Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2022)Volume 13, Issue 4
Background: Xerosis is a common skin manifestation in subjects with diabetes. Severe xerosis with or without fissuration of lower limbs and foot is a risk for the development of foot ulcer. Skin foot care in diabetics is a relevant strategy to prevent further skin damage. Topical urea and other emollient substances are commonly used to improve skin hydration and skin elasticity of lower limbs and feet. Pure Omental Lipids (POL) used topically has shown to improve skin mechanics and strength in fragile skin. Carnosine could interfere with the formation of advance glycated end-products which seem to be responsible of increased stiffness and reduced elasticity of the skin in diabetic subjects. Panthenol is a potent emollient with skin barrier functions defence activity. Recently a topical product containing urea, POL, carnosine and panthenol has been developed (POL-P).
Study aim: To evaluate the efficacy of POL-P in improving foot xerosis in subjects with diabetes.
Subjects and methods: In a randomized, assessor-blinded, parallel group, controlled, prospective 8-week trial, thirtyeight diabetic subjects (10 men and 28 women, mean age 68 ± 7 years) were enrolled after their informed consent. Eight-teen were allocated in the POL-P group (active group). The cream was applied twice daily for 8 consecutive weeks on the lower third of the legs and on the entire feet. Twenty subjects were randomly allocated in the control group (a glycerine-based cream). The study outcomes were evaluation of Dry Area Severity Index (DASI score) assessing xerosis, erythema, scaling and skin fissuration (minimum-maximum score values: 4-20), a patient-assessed itch intensity score using a 10-cm Visual Analog scale (I-VAS). An additional trial outcome was the evaluation by the investigator of the general state of hydration and elasticity of the treated skin areas (HE-VAS score) using a 10-cm VAS scale. All outcomes were evaluated at baseline, after 4 and 8 weeks by an investigator unaware of treatment allocation (active or control groups).
Results: All the enrolled subjects concluded the trial. At baseline, DASI score (mean ± SD) was 8.0 ± 2.5 in active group and 10.5 ± 2.9 in the control group. In the active group, the DASI score was reduced to 3.3 ± 1.8 and to 2.1 ± 2.0 after 4 and 8 weeks of treatment, respectively. In comparison with baseline, the percentage reduction of the DASI score at week 8 was 74%. In the control group the DASI score was reduced to 9.5 ± 2.5 at week 4 (not significant). No further reduction in DASI score in control group was observed at week 8. The absolute difference in DASI score at week 8 in the active group in comparison with control was -7.3 (95% CI of the difference: -5,962 to -8,816; P=0.001). The H-E VAS score at baseline was 6.5 ± 1.4 in the active group and 7.7 ± 2.0 in the control. HE vas score was
Xerosis; Purified omental lipid; Urea; Diabetic foot
Xerosis is a common skin manifestation in subjects with diabetes [1]. Severe xerosis with or without fissuration of lower limbs and foot is a risk for the development of foot ulcer [2]. Skin foot care in diabetics is a relevant strategy to prevent further skin damage [3]. Topical urea and other emollient substances are commonly used to improve skin hydration and skin elasticity of lower limbs and feet [4,5]. Pure Omental lipids (POL) used topically have shown to improve skin mechanics and strength in fragile skin [6]. Carnosine could interfere with the formation of advance glycated end-products which seem to be responsible of increased stiffness and reduced elasticity of the skin in diabetic subjects [7]. Recently a topical product containing urea, POL, carnosine and panthenol has been specifically developed (POL-P) to be used for the treatment of xerosis of the lower limb and foot.
Study aim
We evaluated the efficacy of POL-P in improving lower leg and foot xerosis in subjects with diabetes in comparison with a control hydrating cream.
Study design
We adopted a randomized, assessor-blinded, parallel group, controlled, prospective 8-week trial study design.
The trial was conducted in three tertiary referral hospitals in Italy (Modena, Bolzano and Alcamo). The study was carried out between January 2022 and October 2022. The study protocol, the Subject Information Sheet (SIS) and Informed Consent Form (ICF) were reviewed and approved by the Ethics Committees of the three participating Centres. A total of thirtyeight (28 women and 10 men, mean age 68 years) diabetic subjects were enrolled after their informed consent. The main inclusion criteria were: Male or female subjects with an age between 40 and 75 years; with a diagnosis of type 2 diabetes mellitus for at least 5 years and in dietary treatment or with oral hypoglycaemic agents and willing to participate to the trial with a clinically relevant xerosis of the lower limbs/feet. Main exclusion criteria were: Presence of ulcerative lesions in the lower limb ("diabetic foot"); insulin treatment; presence of significant obstructive peripheral vascular disease (ankle/arm index <0.70) and presence of a clinically relevant peripheral neuropathy. Eight-teen were randomly allocated in the POL-P group (active group). The cream was applied twice daily for 8 consecutive weeks on the lower third of the legs and on the entire feet. Twenty subjects were randomly allocated in the control group (a glycerine-based cream). Randomization list was generated by a dedicated computer program. The study outcomes were: evaluation of Dry Area Severity Index (DASI score), according to Serup et al. [8], assessing xerosis, erythema, scaling and skin fissuration (minimum-maximum score values: 4-20), a patient-assessed itch intensity score using a 10-cm Visual Analog scale (I-VAS). An additional trial outcome was the evaluation by the investigator of the state of hydration and elasticity of the treated skin areas (HE-VAS score) using a 10-cm VAS scale [9]. All outcomes were evaluated at baseline, after 4 and 8 weeks by an investigator unaware of treatment allocation (active or control groups). The DASI score evolution was chosen as the primary outcome of the trial.
