
Cell & Developmental Biology
Open Access
ISSN: 2168-9296

ISSN: 2168-9296
Research Article - (2022)Volume 11, Issue 1
Lung cancer is the leading cause of cancer deaths worldwide [1], about 85% of cases are diagnosed as Non-Small-Cell Lung Cancer (NSCLC) [2]. The median age of NSCLC patients is 70 years and the disease is usually diagnosed in advanced stages, when curative surgery is no longer feasible [3]. In metastasized disease, firstline chemotherapy is often not successful and the 5-year survival rate is only 4.2% [3]. NSCLC is histologically classified into the major subtype’s adenocarcinoma (40%) [4,5], Recurring mutations have been reported in genes coding for Epidermal Growth Factor Receptors (EGFR) in 10%-40% of adenocarcinomas [6-8], EGFR mutations can lead to constitutive activation of anti-apoptotic and proliferation signaling pathways, which promote cancer progression [9], EGFR Tyrosine Kinase Inhibitors (TKI) are the preferred firstline treatment for advanced NSCLC with EGFR mutations [10,11], treating NSCLC is challenging because of the advanced age of patients. As EGFR-TKI avoid the systemic side effects of traditional chemotherapy, they might be more suitable for treating elderly patients [12]. Osimertinib, a third-generation EGFR-TKI that selectively binds the C797 residue inhibiting the T790M mutation, has shown high activity in term of Progression-Free Survival (PFS) and overall response rate in EGFR-T790M positive patients [13,14] and efficacy superior to gefitinib/erlotinip in the first-line treatment by approximately a 9 months advantage in PFS [15]. However, acquired resistance occurs also to osimertinib either in T790Mpositive NSCLC patients or in patients treated in first-line [16,17]. EGFR-dependent or independent mechanisms of resistance have been described even if they remain not completely understood [16]. EGFR G796/C797, L792 and L718/G719 mutations, MET and HER2 amplification, BRAF, KRAS and PIK3CA mutations, oncogenic fusion mutations in FGFR3, RET and NTRK were recently identified in a large cohorts of osimertinib-resistant lung cancer patients either treated in second-line [18,19] and in firstline [20]. Knowledge of these mechanisms is relevant in order to develop new therapeutic strategies to overcome TKI-resistance; however, how prevent or delay the acquisition of resistance remains an important issue. Some data indicated that in PC9 cell line and xenograft models, the combination of gefitinib with pemetrexed or the intermittent combination of pemetrexed and gefitinib prevented some the appearance of gefitinib resistance mediated by T790M mutation and epithelial-mesenchymal transition [20]. However, the combination was ineffective when gefitinib was administered before pemetrexed. Theoretically, chemotherapy, given its different and more generic mechanism of action, can postpone the resistance to EGFR-TKIs by limiting the tumor heterogeneity, thus improving the efficacy of treatment either in first-and second-line. Osimertinib combined or intercalated with chemotherapy deserves to be considered either for patients in progression after first/secondgeneration TKIs or in first-line setting. Our study was undertaken to explore a long-term survival outcomes in the combination of osimertinib with pemetrexed add platinum and the combination of gefitinib/erlotinip with pemetrexed add carboplatin in elderly lung adenocarcinoma patients.
Study design and patients
Our study was a five-arm randomized open-label phase II study, a pemetrexed-carboplatin plus gefitinib-erlotinip group, a pemetrexed-carboplatin plus gefitinib-erlotinip plus osimertinib group, pemetrexed plus carboplatin group, gefitinib-erlotinip group, osimertinib group as first-line therapy for lung adenocarcinoma patients. The study was approved by the Institutional Review Board of the Shan-Xi Bethune Hospital and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided informed consent before any study-related procedure. Patients were eligible for our study if they were 45-84 years old had a histologic or cytological diagnosis of locally advanced or metastatic adenocarcinoma (Stage IIIB or IV). The staging was performed according to the 7th edition of the TNM classification. Patients required at least one measurable lesion meeting Response Evaluation Criteria in Solid Tumors (RECIST) guidelines and an Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 0–1. Patients were excluded with surgical-complex therapy, radio-complex therapy, if they had received systemic anticancer therapy for advanced disease; had unstable systemic disease, including active infection, uncontrolled hypertension, and unstable angina. The study was conducted according to the Declaration of Helsinki and approved by the Shan-Xi Bethune Hospital, Taiyuan City, Shanxi Province Ethics Committee. All participants included in the study signed written informed consent before treatment.
