Journal of Women's Health Care
Open Access
ISSN: 2167-0420
ISSN: 2167-0420
Research - (2023)Volume 12, Issue 11
Background: Polycystic Ovary Syndrome (PCOS) is a complex metabolic disorder affecting 5 to 10% of women of reproductive age group, with key features including; menstrual cycle disturbance, obesity, hyperandrogenism and the commonest cause of an ovulatory infertility. An effective, simple and safe treatment of PCOS is an important public health initiative with the recent introduction of letrozole as against the traditional use of Clomiphene citrate for ovulation induction in affected patients. Objective: The objective of this study was to compare the efficacy and safety of letrozole in the induction of ovulation in infertile an ovulatory PCOS patients in comparison with clomiphene citrate.
Methodology: One hundred and forty-two infertile patients aged 18 to 40 years that met the inclusion criteria for infertile an ovulatory PCOS were recruited and randomized by computer-based techniques to receive letrozole 5mg or clomiphene citrate 100mg on day 2 to day 6 of menstruation respectively. Data was analyzed using Statistical Package for Social Sciences (SPSS) version 25. Chi-square and Fisher’s exact tests were used to compare qualitative variables and the Student-t test to compare the qualities of the two treatment groups where necessary with p <0.05 considered significant statistical association. The research was approved by the Research Ethics Committee of the Federal Medical Centre, Makurdi and all participants gave written informed consent.
Results: Women in both groups were similar with respect to demographic characteristics at randomization. Following treatment, ovulation (p value=0.001), mean endometrial thickness at midcycle of menstruation (p value=0.000) and pregnancy rate (p value=0.019) occurred significantly more often in patients treated with letrozole than did in the clomiphene citrate group. However in a general perspective, Letrozole and clomiphene citrate were effective. No significant differences were observed with adverse effect between the letrozole and clomiphene citrate treatment groups (p value=0.118). However, there were more hot flushes and dizziness with patients treated with CC in comparison with letrozole.
Conclusion: Letrozole improved ovulation, endometrial thickness and pregnancy rates in patients with an ovulatory infertility PCOS without significant adverse effects when compared to treatment with clomiphene citrate. Therefore, Letrozole could replace CC as the first line medication for ovulation induction in infertile patients with PCOS especially where cost implications or other logistic challenges associated with its sourcing are non-restrictive.
Polycystic ovary syndrome, Infertility, Letrozole, Clomiphene Citrate, Ovulation, Endometrial Thickness, Pregnancy
The Polycystic Ovary Syndrome (PCOS) is a complex reproductive metabolic disorder affecting 5 to 10% of women of reproductive age group, with key features including; menstrual cycle disturbance, obesity, hyperandrogenism and infertility. It is commonest cause of an ovulatory infertility [1].
According to the joint European Society for Human Reproduction and Embryology/ American Society for Reproductive Medicine (ESHRE / ASRM), PCOS is defined by at least of two the following criteria: 1) Oligo- and / or anovulation i.e. oligomenorrhoea or amenorrhoea; 2) Hyperandrogenism (clinical features and / or biochemical elevation of testosterone); and / or 3) Polycystic ovaries assessed by ultrasound scan [1].
The ovaries in PCOS may contain multiple sub capsular cysts that are 2 to 9mm in diameter and numbering 12 or more, arranged peripherally (sub capsular) or scattered throughout a hyperplastic stroma. Additionally, most of these cysts contain potentially viable oocytes, within dysfunctional follicles [2]. Atretic follicles are being continuously replaced by new follicles, none of which grows to become the dominant ovulatory follicle. The stroma is abundant, dense, hypervascularised and secretes androgen. While about 20 to 25% of women with sonographically evident polycystic ovaries have no symptoms, not all cases of hyperandrogenic anovulation have sonographically polycystic ovaries [3-7]. Thus, the presence of polycystic ovaries as a maker of PCOS is relative and non-specific.
Although most symptoms of PCOS can be adequately controlled or eliminated with proper diagnosis and adequate management, treatment modalities and ovulation induction protocols must be balanced for optimal results [8]. For decades, clomiphene citrate (CC), a selective estrogen receptor modulator has been the first line treatment for induction of ovulation in PCOS with infertility. However, CC has some acclaimed drawbacks in efficacy. Some studies suggest ovulation rate with use of CC in treatment of infertile an ovulatory PCOS as high as 50% to 80% but rate of pregnancy between 10% to 20% with one cycle use [9, 10] and only about a 22% live birth with up to six cycles [11]. While other studies reported a relatively high multiple pregnancy rate (3 to 8%) as compared with the rate associated with unassisted conception (<1%) and an undesirable side effect profile including mood changes, increased risk for ovarian hyperstimulation syndrome and hot flushes [12] as well as thinning of endometrial lining [13].
