Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2022)Volume 13, Issue 5
Introduction: We evaluated efficacy, tolerability, and cosmetic acceptability of a gel containing growth factors, taurine, caffeine and iron chelating complex (GFmgel) in subjects with hair loss (AGA/FAGA, TE) in a real-life condition.
Materials and methods: 471 subjects (273 women) with hair loss conditions (AGA/FAGA: n=269; or chronic TE, n=202) were enrolled. Eligible subjects could be naïve (no specific hair loss treatments) or treated with anti-hair loss drugs since at least 6 months. GFmgel (12.5 ml per application) should be applied on the scalp once weekly. The primary outcome was the Global Assessment Score (GAS) using a 7-point scale in comparison with baseline condition after 6 months of therapy. At baseline a global picture of the scalp was performed to be used for the GAS evaluation. A total of 229 subjects were treated with GFmgel alone (G1), 136 with GFmgel and topical minoxidil (G2); 34 subjects with GFmgel and oral finasteride (1 mg/die) (G3) and finally 69 subjects with GFmgel and multitherapy (drug and/or adjuvants) (G4).
Results: In the population as a whole GAS at month 6 significantly increased in all groups in comparison with baseline: G1: +1.6 ± 1; G2: +1.8 ± 1; G3: +2 ± 1 and G4: +2.1 ± 1. GAS scores of G4, G3 and G2 were significantly higher (p=0.02) in comparison with G1. In AGA/FAGA subjects the GAS scores were 1.2 ± 1 in G1, 1.7 ± 1 in G2, 2.2± 1 in G3 and 1.7 ± 1 in G4. GAS scores of G4, G3 and G2 groups were significantly higher (p=0.001) in comparison with G1. In TE subjects the GAS scores were: 1.9 ± 1 in G1, 1.9 ± 1 in G2 and 2.0 ± 0.8 in G4. No statistical differences between G1, G2 and G4 groups were observed for the GAS scores. The gel was very well tolerated. Cosmetic acceptability was judged good or very good by 75% of the subjects.
Conclusion: This gel has shown to be very effective as therapeutic strategy in subjects with hair loss (AGA/FAGA) used alone or in combination with drugs treatment. In subjects with chronic TE the use of this gel as monotherapy has shown an efficacy comparable to combination therapies.
Hair loss; Real-life trial; Growth factors; Caffeine; Taurine
Topical uses of mimicking growth factors oligopeptides [1], caffeine [2] and taurine [3] are considered useful in hair loss condition such as Androgenetic Alopecia (AGA) and Telogen Effluvium (TE). A gel containing three growth factors mimicking oligopeptides, caffeine and taurine and an iron chelating complex has been recently available (GFmgel). This gel (one application per week) has shown to be efficacious in subjects with Androgenic Alopecia (AGA) when used in combination with anti-hair loss drugs treatment such as topical minoxidil and oral finasteride [4]. So far, no data in real life conditions are available evaluating efficacy, tolerability, and cosmetic acceptability in subjects with AGA/FAGA or chronic TE when used alone or in combination with other anti-hair loss substances.
Study aim
We evaluated the efficacy, tolerability, and cosmetic acceptability of GFmgel in a real-life condition in subjects with hair loss of different aetiologies.
A total of 39 Italian out-patients hair clinics participated in this trial enrolling, after their informed consent, a total of 471 subjects (273 women and 198 men; mean age: 42 ± 14 years) with hair loss conditions (AGA/FAGA: n=269; or chronic TE, n=202). Inclusion criteria were age 18-65 years, with a clinical diagnosis of mild to moderate AGA/FAGA (Hamilton Scale IIIV; Ludwig Scale I-2-II-1) or chronic TE suitable for a treatment with GFmgel. Eligible subjects could be naïve (no specific hair loss treatments) or treated with anti-hair loss drugs (i.e., oral finasteride or topical minoxidil) since at least 6 months. Exclusion criteria were inflammatory scalp skin conditions, iron deficient anaemia, positive history of minoxidil or finasteride treatments in the previous 6 months before enrolment in the present study GFm gel (12.5 ml per application) should be applied on the scalp once weekly. The primary outcome was the Global Assessment Score (GAS) using a 7-point scale (from-3: Severe worsening to +3: Great improvement) in comparison with baseline condition evaluated by an assessor unaware of the type of treatment after 6 months of therapy. Tolerability and cosmetic acceptability were evaluated using a 7-item questionnaire at month 6. In AGA/FAGA subjects, alopecia grade was evaluated at each visit using the Hamilton (men) and Ludwig scale (women) [5,6]. At baseline a global picture of the scalp was performed to be used for the GAS evaluation. A total of 229 subjects were treated with GFmgel alone (), 136 with GFmgel and topical minoxidil (G2); 34 subjects with GFmgel and oral finasteride (1 mg/die) (G3) and finally 69 subjects with GFmgel and multi-therapy (drug and/or adjuvants) (G4).