Statistical analysis and sample size calculationStatistical analysis was performed using GraphPad statistical software ver 13.0 (La Jolla, CA, USA). Continuous variables were expressed as mean ± Standard Deviation (SD). The primary endpoint of the trial was the evolution of DASI score over the time (week 8 and week 4 in comparison with baseline) and in comparison, between the two groups. The paired t test and the Wilcoxon test were used for the analysis of the study outcomes. According to the primary outcome of the trial (reduction in the DASI score) the sample size calculation was performed assessing a test hypothesis of the difference in this score at the end of the treatment of at least 5 points in the DASI score mean values between active and control group. With an effect size (Cohen’s d value) of 0.5, an alpha value of .05 and a power of 90%, a total of at least 30 subjects (15 per arm) should be enrolled to detect this difference. The sample size was calculated using G-Power statistical software version 3.9 (Kiel, Germany). A p value of <. 05 was considered significant.
All the enrolled subjects concluded the trial. The two groups were well matched according to baseline characteristics. At baseline DASI score (mean ± SD) was 8.0 ± 2.5 in active group and 10.5 ± 2.9 in the control group. In the active group, the DASI score was reduced to 3.3 ± 1.8 and to 2.1 ± 2.0 after 4 and 8 weeks of treatment, respectively (Figure 1a). In comparison with baseline, the percentage reduction of the DASI score at week 8 was 74%. In the control group the DASI score was reduced to 9.5 ± 2.5 at week 4 (not significant in comparison with baseline). No further reduction in DASI score in control group was observed at week 8 (Figure 1b). The absolute difference in DASI score at week 8 in the active group in comparison with control was -7.3 (95% CI of the difference: -5,962 to -8,816; P=0.001). The H-E VAS score at baseline was 6.5 ± 1.4 in the active group and 7.7 ± 2.0 in the control. HE vas score was significantly (P=0.001) reduced to 3.4 ± 1.4, after 4 weeks and to 2.4 ± 1.8 after 8 weeks. No significant reduction in HE-VAS score were observed in the control group (7.2 ± 1.5 at week 4 and 7.2 ± 1.5 after 8 weeks) (Figures 2a and 2b). Figure 3 reports some pictures of subjects treated with active product. No difference on I-VAS score were observed between the two groups and between treatment time points. Figures 3a-3d shows some subjects’ macro and micro pictures of the foot at baseline and after POL-P treatment.
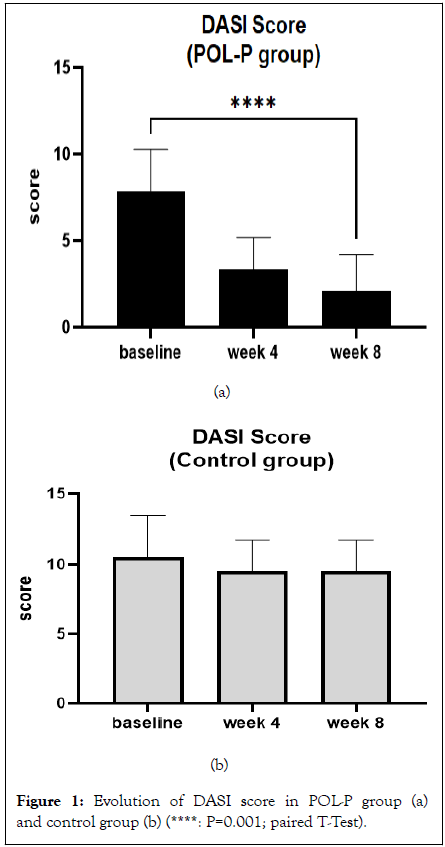
Figure 1: Evolution of DASI score in POL-P group (a) and control group (b) (****: P=0.001; paired T-Test).