Failure either to ovulate with CC treatment (CC resistance) which occurred in about 25% of the patients diagnosed of PCOS with infertility or to conceive (CC failure) often results in the use of more expensive treatment options for infertility that may be associated with higher multiple pregnancy rates and increased risk of the ovarian hyperstimulation syndrome [14]. Clomiphene citrate is not equally successful in all situations, the desire for a more effective alternative and safe ovulatory agent persists [15-19].
Letrozole is a potent, nonsteroidal, aromatase inhibitor, originally used in postmenopausal breast cancer therapy to prevent and treat hormone-responsive breast cancer; at present its only registered indication [18]. It was introduced into infertility practice in the year 2000 and it works by suppressing oestrogen production and has been used to induce ovulation [18]. Letrozole has found acceptance in various clinical situations and the indications for use have expanded. In contrast to clomiphene, letrozole elicits a monofollicular response and does not adversely affect either the endometrium or the cervical mucus, due to an absence of a peripheral oestrogen receptor blockage [18, 19]. Letrozole is also cleared from the circulation more rapidly due to a shorter halflife (48 hours) as compared to clomiphene citrate which may take up to 2 months due to its prolonged half-life (2 weeks) [18]. It is regarded as an alternative treatment to CC with insulin sensitizing effect which has attracted attention [19]. Letrozole, an aromatase inhibitor which blocks oestrogen synthesis, directly affects hypothalamic pituitary ovarian function and might increase ovulation rate as well as pregnancy rates [16]. Potential advantages of letrozole over CC include a more physiologic hormonal stimulation of the endometrium, higher ovulation rate, a lower multiple pregnancy rate through single follicle recruitment, a better side effect profile with more rapid clearance, thus reducing the chances of periconceptional exposure [17].
Infertility is a big societal problem with estimates suggesting that, about one in four couples have difficulty in conceiving successfully [20-22]. There has been some controlled trials (RCTs) and metaanalyses conducted to assess the efficiency and safety letrozole in infertile an ovulatory PCOS patients and in comparison with CC which have been largely inconclusive [23-26].
Anecdotal reports in our Centre suggest an ovulatory infertility to be on an increase with over 75% of cases attributable. There is equally the paucity of studies comparing the efficacy and safety of CC and letrozole in our unit. Therefore, this study sought to compare the use of letrozole and CC in infertile an ovulatory patients due to PCOS undergoing ovulation induction and to evaluate the efficacy and safety profile between the two groups.
This randomized prospective study was carried out at the Gynaecology unit of the Federal Medical Centre, Makurdi (Centre) over the period of twenty-four months (from 17th February, 2017 to 16th February, 2019).
The Centre is four hundred (400) beds tertiary hospital located in Benue State, North Central Nigeria. The Centre serves the two million women residents within Benue State (according to the 2006 Nigerian National population census report and also as a referral center for other hospitals in parts of Nasarawa, Taraba, Ebonyi, Cross Rivers, Kogi states and part of the Federal Capital Territory (FCT). The department of Obstetrics and Gynaecology is equipped with 30 bed spaces for gynaecological patients in¨patient care and run a five day a week clinic for out-patients with gynaecological conditions. The study populations were 142 patients with infertile anovulatory PCOS seen at the Gynaecology Clinic during the study period.
The sample size per group was determined adopting the formula
for calculation of sample size for randomized controlled trial
based on prior studies [15]. A 25% higher rate in ovulation among
patients in the intervention (letrozole) group was assumed to be
clinically relevant with a power of 80% at 95% confidence limit
and statistical significance (α) of 0.05 (5%). The sample size per
group was calculated using 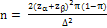
Where:
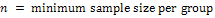
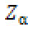 = standard normal deviate corresponding to the probability
α (which is 1.96 at 95% confidence limit, for a two tailed test)
= standard normal deviate corresponding to the probability
α (which is 1.96 at 95% confidence limit, for a two tailed test)
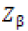 = Standard normal deviate corresponding to the probability
β (which is 0.80 at β = 0.20)
= Standard normal deviate corresponding to the probability
β (which is 0.80 at β = 0.20)
π = the arithmetic average of the proportion of the target population
Δ = the minimum arithmetic difference in the proportion
π = approximately 60% ovulation rate among anovulatory PCOS patients with infertility treated with Clomiphene citrate.
