Corneometer® preferably in the morning between 9:00 and 12:00 hours, in a room with a temperature ranging between 21 and 25°C, and an environmental humidity between 40% and 60%.
Statistical analysis
Statistical analyses were conducted using GraphPad statistical software version 5.0 (GraphPad Software Inc., La Jolla, CA, USA). A non-parametric paired test was used to compare data at baseline and after 15 days and 4 weeks, while a non-parametric unpaired test was used to compare the different groups. Data are expressed as mean ± Standard Deviation (SD), and a p- value<0.05 was considered significant.
A total of 21 participants were enrolled in this study and all the subjects completed the treatment period. Patients were randomized into two groups: Group A (n=14, mean age 67.8 ± 9.3 years, DASI at baseline 20) and group B (n=7, mean age 74.9 ± 9.5 years, DASI at baseline 20). The DASI Score significantly decreased in both groups (p<0.001): In group A (POL-P cream) the DASI score was reduced to 9.9 ± 3.6 (-50%) and 5.2 ± 3.0 (-74%) at T1 and T2, respectively; in group B (glycerol-based emollient cream) the DASI score was reduced to 11.7 ± 1.7 (-41%) and 6.4 ± 2.3 (-68%) at T1 and T2, respectively (Figure 2). After 15 days (T1), more subjects in group A showed a reduction ≥ 10 point in DASI score (8 out of 14, 57%) than subjects in group B (1 out of 7, 14%) (p=0.03, Chi-squared test).
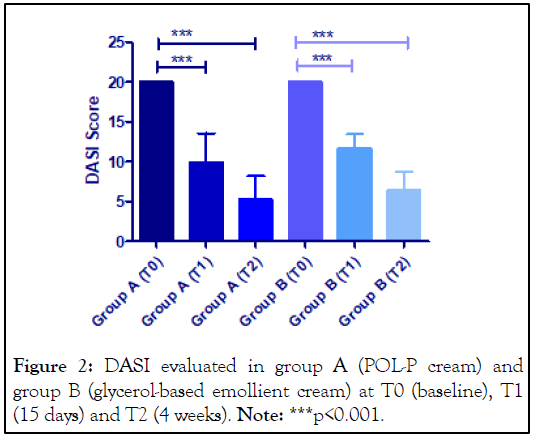
Figure 2: DASI evaluated in group A (POL-P cream) and group B (glycerol-based emollient cream) at T0 (baseline), T1 (15 days) and T2 (4 weeks). Note: ***p<0.001.
Table 1, reports the variation of the single parameters of DASI during the study period. All the parameters decreased during the trial in both groups, however, the score of redness decreased in group A by 73% and 93% at T1 and T2 respectively, compared to group B where the reduction was by 29% and 54%, respectively (differences between two groups statistically significant, p<0.001). Although the difference between the two groups was not significant, a higher reduction of the parameter itching was observed in group A compared to group B (-55% and -80% at T1 and T2 in group A, -43% and -64% at T1 and T2 in group B).
| Group A (n=14) | Group B (n=7) | |||||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | |
| Scaling | 4.0 | 2.5 ± 0.5 | 1.6 ± 0.5 | 4.0 | 2.1 ± 0.4 | 1.3 ± 0.5 |
| Roughness | 4.0 | 2.4 ± 0.7 | 1.2 ± 0.9 | 4.0 | 2.3 ± 0.5 | 1.0 ± 0.8 |
| Redness | 4.0 | 1.1 ± 0.9*** | 0.3 ± 0.5*** | 4.0 | 2.9 ± 0.4 | 1.9 ± 0.4 |
| Cracks | 4.0 | 2.2 ± 1.0 | 1.4 ± 0.6 | 4.0 | 2.1 ± 0.4 | 0.9 ± 0.7 |
| Itching | 4.0 | 1.8 ± 0.9 | 0.8 ± 0.8 | 4.0 | 2.3 ± 0.5 | 1.4 ± 0.5 |
| Total | 20.0 | 9.9 ± 3.6 | 5.2 ± 3.0 | 20.0 | 11.7 ± 1.7 | 6.4 ± 2.3 |
Note: ***p<0.001 statistical difference between group A and group B.