Randomization
Patients were allocated in a 1:1:1:1:1; ratio using minimization software. Randomization was stratified by different treatments throughout the study, clinicians and study participants were not masked to the identity of the study treatment.
Treatment protocol
Before TKI therapy, tumor gene mutation profile, including EGFRT790M, ALK-M, KRAS-M, METM, RETM, ROS and so on gene, was performed. If the test was positive, first-generation TKI therapy drugs, Gefitinib, Erlotinip, were used. Eligible patients were randomized to one of the following treatment arms: Patients with advanced lung adenocarcinoma were randomly assigned to five groups. The combination group A: received pemetrexed (500 mg/m2 on day 1) plus carboplatin (10 mg /m2, on day 1) combined with gefitinib (250 mg/day on days 5–21day) or erlotinip (150 mg/ day on days 5–21day) and repeated every four weeks for up to six cycles and then continued to receive pemetrexed plus carboplatin combined with gefitinib or Erlotinip every four weeks. The combination group B: received pemetrexed and carboplatin plus gefitinib-erlotinip plus osimertinib, pemetrexed and carboplatin plus gefitinib-erlotinib usage as before, when gefitinib-erlotinib plus pemetrexed and carboplatin occurs after resistance, add osimertinib,(80 mg/day on days 5–21day) repeated every four weeks for up to six cycles. The chemotherapy group received the same chemotherapy regimen as the combination group every four weeks for up to six cycles and then continued to receive pemetrexed plus carboplatin alone every four weeks. The gefitinib or erlotinip group received gefitinib or erlotinip alone. Osimertinib group received osimertinib alone; all therapies were continued until progression, unacceptable toxicity or death. Tumor response was assessed by use of CT with RECIST every 4 weeks until treatment cessation or disease progression.
Outcomes measure
The primary end point was PFS. Secondary end points included OS, Overall Response Rate (ORR) and the adverse-event profile. PFS was defined as the time from the date of randomization to the first date of disease progression or death from any cause. For patients not known to have died as of the data cut-off date and who did not have objective progressive disease, PFS was censored at the date of the last objective progression-free disease assessment. OS was measured from the date of randomization to the date of death. For each patient who was not known to have died as of the data inclusion cut-off date for a particular analysis, OS was censored for that analysis at the date of last prior contact. Toxicity was graded according to National Cancer Institute Common Terminology Criteria for adverse events, version 4.0. Analyses were performed on an Intention-To-Treat (ITT) basis.
Statistical analysis
The leading parameter for sample size calculation was PFS. The sample size was set at 200 patients in total using a log-rank-test power analysis, on the basis of several assumptions, clinical parameters and adverse events among five arms were analyzed using the χ2 test. Survival results were summarized as median values and two-sided 95% Confidence Intervals (CIs) and were analyzed using Kaplan–Meier's technique, whereas the log-rank test was used for comparisons among subgroups. Multivariable adjusted Hazard Ratios (HRs) for all-cause mortality by patient and treatment pattern were estimated using Cox regression. HRs was calculated along with their corresponding 95% CIs as measurements of association. the graph sand statistical tests have been made using the Graph Pad Prism software (versions 8.0.1, 9.0.0) and statistical tests were conducted using the SPSS software, version 22 (SPSS, Inc., Chicago, IL),was used for all statistical analyses.