Statistical analysis
GraphPad Prism statistical software (version 9) was utilized for data analysis. Continuous variables were expressed as mean ± Standard Deviation (SD). The primary endpoint of the trial was the evolution of GAS score in comparison with baseline and between the groups. The paired t-test, the Wilcoxon test and the Mann-Whitney test were used, when appropriate, for the analysis of the study outcomes.
Between June 2021 and October 2022, a total of 471 subjects accepted to participate in the trial, after informed consent. The study population was identified in forty out-patient hair clinic in Italy. Table 1 presents the main demographic and clinic characteristics of the subjects at baseline. In the population as a whole (including AGA/FAGA and chronic TE) GAS at month 6 significantly increased in all groups in comparison with baseline: G1: +1.6 ± 1; G2: +1.8 ± 1; G3: +2 ± 1 and G4: +2.1 ± 1 (Figure 1). GAS scores of G4, G3 and G2 were significantly higher (p=0.02) in comparison with G1. In AGA/FAGA subjects the GAS scores were 1.2 ± 1 in G1, 1.7 ± 1 in G2, 2.2 ± 1 in G3 and 1.7 ± 1 in G4. GAS scores of G4, G3 and G2 groups were significantly higher (p=0.001) in comparison with G1 (Figure 2). In TE subjects the GAS scores were: 1.9 ± 1 in G1, 1.9 ± 1 in G2 and 2.0 ± 0.8 in G4 (no chronic TE subjects were treated with finasteride) (Figures 3 and 4). No statistical differences between G1, G2 and G4 groups were observed for the GAS scores. The gel was very well tolerated. Cosmetic acceptability was judged good or very good by 75% of the subjects.
| Total Population | Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|---|
| Number | 471 | 229 | 136 | 34 | 69 |
| Age, years; mean (SD) | 42 (14) | 43(13) | 44(12) | 41(10) | 40(10) |
| Men; women | 198; 273 | 85/144 | 71/65 | 30/4 | 14/55 |
| AGA/FAGA | 269 | 106 | 116 | 34 | 12 |
| Chronic TE | 202 | 123 | 20 | 0 | 57 |
| AGA/FAGA Grade | I=116 | I=61 | I=36 | I=5 | I=4 |
| II=46 | II=21 | II=40 | II=6 | II=8 | |
| III=71 | III=20 | III=30 | III=11 | ||
| IV=34 | IV=4 | IV=10 | IV=12 |
Table 1: Subjects’ characteristics at baseline.
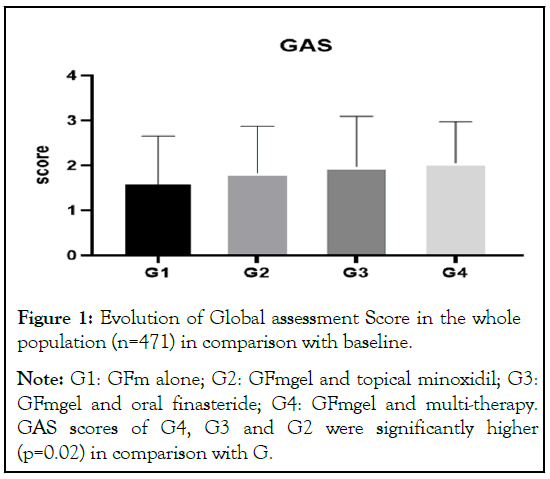
Figure 1: Evolution of Global assessment Score in the whole
population (n=471) in comparison with baseline.
Note: G1: GFm alone; G2: GFmgel and topical minoxidil; G3:
GFmgel and oral finasteride; G4: GFmgel and multi-therapy.
GAS scores of G4, G3 and G2 were significantly higher
(p=0.02) in comparison with G.
Figure 2: Evolution of Global assessment Score in the AGA/
FAGA population (n=269) in comparison with baseline.
Note: G1: GFm alone; G2: GFmgel and topical minoxidil;
G3: GFmgel and oral finasteride; G4: GFmgel and multitherapy.
GAS scores of G4, G3 and G2 were significantly
higher (p=0.01) in comparison with G1.
Figure 3: Evolution of Global assessment Score in the chronic
TE population (n=202) in comparison with baseline.
Note: G1: GFm alone; G2: GFmgel and topical minoxidil;
G4: GFmgel and multi-therapy; No G3 subjects were in this
population. No statistical differences between G1, G2 and G4
groups were observed for the GAS scores.
Figure 4: Colour pictures of two Subjects (A and B) of group 1 treated with GFmgel and one Subject (C) of group 2 treated with (Minoxidil+GFmgel) at baseline and after treatment.