Δ = A 25% higher rate in ovulation among patients in the intervention (letrozole) group which was assumed clinically relevant.
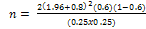
n=58
Attrition rate of 10%
The minimum sample size per group (n) therefore was approximately 65 (i.e. n= 65) and a total of 142 patients were recruited into the study.
The inclusion criteria included;
• Women with polycystic ovary syndrome aged between 18 and 40 years old.
• An ovulatory infertility due to PCOS.
• Both fallopian tubes were patent and normal uterine cavity.
• Normal seminal fluid analysis of male partner.
The exclusion criteria included;
i. Patients with infertility due to uterine and tubal pathologies or male factor.
ii. Women with other causes of an ovulatory infertility such as hyperprolactinaemia, thyroid disease, premature ovarian failure etc.
iii. Patients on centrally acting antihypertensive like apha methyl dopa
iv. Allergy to letrozole or clomiphene citrate.
v. Contraindication to letrozole and clomiphene citrate use
vi. Patients who declined consent for participation
A questionnaire was administered by the principal investigator or trained research assistants to all the patients that met the inclusion criteria to collate relevant data including socio demographics, hospital number, and address of the patient as well as phone contacts. History, vital signs and other important physical findings (such as weight and height) results of relevant investigations and information were also obtained.
All the eligible participants were randomized into two groups (group A and B) using a computer block randomization method. In this method, the computer was used to generate a set of random integers 1 and 2 with blocking which produced equal number of 1 and 2 in a random array pattern. Random numbers 1 correspond with group A while numbers 2 corresponded with group B. The generated random numbers were then sealed in opaque envelopes shuffled and placed in randomization container (a big brown envelope) and without bias, participants were identified and categorized based on picking up from the shuffled envelope the appropriate randomization in one of the two groups.
Group A (LETROZOLE GROUP) received letrozole 5mg (Mamazol manufactured by KHANDELWAL Laboratories PVT Limited, Mumbai, India. Manufactured October, 2016 and with Expiratory date September, 2020) starting on day 2 to day 6 of either spontaneous menstruation or induced menstruation with medroxy progesterone acetate (MPA) 10mg daily for 10 days.
Similarly, the Group B or clomiphene citrate group received 100mg of Clomiphene citrate (Ovumine, manufactured by BAROQUE Pharmaceutical PVT Limited under the License of NOVOPHARM Formulations PVT Limited, Gujarat, India. Manufactured September, 2016 and with an Expiratory date of August, 2019) starting from day 2 to day 6 of the menstruation following either spontaneous menstruation or induced menstruation with MPA 10mg daily for 10 days.
In both groups, a baseline transvaginal ultrasound scan (TVS) was performed on day 2 of the menstrual cycle to measure the number, size and location of the follicles on each ovary, as well as endometrial thickness (ET). A TVS was then repeated on the tenth day of menstrual cycle, to access the presence, number and size of the dominant follicles (DFs). The dominant follicles were defined as follicles measuring at least 12mm on the tenth day of menstruation.
If a dominant follicle (DF) was present (DF≥12mm), then a repeat TVS was performed every two days. Ovulation was diagnosed when a mature DF is approximately 18mm to 24mm size followed by evidence of rupture (disappearance of dominant follicle(s) and presence of fluid in the Pouch of Douglas) approximately two to three days later.
If a DF was absent (DF<12mm) on the tenth day of menstruation, a repeat TVS was performed every 3 to 4 days later and if there was absence of a DF (DF<12mm) up to day 20 of the menstrual cycle, then the patient was considered to be an ovulatory or failed induction of ovulation. Endometrial thickness (ET) was measured at every follow up visit.
The ovulation induction was carried out for one cycle for each patient that was recruited and 10000IU of human chorionic gonadotrophin (HCG) was given intramuscularly to trigger ovulation when at least one mature follicle was 18mm or more in diameter as detected by TVS. Couples were counseled to have sexual intercourse 32 to 36 hours later.
All patients that had continued amenorrhea of at least 3 weeks to 4 weeks duration following documented evidence of ovulation had a urine-based pregnancy test. If the urine-based pregnancy test was positive, they were followed up with pelvic ultrasound scan 2 to 4 weeks later for documentation of the viability of the pregnancy as well as the number of gestational sacs present. Patients with confirmed clinical pregnancy were counseled and booked for antenatal care and supervision of their pregnancies. Those who failed to conceive following documented evidence of ovulation had repeated ovulation induction cycles with either letrozole or CC but they were not recruited into the study again.