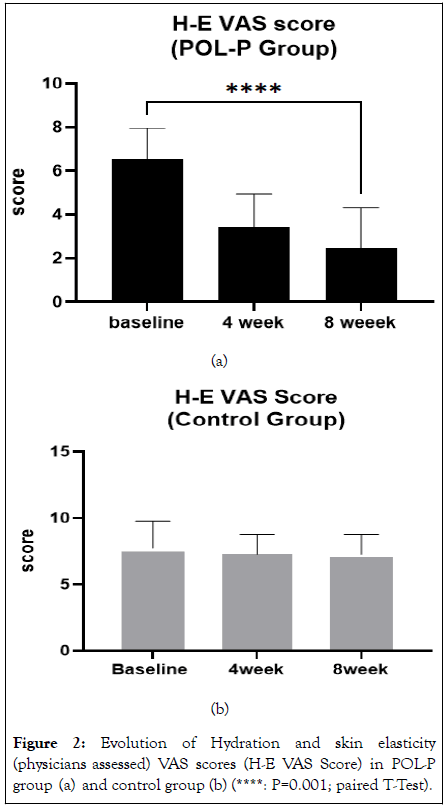
Figure 2: Evolution of Hydration and skin elasticity (physicians assessed) VAS scores (H-E VAS Score) in POL-P group (a) and control group (b) (****: P=0.001; paired T-Test).
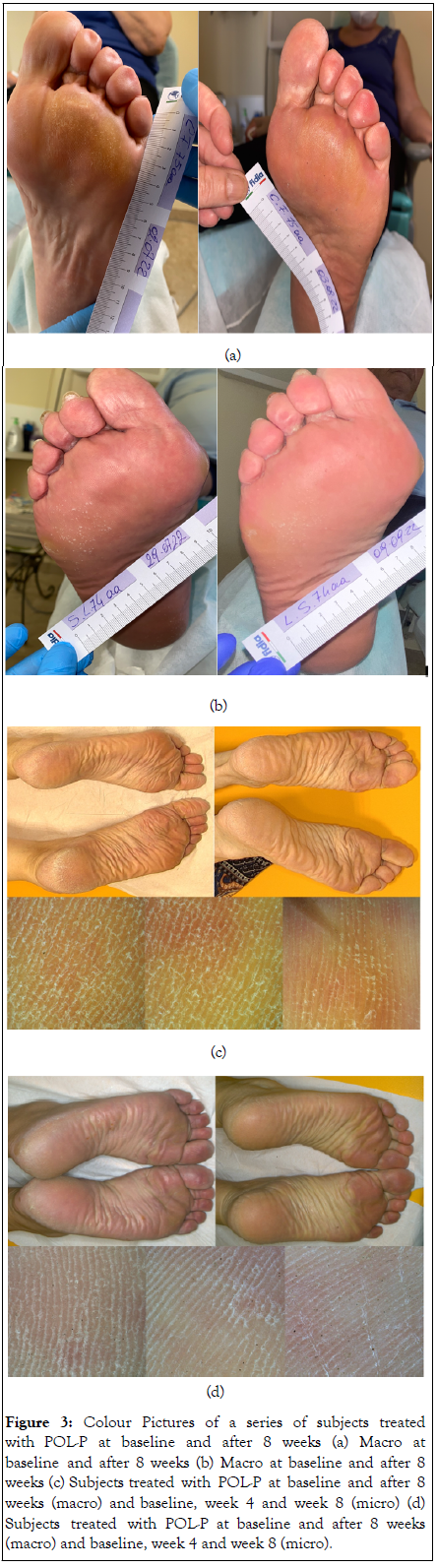
Figure 3: Colour Pictures of a series of subjects treated with POL-P at baseline and after 8 weeks (a) Macro at baseline and after 8 weeks (b) Macro at baseline and after 8 weeks (c) Subjects treated with POL-P at baseline and after 8 weeks (macro) and baseline, week 4 and week 8 (micro) (d) Subjects treated with POL-P at baseline and after 8 weeks (macro) and baseline, week 4 and week 8 (micro).
Diabetes mellitus is commonly responsible for relevant pathophysiologic changes of the skin [10]. Xerosis is a frequently observed skin alteration in subjects with diabetes especially at the extremities such as legs and feet [11]. Severe xerosis and fissuration are considered relevant risk factor conditions for the development of ulcers [12]. Xerosis of the foot in diabetic patients could represent early stage of the ulceration process through the development of fissure, cracking, and hyperkeratosis [13]. Treatment of skin dryness in diabetic subjects trough specific skin care protocols are of great relevance and should be implemented as soon as possible [14]. A good foot care is crucial in order to reduce the risk of ulcers [15]. Skin xerosis is usually treated with the long-standing use of emollients and moisturizers, for example urea [16]. The product we assessed in this study contains urea, purified omental lipid, panthenol and carnosine. Urea is considered a very effective hydrating and emollient topical product [17]. Several studies have shown that topical urea markedly improve skin hydration [18,19]. Topical omental lipids present a potent emollient and repairing activity in fragile skin [20]. A recent narrative review summarizes all the clinical evidence available for this compound in the treatment of fragile skin [21]. Carnosine is considered a molecule able to interfere with the formation of Advanced Glycosylated End-products (AGE) [22,23]. Accumulation of AGE is related with skin ageing, stiffness and in general with skin modifications in diabetics [24]. Finally, the product we evaluated contains also panthenol. Panthenol-based formulations increased skin moisture and had a significant effect on skin barrier function by decreasing TEWL values [25]. So, the composition of the tested product offers a strong rational for its use in treating the diabetic skin at risk of more severe lesions (i.e ulcer). Some study limitation should be taken in account in evaluating our results. First, this was not a double-blinded study. However, to increase the internal validity of our results we decided to overcome this problem using, in evaluating all the main study outcomes (DASI, H-E VAS scores), with an assessor-blinded approach. A second aspect to be considered is that treatment duration was 8 weeks, and therefore, no data regarding efficacy and tolerability for longer treatment duration could be inferred from this study.
Diabetes mellitus is commonly responsible for relevant pathophysiologic changes of the skin [10]. Xerosis is a frequently observed skin alteration in subjects with diabetes especially at the extremities such as legs and feet [11]. Severe xerosis and fissuration are considered relevant risk factor conditions for the development of ulcers [12]. Xerosis of the foot in diabetic patients could represent early stage of the ulceration process through the development of fissure, cracking, and hyperkeratosis [13]. Treatment of skin dryness in diabetic subjects trough specific skin care protocols are of great relevance and should be implemented as soon as possible [14]. A good foot care is crucial in order to reduce the risk of ulcers [15]. Skin xerosis is usually treated with the long-standing use of emollients and moisturizers, for example urea [16]. The product we assessed in this study contains urea, purified omental lipid, panthenol and carnosine. Urea is considered a very effective hydrating and emollient topical product [17]. Several studies have shown that topical urea markedly improve skin hydration [18,19]. Topical omental lipids present a potent emollient and repairing activity in fragile skin [20]. A recent narrative review summarizes all the clinical evidence available for this compound in the treatment of fragile skin [21]. Carnosine is considered a molecule able to interfere with the formation of Advanced Glycosylated End-products (AGE) [22,23]. Accumulation of AGE is related with skin ageing, stiffness and in general with skin modifications in diabetics [24]. Finally, the product we evaluated contains also panthenol. Panthenol-based formulations increased skin moisture and had a significant effect on skin barrier function by decreasing TEWL values [25]. So, the composition of the tested product offers a strong rational for its use in treating the diabetic skin at risk of more severe lesions (i.e ulcer). Some study limitation should be taken in account in evaluating our results. First, this was not a double-blinded study. However, to increase the internal validity of our results we decided to overcome this problem using, in evaluating all the main study outcomes (DASI, H-E VAS scores), with an assessor-blinded approach. A second aspect to be considered is that treatment duration was 8 weeks, and therefore, no data regarding efficacy and tolerability for longer treatment duration could be inferred from this study.
Emollient is a cornerstone strategy for foot care in diabetic subjects with the aim to reduce the risk of developing severe skin injuries and ulcer. A crucial physiopathology mechanism in skin alteration in diabetes is the formation of AGE (Advance Glycation End products). AGE accumulation skin has been associated with skin aging and diabetic dermopathy. Substances like urea, panthenol can improve emollience and skin hydration. Topical carnosine is able to reduce the accumulation of AGE at dermal level. This new topical product containing urea, purified omental lipids, panthenol and carnosine has shown to have a greater emollient and hydrating efficacy in the treatment of xerosis of lower legs and feet in diabetic subjects in comparison with a glycerine-based cream.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Puviani M, Eisendle K, Adamo L, Milani M (2022) Efficacy of a Urea, Omental Lipids, Carnosine and Panthenol Cream in the Treatment of Severe Foot Xerosis in Diabetic Subjects: A Randomised, Controlled, Assessor-Blinded Prospective Trial. J Clin Exp Dermatol Res. 13: 616.
Received: 26-Oct-2022, Manuscript No. JCEDR-22-19849; Editor assigned: 28-Oct-2022, Pre QC No. JCEDR-22-19849 (PQ); Reviewed: 11-Nov-2022, QC No. JCEDR-22-19849; Revised: 18-Nov-2022, Manuscript No. JCEDR-22-19849 (R); Published: 28-Nov-2022 , DOI: 10.35841/2329-9509.22.13.616
Copyright: © 2022 Puviani M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.