In men and women, the most common form of alopecia is AGA/FAGA [7,8]. The Telogen effluvium, in both acute and chronic forms, is a hair loss condition commonly seen in women [9]. The only medications approved for AGA/FAGA are topical minoxidil (for both men and women) and oral finasteride (men) [10]. The therapeutic approach in chronic TE is wider with the use of both drugs (minoxidil), dietary supplementation or dermo cosmetic products [11,12]. In AGA/ FAGA subjects but also in chronic TE the clinical efficacy of a monotherapy approach is often disappointing [13]. For this reason, in these hair loss conditions the dermatologist adopted a combination therapeutic strategy with the aim to improve the clinical outcome [14,15]. In this real-life trial we assessed the efficacy of a gel containing different components which can have positive effects on hair growth. This gel contains: Growth factors mimicking oligopeptides, caffeine, taurine and an iron-chelating complex. In vitro studies have shown that caffeine prolonged the duration of the anagen phase and counteracted testosteroneinduced TGF-β2 protein expression in hair follicles [16]. Caffeine used topically can increase intracellular cAMP concentrations, resulting in stimulatory effects on cell metabolism and proliferation [17]. In both males and females, IGF-1 protein expression was potentiated by caffeine, promoting anagen phase [18]. A caffeine containing lotion has demonstrated in a randomised double blind trial similar efficacy of topical minoxidil in AGA subjects [19]. Taurine is a betaamino acid produced by methionine and cysteine metabolism [20]. It is involved in a variety of physiological functions, including immunomodulatory and antifibrotic [21]. Taurine has a specific tropism for the hair bulb [22]. 4
In vitro studies have shown that taurine can counteract the negative effect of TGF-beta on hair growth [23]. In vitro, taurine prolongs the survival rate of human hair follicles [24]. The test gel also contains three components with iron chelating activity (lactoferrin, EDTA and sodium gluconate). The Hypoxia- Inducible Factor-1a (HIF-1α) may counteract hair loss [25]. Its activity could be blocked by iron molecules [26]. When iron at scalp level is chelated the HIF-1α activity is enhanced [27]. This could represent a rational for the topical use of chelating substances in the strategic therapeutic approach of hair loss. Finally, this gel contains a mix of four high-purified, growth factors-like polypeptides (octapeptide 2, acetyl decapeptide 3, oligopeptide 20 and copper tripeptide). Copper tripeptide has anti-inflammatory, antioxidant and blood vessel growth promoting action [28]. In addition, it can increase the activity of FGF (Fibroblast Growth Factor) and VEGF [29]. Interestingly, copper tripeptide decreases the secretion of dermal fibroblasts TGF-β, a pro-catagen factor [30]. Finally, copper tripeptide can also interfere with the activity of 5-α-reductase, therefore reducing the production of DHT [31]. This peptide is also able to stimulate the production of decorin [32]. Decorin at scalp level improves the anagen phase and consequently hair growth [33]. The acetyl decapeptide is a synthetic peptide that mimics the action of two growth factors: The Keratinocyte Growth Factor (KGF) and the Epidermal Growth Factor (EGF), which acts on the follicle, promoting the anagen growth phase, through an antiapoptotic effect, maintaining the biochemical activity of the bulge stem cells [34]. Octapeptide 2, can promote hair growth, to reduce apoptosis and to increase keratinocyte proliferation [35]. When used in humans this gel has also demonstrated to improve the hair growth effects of platelet rich plasma therapy in subjects with androgenic alopecia [4].
Hair loss is a common dermatological condition. Androgenic alopecia and telogen effluvium are the most frequent hair loss pathological situations. TE in the acute form could be a selflimiting condition, whereas the chronic form of TE and AGA/ FAGA are frequently difficult to treat. In AGA/FAGA patients effective drugs treatments are minoxidil (in topical formulation at 2 and 5% or more recently oral) and finasteride (both oraland topical). In chronic TE a multi-therapy regimen is commonly adopted by dermatologist using both drugs (like minoxidil) and adjuvant products. This gel containing growth factors mimicking oligopeptides, caffeine and taurine and an iron chelating complex has shown to be very effective as therapeutic strategy in subjects with hair loss (AGA/FAGA) used alone or in combination with drugs treatment. In subjects with chronic TE the use of this gel as monotherapy has shown an efficacy comparable to combination therapies. Finally, it is important to underline that these results were obtained in a reallife scenario in a quite large population sample, thus increasing the evaluation of the effectiveness of this product.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Caruso A, D'Erme AM, Bordin A, Balducci A, Bonifati A, Agolzer A, et al. (2022) Efficacy, Tolerability and Cosmetic Acceptability of a Growth Factors, Caffeine, Taurine and a Chelating Complex-Based Gel in Subjects with Hair Loss Alone or In Combination with Drugs Treatments: A Real Life, Multicentre, Assessor-Blinded, 6-Month Trial on 471 Subjects. J Clin Exp Dermatol Res. 13:623.
Received: 28-Nov-2022, Manuscript No. JCEDR-22-20495; Editor assigned: 30-Nov-2022, Pre QC No. JCEDR-22-20495 (PQ); Reviewed: 14-Dec-2022, QC No. JCEDR-22-20495; Revised: 21-Dec-2022, Manuscript No. JCEDR-22-20495 (R); Published: 28-Dec-2022 , DOI: 10.35841/2329-9509.22.13.623
Copyright: © 2022 Caruso A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.