Data Analysis
Data entry and statistical analysis were performed using Statistical Program (Package) for Social Sciences version 20. Descriptive statistics comprising of means and standard deviations for numerical variables, and frequency and proportion for categorical variables were reported. Analytical statistics comprising the Student-t test were used to make comparisons between means. Chi-square test (or Fisher exact test if expected frequency count was less than 5) was used for testing differences between the two treatment groups. For all tests, a p value <0.05 was considered significant Figure 1.

Figure 1. Study Flow chart showing the progression of participants through each stage of the trial. (Adopted from CONSORT- Consolidated Standards of Reporting Trials).
Ethical Clearance
The ethical clearance was obtained from the Research Ethics Committee of Federal Medical Centre, Makurdi on 14th February 2017. (Appendix III) Additionally, participants gave an informed written consent (Appendix I) before being enrolled into the study haven being exposed to its purpose, importance and participant’s capacity to withdraw from it at time without any prejudice to their subsequent care.
This randomized controlled trial for induction of ovulation in anovulatory infertility POCS using letrozole or clomiphene citrate has demonstrated efficacy of both drugs (p= ). However, this study has shown that ovulation rate was significantly higher in the letrozole group 62 (87.3%) than clomiphene citrate group 45 (63.4%) and with a statistically significant association p=0.001(p<0.05). The study further showed that, subjects in the letrozole treatment group had four times chance of ovulating (Relative ratio of 3.98 at 95% CI in comparison with the Clomiphene citrate group. The findings in this study are similar with Nik Hazlina N.H. et al that reported a significantly higher ovulation rate in the letrozole group 59(78.7%) in comparison with CC group 40(53.3%) [27]. Previous studies also reported ovulation rates for letrozole group of 82.4% and CC group of 63.2%7. Another study reported that among women with PCOS with infertility taking letrozole, 62.5% had achieved ovulatory cycles as compared to 37.5% of women taking CC, which was however not statistically significant [28].
The few systematic review and meta-analysis showed an evidence of a statistical significant difference in ovulation between women with PCOS receiving either letrozole or CC [16]. Evidently, albeit minimal, letrozole induced a higher ovulation rate per cycle than CC. The current evidence by meta-analysis was said to be insufficient to support either letrozole or CC as being superior in terms of ovulation rate, but it adequately reinforces and emphasizes the favourable potential of both therapies for ovulation induction in women with PCOS [19, 20]. Additionally, another study comparing letrozole and combined metformin-clomiphene citrate treatment in anovulatory PCOS patients with infertility also showed no significant different in rates of ovulation and pregnancy but higher ovulation rate among letrozole group [29]. In this study, the findings supported both drugs to be effective in inducing ovulation in infertile an ovulatory PCOS patients but the rate of ovulation was higher in the letrozole treatment group than CC treatment group.
Endometrial thickness (ET) measurement has been shown to be a predictor for successful implantation following ovulation induction, with many studies reporting more success with ET of 9mm to 10mm. In this study, mean ET values of 8.59mm (SD of 2.22) in the letrozole group was thicker than the values of 8mm (SD of 2.45) in the CC group at midcycle of menstruation (day 12 to 16), This difference was statistically significant p=0.000. Furthermore, the result showed a 1.5mm thicker endometrium (Mean difference (mm) with its 95% CI in patients who underwent ovulation induction using letrozole compared with those in Clomiphene citrate treatment group. This finding was consistent with other studies reporting that most patients taking letrozole for ovulation induction due to anovulatory PCOS had a thicker endometrium (8.4mm) and conceived more frequently (21.6% pregnancy rate) when compared to those taking CC (5.2mm ET and 9.1% pregnancy rate) [12]. The finding in this study was also similar to the study by Nik Hazlina N.H. and coworkers who also found out that mean ET in the letrozole group was thicker than in the CC group at midcycle of menstruation, with endometrial thickness of 9.2mm and 8.4mm respectively and the difference was statistically significant [7]. Similar findings were also shown as letrozole had an overall greater beneficial effect on the endometrium as compared to CC in PCOS patients undergoing ovulation induction [9, 10]. However, another study reported that the ET was statistically significantly thicker in the CC group than letrozole group, possibly due to an increase in the number of growing follicles and thus a higher level of estrogen and progesterone, although ET in both study groups was >5mm or 6mm [6, 7].