Table 1: Variation of single parameters of DASI evaluated at baseline (T0); after 15 days (T1); and 4 weeks (T2) and data are expressed as mean ± SD.
The Cutometer® is a non-invasive device that can evaluate the biomechanical properties of the skin in vivo, such as distensibility, elasticity, and viscoelasticity. It measures the vertical deformation of the skin surface when the skin is pulled in. The parameters considered in this study were: The distensibility (R0=Uf, final distension, an improvement in distensibility generally showed a reduction in R0 value); the elasticity (R2=Ua/Uf, gross elasticity, improvement of elastic properties of skin is usually associated with an increase of R2;
R5=Ur/Ue, net-elasticity, a higher elasticity is associated with an increased value of R5; R7=Ur/Uf, biologic elasticity, improvement of elastic properties of skin is generally associated with an increase of R7).
A non-statistical increase of distensibility (R0) was observed in both groups, however, the increase was higher in Group B (+71% and +60% at T1 and T2 on the right midfoot; +82% and +90% at T1 and T2 on the left midfoot) compared to group A (+12% and +20% at T1 and T2 on the right midfoot; +33% and +42% at T1 and T2 on the left midfoot). The elasticity (gross elasticity, net elasticity, biologic elasticity) tends to increase only in group A after 4 weeks of treatment in both feet (left and right). A worsening of all parameters was observed in group B both after 15 days and 4 weeks (data on file).
The hydration of the skin (the stratum corneum water content) was evaluated using a non-invasive in vivo technique (Corneometer®) which measures the capacitance that is entirely dependent on the water content in the skin, expressed into a digitally measured value Arbitrary Units (AU) that is proportional to the skin humidity, which are estimated to correspond to 1 AU in 0.2–0.9 mg of water per gram of the stratum corneum [12].
The results of the skin hydration of hell, midfoot and forefoot of both right and left feet are illustrated in Figure 3.
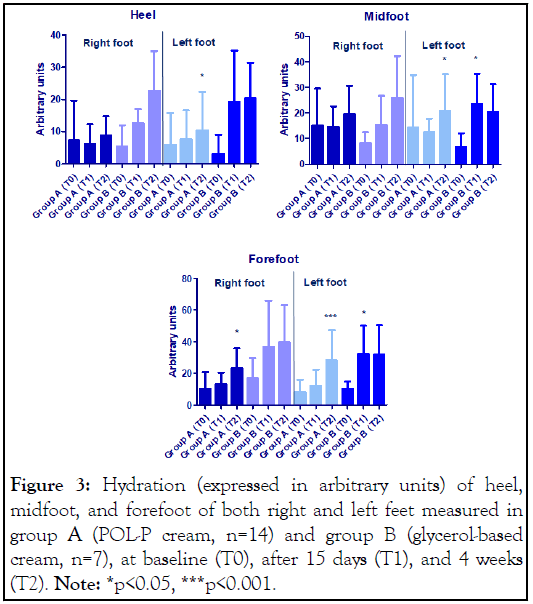
Figure 3: Hydration (expressed in arbitrary units) of heel, midfoot, and forefoot of both right and left feet measured in group A (POL-P cream, n=14) and group B (glycerol-based cream, n=7), at baseline (T0), after 15 days (T1), and 4 weeks (T2). Note: *p<0.05, ***p<0.001.
The treatments determined in both groups an increase in skin hydration in all the parts of feet considered (heel, midfoot, forefoot), however after 4 weeks of the treatment the increase was statistically significant only for group A in the left foot, and in the forefoot of the right foot. Not statistical differences were observed for group B, probably due to the limited number of assigned patients. A very good tolerability was reported in both groups.
Xerosis is a common skin manifestation in patients with diabetes and represents a risk factor for the development of foot ulcers [7,13,14]. Skin foot care for diabetics is an important strategy to prevent further skin damage. POL-P cream, containing urea, POL technology, carnosine, and panthenol offers a strong scientific rationale for its use in treating diabetic skin. Urea and panthenol can improve emollience and skin hydration [9,15]. Topical carnosine is able to reduce the AGE accumulation at the dermal level [7,16,17]. Finally, POL has been shown to improve skin mechanics and strength in fragile skin [7].