Between January 1, 2017 and June 30, 2021, 200 patients were randomly assigned to chemotherapy plus gefitinib-erlotinib group (N=40), chemotherapy plus gefitinib-erlotinib osimertinib group (N=40), chemotherapy group (N=40) or gefitinib-erlotinib alone group (N=40), osimertinib alone group (N=40).The demographics were balanced between the treatment arms (Tables 1 and 2). At the time of analysis of our study, due to disease progression, protocol violation (Figure 1); 30 gefitinib-erlotinib combination chemotherapy, 28 osimertinib gefitinib-erlotinib combination chemotherapy and 30 alone chemotherapy, 33 gefitinib-erlotinib, 29 Osimertinib discontinued the first-line combination respectively, have OS events. Confidence interval, Cox regression models with adjustment for single factors showed a significant influence of age (year) (p=0.005), gender (p=0.04) and EGFR status (p=0.01), first add third generation TKI therapy with chemical therapy (p=0.02) on OS. Accordingly, Age (year) 55-69 had an 51% reduced risk of death compared to ≥ 70 (year) (hazard ratio ((HR) 0.49, 95% CI 0.30–0.81). Females had an almost 30% reduced risk of death compared to males (hazard ratio ((HR) 0.71, 95% CI 0.53–0.96). Patients with an EGFR mutation had an almost 28% reduced risk of death compared to negative patients ((HR) 0.72, 95% CI 0.07-0.25). First add third generation TKI therapy with chemical therapy had an almost 54% reduced risk of death compared to first add third generation TKI therapy alone. The mutant patients had a longer Overall Survival (OS) than the wild-type patients; our patient with 60% positive EGFR gene mutations demonstrated a longer progress-free OS survival than those with negative and wild-type gene. Nevertheless, EGFR mutations were more frequent in patients over 75 than in younger patients: 17% versus 8.1% (p<0.001) (Figures 2A-2D and 3) (Table 3).
| Characteristic | No. (%) |
|---|---|
| Age (yr) | |
| 45-69 | 126 (63.00) |
| ≥ 70 | 74 (37.00) |
| Gender | |
| Male | 89 (44.73) |
| Female | 110 (55.26) |
| Gene mutation(Tested)< | |
| EGFR + | 120 (60.00) |
| EGFR - | 36 (18.00) |
| Wild-type | 4 (2.00) |
| T790M mutations + | 37 (18.50) |
| ALK mutations+ | 5 (2.50) |
| KRAS mutations+ | 11 (5.50) |
| RET mutations+ | 7 (3.50) |
| MET mutations+ | 7 (3.50) |
| EGFR gene mutation site-n | |
| Exon18 | 9 (4.50) |
| Exon19 | 47 (23.50) |
| Exon19 + Exon21 | 10 (5.00) |
| Exon 20 + | 6 (3.00) |
| Exon21L858R | 70 (35.00) |
| Chemical-TKI therapy | |
| Yes | 80 (40.00) |
| No | 120 (60,00) |
| First-generation Chemical-TKI therapy | 40 (20.00) |
| First-Third generation Chemical-TKI therapy | 40 (20.00) |
| TKI therapy alone | |
| Yes | 80 (40.00) |
| No | 120 (60.00) |
| First generation TKI therapy alone | 40 (20.00) |
| Third generation TKI therapy alone | 40 (20.00) |
| Chemical-therapy alone | 40 (20.00) |
Table 1: Patient baseline characteristics (N=200)
| Characteristic | Univariable analyses | Multivariable analyses | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Age (year) | ||||
| 45-69 | Reference | Reference | ||
| ≥ 70 | 0.49 (0.30-0.81) | 0.005 | 0.49 (0.28-0.89) | 0.02 |
| Gender | ||||
| Male | Reference | Reference | ||
| Female | 0.77 (0.53–0.96) | 0.04 | 0.92 (0.56-1.53) | 0.76 |
| Gene mutation | ||||
| No | Reference | Reference | ||
| Yes | 0.72 (0.07-0.25) | 0.01 | 0.15 (0.07-0.29) | 0.01 |
| First add third generation TKI therapy with Chemical therapy | ||||
| No | Reference | Reference | ||
| Yes | 0.56 (0.35-0.93) | 0.02 | 1.50 (0.42-5.31) | 0.52 |
| First add third generation TKI therapy alone | ||||
| No | Reference | Reference | ||
| Yes | 1.11 (0.64-1.91) | 0.72 | 0.71 (0.18-2.71) | 0.62 |
| Chemical therapy alone | ||||
| No | Reference | Reference | ||
| Yes | 8.05 (4.07-15.93) | 0.001 | 5.97 (1.45-24.44) | 0.01 |
Table 2: Results of Cox univariate and multivariate regression analysis
| First generation Chemical- TKItherapy group (N=40) | First generation TKItherapy group (N=40) | p | First-Third generation Chemical- TKItherapy group (N=40) | Third generation TKItherapy group (N=40) | p | ChemicalItherapy group (N=40) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.001 | All (%) | Grade 3–4 (%) | All (%) | Grade 3–4 (%) | Grade 3-4 | All (%) | Grade 3–4 (%) | All (%) | Grade 3–4 (%) | Grade 3–4 | All (%) | Grade 3–4 (%) |
| Skin rash | 25 (63.12) | 4 (10.0) | 25 (61.48) | 2 (5.00) | 0.25 | 26 (65.00) | 6 (15.00) | 21 (53.02) | 0 (0.00) | 1 | 8 (20.0) | 0 (0.00) |
| Anorexia | 21 (53.12) | 0 (0.00) | 17 (42.21) | 0 (0.00) | 1 | 24 (60.00) | 0 (0.00) | 13 (31.81) | 0 (0.00) | 1 | 16 (40.0) | 0 (0.00) |
| Cough | 18 (46.12) | 0 (0.00) | 16 (40.23) | 0 (0.00) | 1 | 19 (45.00) | 0 (0.00) | 13 (33.31) | 0 (0.00) | 1 | 13 (33.31) | 0 (0.00) |
| Nausea | 18 (43.12) | 0 (0.00) | 12 (30.23) | 0 (0.00) | 1 | 19 (47.50) | 0 (0.00) | 10 (24.21) | 0 (0.00) | 1 | 16 (40.0) | 0 (0.00) |
| Fatigue | 12 (30.00) | 5 (12.5) | 11 (27.58) | 3 (7.50) | 1 | 15 (37.50) | 6 (15.00) | 4 (10.61) | 0 (0.00) | 0.22 | 11 (27.5) | 2 (5.0) |
| Diarrhea | 19 (47.12) | 0 (0.00) | 17 (43.34) | 0 (0.00) | 1 | 24 (60.00) | 0 (0.00) | 13 (31.81) | 0 (0.00) | 1 | 8 (20.0) | 0 (0.00) |
| Neutrop | 13 (32.12) | 7 (12.00) | 11 (27.12) | 4 (10.00) | 0.15 | 15 (37.50) | 10 (25.00) | 7 (18.22) | 0 (0.00) | 1 | 14 (35.0) | 5 (12.5) |
| enia | ||||||||||||
| Anemia | 17 (43.00) | 0 (0.00) | 14 (35.12) | 0 (0.00) | 1 | 18 (45.00) | 0 (0.00) | 7 (27.31) | 0 (0.00) | 1 | 16 (40.0) | 0 (0.00) |
| Thrombocy | 17 (40.07) | 0 (0.00) | 13 (32.12) | 0 (0.00) | 1 | 18 (45.00) | 0 (0.00) | 11 (27.31) | 0 (0.00) | 1 | 10 (25.0) | 0 (0.00) |
| topenia | ||||||||||||
| Increased LFT | 22 (54.54) | 4 (10.0) | 17 (41.72) | 3 (8.0) | 0.35 | 24 (60.00) | 5 (12.50) | 5 (13.61) | 0 (0.00) | 1 | 10 (27.5) | 0 (0.00) |
Table 3: The most common grade 3–4 adverse events were Neutropenia: 7patients (12.0%) in First generation Chemical-TKI therapy group, 5 patients (12.5%) in the chemotherapy group and 10 patients(25.00%) in First-Third generation Chemical-TKI therapy group, Fatigue: 5 patients(7.5%)in First generation Chemical-TKI therapy group,6 patients(15.00%) in First-Third generation Chemical-TKI therapy group,3 patients[7.5%] in First generation target group, 2 patients [5.0%] in the chemotherapy group, Liver dysfunction: 4 patients [10.0%] in First generation Chemical-TKI therapy group and 3 patient [8%] in First generation target group and 5 patient [12.50%] in First-Third generation Chemical-TKI therapy. Skin rash: 4 patients (10.0%) in First generation Chemical-TKI therapy group,6 patients(15.00)in First-Third generation Chemical-TKI therapy group, Comparisons of grade 3–4 adverse events among five arms were performed ,using χ2 test. None of the p-value is small than 0.05.There was no grade 5 adverse events.