Historically, the overall success in pregnancy rates after ovulation induction is between 9-25% [14, 15]. These controversies may stem from differences in the treatment groups, diagnostic criteria, techniques used, age of the cohort, cause of infertility and the number of treatment cycles. In this study, consistent with the number of successful ovulatory cycles, pregnancy rate was notably higher in the letrozole treatment group compared to the CC group with 19 (26.8%) and 8 (11.3%) pregnancies respectively [Table 1]. This was statistically significantly different with p value of 0.019. The result further showed that, patients in the letrozole treatment group had a 3 times chance (at 95% CI of achieving pregnancy compared to those in CC treatment group. The pregnancy rate observed in this study was consistent with other reported studies such as that by Mitwally, in which a pregnancy rate of 25% was observed for PCOS patients treated with 2.5mg letrozole [16]. Also, another study found a pregnancy rate of 25.3% in the letrozole treated group and 16.0% in the CC treatment group. However, this did not show a statistically significant difference [14]. Additionally, Atay et al also found a pregnancy rate of 21.6% after treatment with 2.5mg letrozole and 9.1% after treatment with 100mg CC, which was statistically significant [12]. The metaanalysis favoured letrozole by confirming a significant increase in clinical pregnancy in the group treated with letrozole. This is in agreement with a highly robust review conducted by Franik et al. as reported in The Cochrane Library and the review further showed that, because of the much shorter life span of letrozole (2 days) and absence of anti-oestrogenic effects in the late follicular phase, oestradiol concentration increased and consequently the endometrium grew faster, and thus a greater endometrial thickness on the day of HCG administration, in favor of nidation, and a higher pregnancy rate was to be expected [18,19]. There are other studies that also showed significantly higher pregnancy rates with used of letrozole for ovulation induction in an ovulatory infertile woman due to PCOS than with used of CC for the same purpose [22-24]. Pregnancy rate may be altered by other confounding factors, for example, approximately 41% of women recruited in this study were 35 years or more in age. This factor might be a cofactor that lowered the number of pregnancies and made the studied population a relatively less fertile group.
| Variables | Trial group | All variables | Test Statistics | p-value | |
|---|---|---|---|---|---|
| Letrozole n= 71 (%) |
Clomiphene Citrate n= 71 (%) |
n= 142 (%) | |||
| Age (in years) mean(SD) | 33.07(4.18) | 32.41(5.04) | 32.74(4.63) | t=0.85 | 0.396 |
| <35 | 44(62.0) | 40(56.3) | 84(59.2) | χ2=0.47 | 0.495 |
| ≥35 | 27(38.0) | 31(43.7) | 58(40.8) | ||
| Parity | |||||
| 0 | 40(56.3) | 36(50.6) | 76(53.5) | χ2=0.45 | 0.501 |
| ≥1 | 31(43.7) | 35(49.3) | 66(46.5) | ||
| Duration of infertility (in years) mean (SD) | 4.02(2.59) | 4.19(2.97) | 4.10(2.77) | t=-0.37 | 0.711 |
| <4 | 38(53.5) | 40(56.3) | 78(54.9) | χ2=0.11 | 0.736 |
| ≥4 | 33(46.5) | 31(43.7) | 64(45.1) | ||
| BMI mean(SD) | 28.36(4.27) | 28.35(4.00) | 28.36(4.12) | t=0.04 | 0.997 |
| Normal | 12(16.9) | 16(22.5) | 28(19.7) | Fisher’s Exact =1.21 | 0.561 |
| Over weight | 36(50.7) | 30(42.3) | 66(46.5) | ||
| Obese | 23(32.4) | 25(35.2) | 48(33.8) | ||
| Number of follicles | |||||
| mean(SD) | 14.49(4.71) | 13.58(5.21) | 14.04(4.97) | t=1.10 | 0.274 |
| <14 | 34(47.9) | 34(47.9) | 68(47.9) | χ2=0.00 | 1 |
| ≥14 | 37(52.1) | 37(52.1) | 74(52.1) | ||
| Endometrial thickness mean(SD) | 4.43(1.00) | 4.40(1.17) | 4.42(1.09) | t=0.12 | 0.904 |
Table 1: Baseline demographic and clinical characteristics of patient for each group
Clomiphene citrate has been in use as ovulation induction agent for several decades with an acclaimed safety profile. Known complications of CC use include multifetal pregnancy, pregnancy wastage and the major complication associated with its use is the occurrence of ovarian hyperstimulation syndrome (OHSS). Current studies have shown that letrozole is generally safe for use as ovulation agent in an ovulatory infertile women [25]. In this study, most of the drug adverse effects observed were minor and showed no statistically significant difference between the letrozole and CC treatment groups p=0.118 [Table 2]. However, there were more hot flushes and dizziness with patients treated with CC compared to those treated with letrozole. It was also observed that, letrozole was associated with more incidence of minor rash around the fingers. No major complication such as OHSS was observed among the two treatment groups.