The efficacy of POL-P cream in improving foot xerosis in patients with diabetes was confirmed in this trial. The DASI score significantly decreased in both groups (p<0.001): Particularly in group A (POL-P cream) the DASI score was reduced to -50% and -74% at T1 and T2, respectively; and at T1 a majority of subjects showed a reduction ≥ 10 points in DASI score (57%) than subjects in group B (14%) (p=0.03), suggesting a faster clinical response with POL-P cream compared with the glycerol-based cream. These data agree with the results of Puviani et al., where the use of POL-P cream in diabetic patients determined a reduction in DASI score of -59% and -74% after 4 and 8 weeks respectively [7].
The efficacy of POL-P cream was also quantitatively evaluated instrumentally using the devices Cutometer® and Corneometer®.
Alteration of the mechanical properties of the skin is a common manifestation of diabetes mellitus. The elasticity of the skin, related to skin composition and structure, could be quantitatively monitored using the Cutometer®. Different studies reported that diabetic subjects showed lower skin elasticity compared to the control population, including increased distensibility (increased R0), and decreased elasticity (decreased R2 and R7) [18,19]. In this study, the elasticity (gross elasticity, net elasticity, biologic elasticity) tends to increase only in group A (POL-P cream) after 4 weeks of treatment in both feet (left and right), while a worsening of all parameters was observed in group B (glycerol-based cream).
As reported in the literature feet are generally very dry (AU<30) [19]. The treatments determined in both groups an increase in skin hydration in all the parts of the feet considered (heel, midfoot, forefoot), however statistical improvements were observed after 4 weeks in different parts of the feet only in POL- P cream group. Summarizing the complex instrumental data that we have collected in this trial, we can found that in comparison with the glycerol-based cream, POL-P cream improved the following parameters: Gross elasticity, net elasticity, biologic elasticity and skin hydration. Some limitations should be considered in evaluating our study results. The main limitation was that this study is an open trial, however, to increase the internal validity of our results, we adopted an assessor-blinded evaluation. A second aspect to be considered is that the treatment duration was 4 weeks, and therefore, no data regarding efficacy for longer treatment duration could be inferred from this study.
Emollients are a key strategy to treat xerosis in diabetic subjects reducing the risk of developing severe skin injuries and ulcers. The POL-P cream contains POL technology, urea, panthenol and carnosine, represent a product with a strong scientific rationale for its use in treating diabetic skin. POL-P cream has shown to have a greater emollient and hydrating efficacy in the treatment of xerosis of diabetic feet compared to a glycerol-based emollient cream. This greater effect was supported both clinically and instrumentally. This POL, urea, carnosine and panthenol cream could be considered a useful tool in improving xerosis of the lower limbs in subjects with diabetes.
Marianna D’Armetta participates to the investigation. Stefano Alfano and Colombo Francesca participated in the study design and drafting of the manuscript. All authors contributed to the review and final approval of the manuscript. All authors have read and agreed to the published version of the manuscript.
No ethical approval was required since our study was based on the application of a cosmetic. All participants provided written informed consent before starting the study.
Stefano Alfano and Colombo Francesca are employees of Difa Cooper Cantabria Labs which commercialized the product used in the trial.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Armetta MD, Alfano S, Francesca C (2024). Efficacy of a Pure Omental Lipids Based Cream in the Treatment of Diabetic Xerosis: A Randomized, Controlled Assessor-Blinded Trial. J Clin Exp Dermatol Res. 15:679.
Received: 07-Nov-2024, Manuscript No. JCEDR-24-34504; Editor assigned: 09-Oct-2024, Pre QC No. JCEDR-24-34504 (PQ); Reviewed: 23-Oct-2024, QC No. JCEDR-24-34504; Revised: 30-Oct-2024, Manuscript No. JCEDR-24-34504 (R); Published: 06-Nov-2024 , DOI: 10.35841/2155-9554.24.15.679
Copyright: © 2024 Armetta MD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.