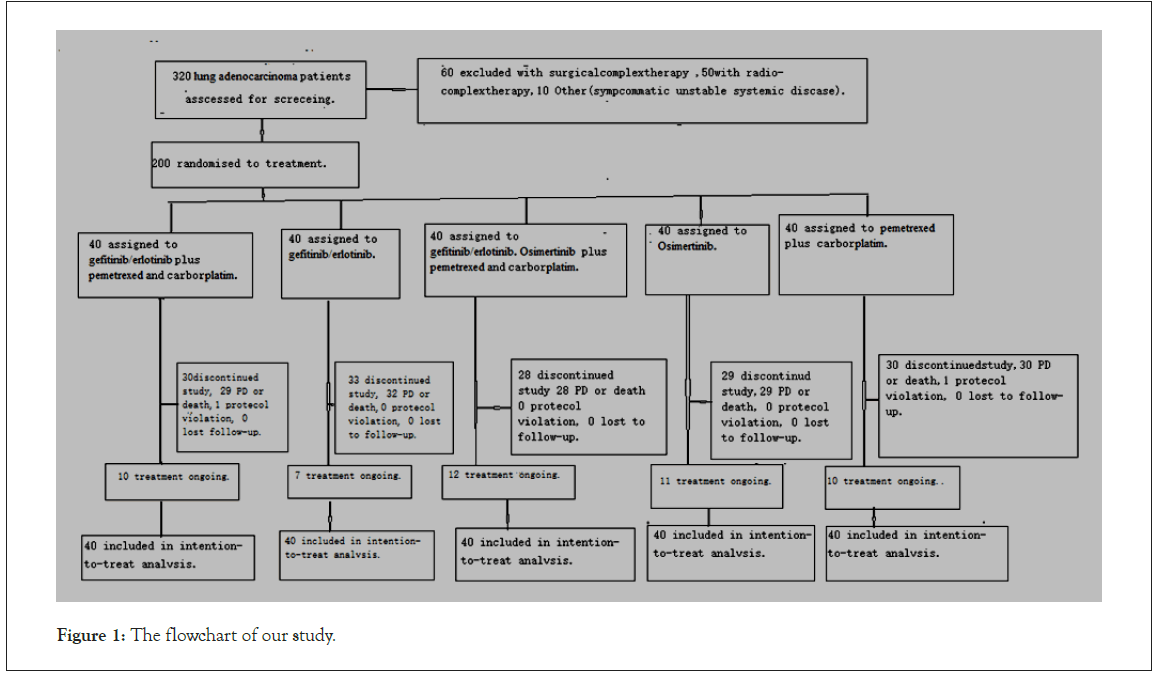
Figure 1: The flowchart of our study.
Figure 2A: First generation Chemical-TKI therapy group PFS vs First generation
TKI therapy group PFS vs chemotherapy (pemetrexed plus carborplatim ) group PFS
P<0.05.Mean Survival Time 22.00 month,95%CI [16.29,27.70] vs 14.00 month 95%CI
[11.98,20.01]vs 10,81 months, 95% CI [ 8.99–12.64]. 
Figure 2B: First generation Chemical-TKI therapy group OS vs First generation TKI
therapy alone OS vs chemotherapy (pemetrexed plus carborplatim) group OS P<0.05. Mean
Survival Time 32.00 month,95%CI[25.29,3871]vs 28.00 month,95%CI[14.58,41.41]vs
11,51 months, 95% CI, [8.96–13.64]. 
Figure 2C: First-Third generation Chemical-TKI therapy group PFS vs Third generation
TKI therapy PFS vs chemotherapy (pemetrexed plus carborplatim) group PFS.P<0.001.
Mean Survival Time 40.00,95%CI[28.12,51.87] vs 26.66,95%CI[24.77,29.22] vs
10,81 months, 95% CI,[ 8.99–12.64]. 
Figure 2D: First-Third generation Chemical-TKI therapy group OS vs Third generation
TKI therapy group OS vs chemotherapy (pemetrexed plus carborplatim) group. P<0.05.
Mean Survival Time 48.00.95%CI[42.81,53.18] vs 36.00. 95%CI[34.71,38.28]vs 11,51
months, 95% CI, [8.96–13.64]. 
Figure 3: Waterfall plot of the best per cent change in target lesions from baseline for 200 patients based on investigator assessment. shows
the best per cent change from baseline in terms of size of target lesion for patients with measurable disease.AC+G:First generation Chemical-
TKI therapy group+First-Third generation Chemical-TKI therapy group, G:First generation TKI therapy group+Third generation TKI therapy
group, AC; Chemical therapy group. Tumor response was assessed as Complete Response (CR), Partial Response (PR), Stable Disease (SD),
and Progression Disease (PD) in accordance with the standard of RECIST [21]. 
Our study examined the efficacy of gefitinib-erlotinib combined with carboplatin and pemetrexed for first-line treatment of advanced NSCLC with sensitive EGFR mutations. The study met its Primary End Point (PFS) at the last day of follow-up (June 30, 2021). The PFS results for the five treatment arms are clearly divided by the performed test. The PFS of the combination group was significantly longer than those in the gefitinib-erlotinib alone group and chemotherapy alone group. The results of our study indicate that gefitinib-erlotinib combined with carboplatin and pemetrexed as first-line therapy provides better survival benefits than gefitinib-erlotinib alone. Similar results were also achieved in a previous study in which gefitinib plus pemetrexed provided greater PFS than gefitinib alone (15.8 months vs.10.9 months, HR=0.69) as first-line therapy for EGFR-mutated NSCLC [21,22]. The results in another prospective single arm clinical trial achieved a median PFS of 18.0 months for EGFR-mutated patients using pemetrexed plus gefitinib [23]. The median OS for our combination group was 32.0 months, which is similar to that reported in the FASTACT-2 study, intercalating chemotherapy with erlotinib [24], Han reported the combination group PFS17.5months, OS32.6 month was longer than those in the gefitinib-group IPFS11.9months, OS 25.8 month [25]. In another study are same [26-31]. In this setting, the most important question is whether the combination strategy is better than EGFR TKI alone. Somatic mutations of EGFR have been associated with sensitivity to EGFR-TKIs in patients with advanced NSCLC. However, most of those EGFR mutated NSCLC patients develop acquired EGFR TKI resistance after 10-14 months of firstline TKI treatment. It was reported that simultaneous treatment with gefitinib and pemetrexed prevented the appearance of gefitinib resistance mediated by the T790M mutation or Epithelialto- Mesenchymal Transition (EMT) in PC9 and HCC827 cells, respectively [32]. Sequential use of chemotherapy followed by gefitinib enhances the antitumor effect in NSCLC cell lines poorly responsive to reversible EGFR TKIs [33]. According to a previous study, the combination of EGFR TKIs and cisplatin is also an effective treatment against EGFR TKI-resistant cancer [34]. In addition, FASTACT2 used to evaluate erlotinib plus gemcitabine and platinum therapy in NSCLC patients showed that the combination of platinum-based chemotherapy and TKI exhibited favorable tolerability and promising efficacy [24]. In this study, the median PFS, OS of patients reached 22months, 32 months, in the combination group, but only 14 months, 26 months in the TKI mono therapy group, thus confirming the superiority of the combination therapy mentioned before. In this study, we also discuss the efficacy of osimertinib as a second-line treatment in patients with EGFR T790M-positive advanced NSCLC who experience disease progression with prior first-generation EGFR-TKI treatment. Finally, 40 patients met the enrollment criteria and were analyzed. The EGFR T790M secondary mutation is the most common resistance alteration in EGFR-mutant NSCLC patients treated with the first-or-secondgeneration TKIs [35-38]. Our findings showed that in advanced adenocarcinoma patients with EGFR T790M mutation after obtaining first-generation EGFR-TKI resistance; add osimertinib alone and osimertinib combination chemotherapy arms as the second-line treatment to control recurrent foci may be more effective in its action than in first-generation EGFR-TKI treatment alone and combination chemotherapy arms. It is noteworthy that osimertinib combined chemotherapy and osimertinib alone may exert survival benefits in advanced adenocarcinoma. In a clinical trial of AURA3, when patients who harbored T790M after drug resistance to first-line EGFR-TKI received osimertinib treatment, the median PFS was 10.1 months and the median OS was 26.8 months [39,40]. In a investigation151patients T790M mutation NSCLC with an acquired resistance to first-line EGFR-TKIs then received osimertinib as subsequent treatment, defined first-line EGFR-TKI (PFS1) as the time from the first dose of first-line EGFRTKI to progression or death, while the estimated median PFS of osimertinib (PFS2) was determined as the time from the first dose of osimertinib to progression or death. The ORR and DCR of osimertinib was 56.3% and 88.0%, respectively. The median PFS2 was 10.1 months, OS of osimertinib was 30.2 months, median PFS1+PFS2 was 27.5 months and the median OS from first-line EGFR-TKI to death was 61.3 months [41]. The current standard-ofcare for such patients is the use of the osimertinib which irreversibly inhibits the activity of both the EGFR-activating (L858R, exon 19 deletion) [42,43]. In 2019, at the European Medical Oncology Annual Meeting (ESMO) reported, Osimertinib alone had a significant reduction 60% (ORR) in adenocarcinoma, PFS 18.9 month, OS 38.6 month. A phase III trial evaluating osimertinib combined with platinum-pemetrexed vs. osimertinib mono therapy could be the right step forward to significantly prolong the survival of EGFR-mutated NSCLC patients [44]. Osimertinib had been frequently used as the backbone of combination treatment [45-47]. After eradicating tumors with heterogeneity, adding chemotherapy to osimertinib might increase the response rate and improve PFS and OS with a low incidence of grade ≥ 3 AEs [48], at the same time, most of Brain Metastasis (BM) courses in after firstgeneration EGFR-TKI treatment resistance. A large randomized trial comparing osimertinib to gefitinib or erlotinib reported that PFS was significantly longer in the osimertinib arms and time to CNS metastases was significantly delayed because osimertinib crosses the blood-brain barrier [49]. The efficacy of osimertinib in BM patients may be the increased concentration of osimertinib in CSF as compared with those of previous drugs (such as first and second-generation EGFR-TKI or chemotherapy) [13,14]. This extended its survival period; this intercalated combination strategy only caused a minimal increase in toxicities compared to gefitiniberlotinib with chemotherapy [50]. The common grade 3-4 treatmentrelated adverse events in combination arm were liver dysfunction, neutropenia and fatigue and skin rash. Combinational strategy resulted in a modest increase in liver dysfunction than gefitiniberlotinib arm, which is consistent with the results in JMIT study [51].
Our finding suggested that treatment with pemetrexed plus carboplatin combined with gefitinib-erlotinip and pemetrexed plus carboplatin combined with gefitinib-erlotinip osimertinib group could provide better survival benefits for patients with lung adenocarcinoma harboring sensitive EGFR mutations. Our study demonstrated that pemetrexed plus carboplatin combined with gefitinib-erlotinib osimertinib provided longer PFS, OS compared with either agent alone in patients with sensitive EGFR-mutant lung adenocarcinoma. The toxicity profiles show good tolerance by patients. First-Third generation Chemical-TKI therapy group was a good treatment option. This combinational strategy may yield new choices for the treatment of sensitive EGFR-mutant NSCLC. This trial was a mono-center study and the results should be interpreted considering this limitation. These results need to be confirmed through a multicenter study.
[Cross Ref] [Google scholar] [Pub med]
[Cross Ref] [Google scholar] [Pub med]
[GoogleScholar] [Pubmed]
[Cross Ref] [Google scholar] [Pub med]
[Cross Ref] [Google scholar] [Pub med]
[Cross Ref] [Google scholar] [Pub med]
[Cross Ref] [Google scholar] [Pub med]
[Cross Ref] [Google scholar] [Pub med]
[Cross Ref] [Google scholar] [Pub med]
[Cross Ref] [Google scholar] [Pub med]
[Cross Ref] [Google scholar] [Pub med]
[Cross Ref] [Google scholar] [Pub med]
[Cross Ref] [Google scholar] [Pub med]
[Cross Ref] [Google scholar] [Pub med]
Citation: Chen MW, He AT, Pei Y (2022) Efficacy of First-Third Generation EGFR Inhibitor Combined with Chemotherapy as First-Line Treatment for Advanced Lung Adenocarcinoma in a Real-World Setting. Cell Dev Biol. 11:245.
Received: 05-Jan-2022, Manuscript No. CDB-22-15734; Editor assigned: 07-Jan-2022, Pre QC No. CDB-22-15734 (PQ); Reviewed: 21-Jan-2022, QC No. CDB-22-15734; Revised: 24-Jan-2022, Manuscript No. CDB-22-15734 (R); Published: 31-Jan-2022 , DOI: 10.35248/ 2168-9296.22.11.245
Copyright: © 2022 He AT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.