| Variables | Trial group | Relative ratio (95% CI) | Test Statistics | p-value | |
|---|---|---|---|---|---|
| Letrozole n= 71 (%) |
Clomiphene Citrate n= 71 (%) |
||||
| Ovulation rate | |||||
| Yes | 62(87.3) | 45(63.4) | 3.98(1.70-9.31) | χ2=10.96 | 0.001 |
| No | 9(12.7) | 26(36.6) | |||
| Endometrial thickness | 8.59(2.22) | 7.08(2.45) | 1.51(0.73-2.28)a | t=3.84 | 0 |
| Pregnancy rate | |||||
| Positive | 19(26.8) | 8(11.3) | 2.88(1.17-7.11) | χ2=5.53 | 0.019 |
| Negative | 52(73.2) | 63(88.7) | |||
| Drug reaction | |||||
| Yes | 22(31.0) | 31(43.7) | 0.58(0.29-1.15) | χ2=2.44 | 0.118 |
| No | 49(69.0) | 40(56.3) | |||
| Adverse reaction | |||||
| Headache | 14(19.7) | 15(21.1) | |||
| Dizziness | 17(23.9) | 29(40.8) | |||
| Hot flushes | 3(0.04) | 19(26.8) | |||
| Rash | 3(0.04) | 0(0.0) | |||
| Vomiting | 9(12.7) | 8(11.3) | |||
Table 2: Comparison of outcome (Ovulation rate, endometrial thickness, pregnancy rate and adverse reaction) between Letrozole and Clomiphene citrate group
Follicular maturation at midcycle of menstruation is higher and with improved ovulation, endometrial thickness, pregnancy outcomes and without significant adverse effects in patients with an ovulatory infertility PCOS treated with letrozole in comparison with those on clomiphene citrate.
Compared to CC, letrozole appears to be a more favorable first line treatment in an ovulatory infertility PCOS that should be considered as a reliable alternative fertility treatment to CC where it is feasible and after a robust evaluation of this drug in different clinical settings.
The pregnant women exposed to these drugs were not followed up for long period due to time constraint, perhaps a long time follow up might have added to the strength of our study
Transvaginal ultrasound scan was the only method used in the determination of ovulation in this study; perhaps, a combination of this with other methods like 21-day progesterone estimation or laparoscopy might have increased the outcomes.
Urine based pregnancy test was done for patients with continued amenorrhea; probably serum pregnancy test might have yield a better outcomes than urine pregnancy test.
This study was only conducted in gynecology unit of Federal Medical Centre, Makurdi with a small population of infertile an ovulatory PCOS. Probably, a study with larger population and multicenter might have better outcomes and a definite conclusion.
Letrozole could replace clomiphene citrate as the primary (first line) medication for ovulation induction in infertile patients with PCOS. However, in the absence of letrazole due to cost implications or other logistic challenges, CC may be adopted.
Further studies are needed to determine the optimal dosing and the long-term safety for pregnant women treated with letrozole and their babies exposed to these agents in different parts of the world.
More studies using larger populations and sample sizes in multicenter collaborations in order to recommend letrozole use beyond the gynecological unit of our facility.
Further researches using combined methods of checking for ovulation (such as follicular tracking, diagnostic laparoscopy, 21- day progesterone estimation and use of ovulation kits) could help to determine more appropriate efficacy of these drugs in different populations.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Citation: Joseph AC, Lahaga NI, Oshiotse AP, Irowa O (2023) Efficacy of Letrozole in Comparison with Clomiphene Citrate for Induction of Ovulation in Polycystic Ovary Syndrome with Infertility. 12(11):695.
Received: 18-Oct-2023, Manuscript No. 27639; Editor assigned: 20-Oct-2023, Pre QC No. 27639; Reviewed: 03-Nov-2023, QC No. 27639; Revised: 08-Nov-2023, Manuscript No. 27639; Published: 16-Nov-2023 , DOI: 10.35248/2167-0420.23.12.695
Copyright: © 2023 Joseph AC et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited