Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2019)Volume 10, Issue 5
Introduction and objectives: Autologous Platelet-Rich Plasma (PRP) dermal injections are considered as a therapeutic option for the medical treatment of androgenic alopecia (AGA). However, clinical efficacy of this approach in some cases could be disappointing. Positive effects of PRP in AGA seem to correlate, at least in part, with a growth factors mimicking action. A hair scalp lotion containing high-purified, growth factors-like polypeptides (octapeptide 2, acetyl decapeptide 3, oligopeptide 20 and copper tripeptide) with the addition of glycine and taurine (GFM-L) is available. So far, no data regarding the potential synergistic effect of autologous PRP treatment combined with GFM-L are available.
We therefore conducted a prospective, randomized, assessor-blinded trial to compare the efficacy and tolerability of PRP alone vs. PRP and GFM-L treatment in men and women with AGA.
Materials and methods: The study was a prospective, randomized (with a balance ratio of 2:1), assessor-blinded, 3- month trial. Thirty subjects (18 men and 12 women; mean age: 41 years) were enrolled after their written informed consent. Twenty subjects were allocated to PRP treatment cycle followed by GFM-L application for 3 months (2 ml of lotion every other day) (Group A) and 10 subjects were allocated to PRP treatment alone (Group B).
In both groups autologous PRP injections on the affected area were performed over a period of 3 months at interval of 3-4 weeks, using a volume of 10 cc of PRP for each session treatment. Primary end point was the Investigator Global Assessment (IGA) score of clinical efficacy at month 3 in comparison with baseline.
IGA score ranged from 0 (no improvement) to 3 (strong improvement). Secondary endpoint was the evolution of Hamilton scale grading (HSG), evaluated at baseline and at the end of study period. IGA score and HSG were performed by an investigator unaware of the treatment allocation group.
Results: All the enrolled subjects concluded the study period. At baseline HSG was 2.0 ± 0.6 in Group A and 2.1 ± 0.3 in Group B. At month 3, a significant reduction of HSG was observed in Group A subjects only, both in comparison with baseline and in comparison, with Group B values (1.3 ± 0.5 vs. 2.1 ± 0.37; P=0.0028). At month 3, IGA score was 2.1 ± 0.9 in group A and 0.8 ± 0.3 in group B (P=0.0031). GFM-lotion was well tolerated.
Conclusion: The combination of GFM-lotion containing growth factors like peptides and taurine with autologous PRP treatment is superior in term of efficacy to the treatment with PRP alone in subjects with AGA.
Androgenic alopecia; Platelet-rich-plasma; Growth factor; Controlled trial
Androgenic alopecia (AGA) (also known as pattern alopecia) is the most common form a chronic non-scarring hair loss, affecting both men and women [1]. AGA is characterized by progressive hair loss, especially of scalp hair, and has distinctive patterns of loss in women versus men, but in both genders the central scalp is most severely affected [2].
Follicular miniaturization and local high dihydrotestosterone (DHT) activity are considered the main pathological mechanisms involved in AGA [3]. Microinflammation is also a relevant pathogenetic mechanism involved in this clinical condition [4].
Approved therapeutic options are limited to topical minoxidil [5] for both men and women and oral finasteride [6] for men. However, both drugs possess side effects and are effective in less than 50% of treated patients [7].
Autologous Platelet-Rich Plasma (PRP) dermal injection is an additional therapeutic option for the medical treatment of AGA [8]. However, clinical efficacy of this approach in some cases could be disappointing [9]. Positive effects of PRP in AGA seem correlated, at least in part, with a growth factors mimicking action [10].
PRP is rich in growth factors like PDGF (platelet derived growth factor), TGF (transforming growth Factor Beta) and VEGF (Vascular endothelial growth Factor) [11]. A hair scalp lotion containing high-purified, growth factors-like polypeptides (octapeptide 2, acetyl decapeptide 3, oligopeptide 20 and copper tripeptide) with the addition of glycine and taurine (GFM-L) is available.
Copper tripeptide has shown to have antioxidant [12], antiinflammatory and blood vessel growth promoting action [13]. Copper tripeptide can increase the activity of FGF (Fibroblast Growth Factor) and VEGF [14]. Interestingly, copper tripeptide decreases the secretion of TGF-β [15] from dermal fibroblasts. TGF-β is involved in inducing catagen phase [16].
Copper tripeptide can also interfere with the activity of 5-α- reductase, therefore reducing the production of DHT [17]. Finally, this peptide is also able to stimulate the production of decorin [18].
Decorin, at scalp level, improves the anagen phase [19] and consequently hair growth [20]. The acetyl decapeptide is a synthetic peptide that mimics the action of two growth factors: the keratinocyte growth factor (KGF) and the epidermal growth factor (EGF), which acts on the follicle, promoting the anagen growth phase, through an antiapoptotic effect [21], maintaining the bulge stem cells active [22].
Another component of the growth factor like mixture, octapeptide 2, is able to promote hair growth [23], to reduce apoptosis and to increase keratinocyte proliferation [24].
In this formulation all the 4 oligopeptides are vehiculated in nanosomes of 250 nm in diameter. Published data state that nanosomes with a diameter<500 nm can transport molecules deep in the hair bulb, after topical application [25].
Taurine is a relevant amino acid with interesting hair growth promoting mechanisms [26]. This lotion has shown to be effective as stand-alone approach in the treatment of excessive hair loss in men and women [27].
The peculiar composition of this lotion (mixture of growth factor like peptides and taurine) suggests a potential synergistic effect on hair growth with other therapeutic strategies like PRP treatment. So far, no data regarding the potential synergistic effect of autologous PRP treatment combined with GFM-L are available.
We therefore conducted a prospective, randomized, assessorblinded trial to compare the efficacy and tolerability of PRP alone vs. PRP and GFM-L treatment in men and women with AGA.
We conducted a prospective, randomized, assessor-blinded, monocenter trial to evaluate and compare the potential additional clinical effect of a lotion containing a growth-factor like mixture and taurine (GFM-L) in subjects with AGA treated with PRP.
Study design
The clinical setting was the Dermatology and Hair pathology Unit outpatient service at the Montallergo Hospital; Genoa (Italy). The local IRB approved the study protocol in April 2018. The trial was conducted according to Good Clinical Practice Guidelines and Helsinki Declaration (update 2014) [28]. There was no change to the trial protocol after it commenced.
The study took place from June 2018 and March 2019. The study was a prospective, randomized (with a balance ratio of 2:1), assessor-blinded, 3-month trial. For allocation of the participants, a computer-generated list of random numbers was used.
Subjects
Eligible subjects included men and women aged ≥ 18 years with a diagnosis of AGA, suitable for a Platelet Rich Plasma (PRP) treatment. Exclusion criteria were other forms of hair loss, dermatitis of the scalp and a positive history of allergy or intolerance with one or more components of the tested lotion, pregnancy or breast feeding.
Thirty subjects (18 men and 12 women; mean age: 41 years) were enrolled in the trial after their written informed consent. For both groups, autologous PRP injections on the affected area were performed over a period of 3 months at interval of 3 weeks, using a volume of 10 cc of PRP for each session treatment.
In total 4 PRP injections were performed in each enrolled subject. In group A the GFM-L was applied using the dosage of 2 ml every other day for a total of 3 months.
Study outcomes
Primary end point of the trial was the Investigator Global Assessment (IGA) score of clinical efficacy at month 3 in comparison with baseline. IGA score ranged from 0 (no improvement) to 3 (strong improvement).
Secondary endpoint was the evolution of Hamilton scale grading (HSG), evaluated at baseline and at the end of study period. IGA score and HSG were performed by an investigator unaware of the treatment group (PRP alone vs. PRP and GFML).
Statistical analysis
Statistical analysis was performed using GraphPad Statistical Software (GraphPad Software, Inc., La Jolla, CA, USA). Continuous variables were expressed as mean ± standard deviation (SD). The primary outcomes of the study to evaluate and compare the evolution of IGA score.
For inferential statistical analysis we use the ANOVA multiple comparison test and the Paired Student T Test. We calculated also the 95% Confidence Intervals (CI) of the difference in all the variables evaluated.
This was a superiority trial. Sample size calculation was performed calculating the hypothized difference in IGA score between the two groups (A and B) at end of the treatment: with an effect size (Cohen’s d value) of 0.38, with an alpha value of 0.05 and a power of 80% a total of at least 30 subjects should be enrolled to detect this difference.
The sample size was calculated using G-Power statistical software version 3.9 (Kiel, Germany). A p-value of <.05 was considered as significant. Analysis was performed based on intention-to-treat (ITT) principle.
A total of 30 subjects were enrolled in the trial. Twenty subjects were allocated to PRP treatment followed by GFM-L application for 3 months (Group A) and 10 subjects were allocated to PRP treatment alone (Group B). At baseline HSG was 2.0 ± 0.6 in Group A and 2.1 ± 0.3 in Group B. All the enrolled subjects concluded the trial. In comparison to group B, group A showed a greater increase of IGA score and a more pronounced reduction in HSG.
At month 3, IGA score was 2.1 ± 0.9 in group A and 0.8 ± 0.3 in group B (P=0.0031) (Figure 1) with an absolute difference of 1.3 (95% CI: from 2 to 0.5).
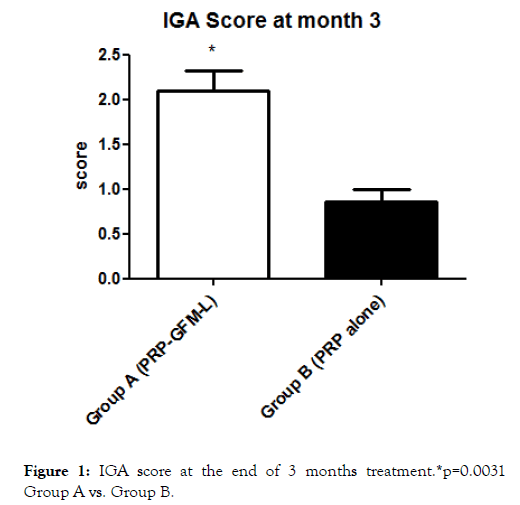
Figure 1. IGA score at the end of 3 months treatment.*p=0.0031 Group A vs. Group B.
At month 3, a significant reduction of HSG was observed in Group A subjects only, both in comparison with baseline values and in comparison with Group B values (1.3 ± 0.5 vs. 2.1 ± 0.37; P=0.0028) with an absolute difference of -0.7 (95% CI: from -1.1 to -0.2) (Figure 2).
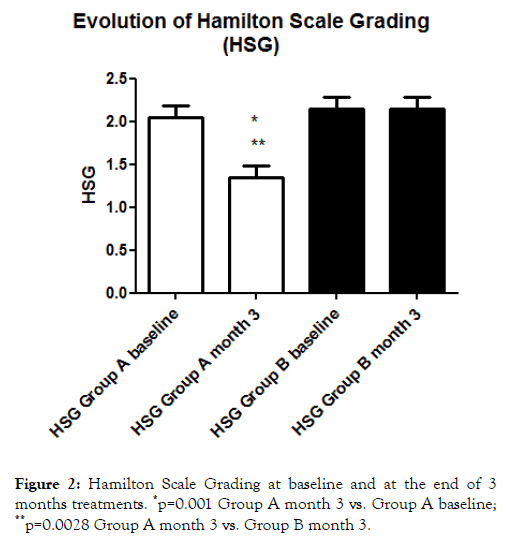
Figure 2. Hamilton Scale Grading at baseline and at the end of 3 months treatments. *p=0.001 Group A month 3 vs. Group A baseline; **p=0.0028 Group A month 3 vs. Group B month 3.
Figure 3 shows the scalp pictures of three enrolled subjects at baseline and after treatment, (two in the group A and one in group B). GFM-lotion was well tolerated. No serious side effects were reported.
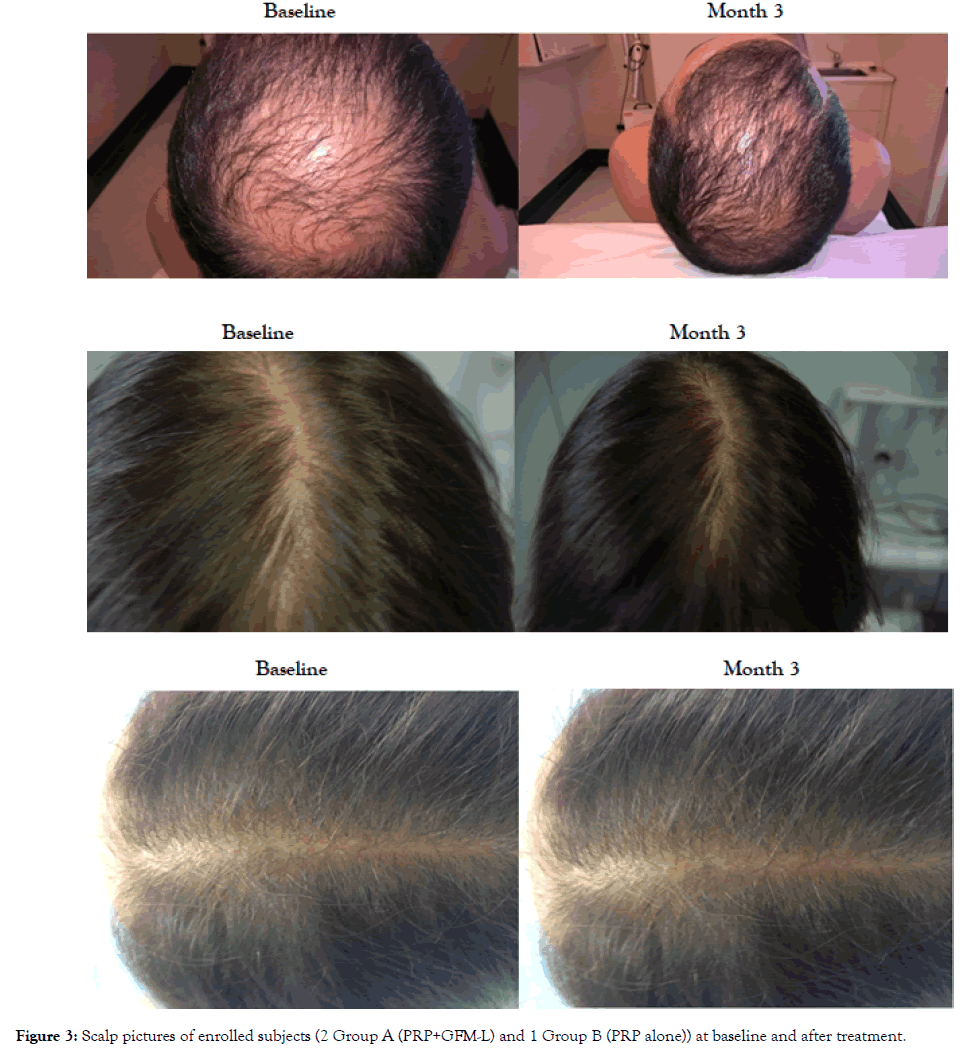
Figure 3. Scalp pictures of enrolled subjects (2 Group A (PRP+GFM-L) and 1 Group B (PRP alone)) at baseline and after treatment.
AGA is a very common hair disorder with a relevant impact on quality of life [29]. Oral finasteride and topical minoxidil are the only two drugs approved for the treatment of AGA [30]. Both drugs, however, possess side effects [31] and are effective in less than 50% of treated patients [32]. PRP is considered an alternative therapeutic approach for AGA [33]. PRP is an autologous preparation of plasma with platelets that are capable of secreting growth factors and cytokines, which stimulate stem cells [34]. The stimulatory factors in PRP, including fibrin, fibronectin and vitronectin, may be involved in hair follicle growth and development [35]. PRP contains a mixture of growth factors molecules such as PDGF, IGF and VEGF [36]. However, the results of studies on the use of PRP in AGA treatment remain controversial [37]. PRP contains also TGF-β which could have a detrimental effect on hair growth [9], promoting the catagen phase and fibrotic processes. Rodrigues has shown that efficacy of PRP in AGA seems do not correlate with the amount of the most common growth factors present in the PRP preparation, suggesting that the efficacy could be, at least in part, ascribed to additional mechanisms of action [38]. In this trial we demonstrated that the addition of a lotion containing a mixture of 4 growth-factor like polypeptides, carried in nanosomes, and taurine significantly increase the clinical effect of PRP treatment in subjects with AGA. The complex of growthfactor like polypeptides, present in the tested lotion, contains copper-tripeptide, octapeptide 2, acetyl-decapeptide and oligopeptide 20. Copper tripeptide is able to increase the activity of FGF and VEGF and it also has a potent anti-oxidant and antiinflammatory effect [12,13]. Octapeptide 2, known also as prohairin, can increase the hair growth rate, probably increasing the anagen phase, to reduce apoptosis and to increase keratinocyte proliferation [23,24]. Oligopeptide 20 can increase the insulinlike growth factor (IGF-1) production [39]. IGF-1 produced by the mesenchymal cells, is an important growth factor in different biological systems: at the level of the hair follicle, it is produced by the dermal papilla and has the function of promoting the duration of the anagen [40]. This lotion contains also taurine. This amino acid has several positive effects on hair bulb and hair growth [26]. These active components of the GFM-L are conveyed in nanosomes, which allow the rapid absorption and a deep release into the follicle. The nanosomes are small micelles within which are enclosed substances to be conducted at depths up to the follicular matrix and bulge, protecting them by the action of the endogenous protease during penetration [41]. Therefore, the composition and the formulation of this lotion (mixture of growth-factor like peptides in nanosomes and taurine) suggest a potential synergistic effect on hair growth with other therapeutic strategies like PRP treatment. The results of our study seem to confirm this hypothesis. At the moment is difficult to explain the mechanism of this additive clinical effect. The combined use of this lotion in subjects treated with PRP can amplify the growth hair stimulus but also counteract some negative actions on hair physiology of some components of PRP for example the TGF-β. Collins et al [26] have demonstrated that taurine blocks the deleterious effect of TGF-β on human hair follicule. Some limitations should be taken in account in evaluating our results. First, this trial was not a double-blind study. To increase the internal validity of the trial results, we decided to adopt an assessor-blinded approach in evaluating the main clinical efficacy outcomes of the study (evolution of IGA score). Another limit of the present trial is the relatively small sample size (30 patients in total). However, taking in account previous trials, we have performed an accurate sample size calculation which justifies the number of subjects we have enrolled in the trial.
The combination of GFM-lotion containing growth factors like peptides and taurine with autologous PRP treatment is superior in term of clinical efficacy to the treatment with PRP alone in subjects with AGA.
MM is an employee of Cantabria Labs Difa Cooper; a pharma company, producing and marketing the tested product (GFM™ Cantabria Labs Difa Cooper). MM participated to the study protocol preparation and was involved in the final version of the manuscript. MM was not involved in data analysis and statistical evaluation. TL was involved in the conduction of the trial (selection of subjects, visits, medical evaluation, data collection, and picture coding and data analysis).
Citation: Lazzari T, Milani M (2019). Efficacy of Autologous Platelet-Rich Plasma alone or in Combination with a Lotion Containing Growth- Factor like Polypeptides and Taurine in the Treatment of Androgenic Alopecia: A Randomized, Prospective, Assessor-Blinded Trial. J Clin Exp Dermatol Res. 10:506. Doi: 10.35248/2155-9554.19.10.506
Received: 06-Sep-2019 Accepted: 16-Sep-2019 Published: 24-Sep-2019 , DOI: 10.35248/2155-9554.19.10.506
Copyright: © 2019 Lazzari T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.