International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Research Article - (2024)Volume 12, Issue 2
Background: Exercise provides countless benefits, including better sleep, enhanced physical function, healthier body weight, as well as a reduced risk of a number of chronic diseases. Despite these well-studied benefits, participation rates in physical activity in much of the westernized world remain low. This study was designed to evaluate whether NEM® brand eggshell membrane would reduce cartilage turnover or alleviate joint pain or stiffness, some of the major barriers to participation, either directly following exercise or 12 hours’ post-exercise, in healthy men and women versus placebo.
Methods: Eighty-five healthy, men and women (aged 40-72) were randomly assigned to receive either oral NEM® 500 mg (n=43) or placebo (n=42) once daily for two weeks while performing an exercise regimen (40 to 100 steps per leg) on alternating days. The primary endpoint was any statistically significant reduction in exerciseinduced cartilage turnover via the biomarker c-terminal cross-linked Telopeptide of Type-II collagen (CTX-II) versus placebo, evaluated at 1 week and 2 weeks of treatment. Secondary endpoints were any reductions in either exercise-induced joint pain or stiffness versus placebo, evaluated daily, via participant questionnaire. The clinical assessment was performed on the per protocol population.
Findings: Supplementation with NEM® produced a significant absolute treatment effect (TEabs) versus placebo after both one week (TEabs -19.2%, p=0.008) and two weeks of exercise (TEabs -18.8%, p=0.031) for the primary endpoint, CTX-II. Rapid treatment responses were observed for both immediate pain (p=0.004 versus placebo) and stiffness (p=0.028) with results occurring as early as Day 1 and Day 5, respectively. Recovery pain and stiffness were not significantly different from placebo, despite the fact that recovery pain (Day 14, TEabs -27.6%) had returned to resting levels and recovery stiffness (Day 14, TEabs -18.2%) had fallen below resting levels for the NEM® treatment group. There were no serious adverse events reported during the study and the treatment was reported to be well tolerated by study participants.
Conclusion: NEM® brand eggshell membrane, 500 mg once daily, rapidly improved exercise-induced joint pain (Day 1) and stiffness (Day 5). Moreover, a substantial chondroprotective effect was demonstrated from supplementation with NEM® through a lasting decrease in the cartilage degradation biomarker CTX-II. Reducing pain and stiffness from exercise and the concern of damaging cartilage is tantamount to reducing these major barriers to participation in increased physical activity and exercise. The Clinical Trial Registration number for this study is: NCT03679923.
CTX-II; Joint pain; Chondroprotection; Eggshell membrane; Exercise
Exercise provides countless benefits, including better sleep, enhanced physical and potentially cognitive function, healthier body weight, as well as a reduced risk of a number of chronic diseases like heart disease, stroke, or type 2 diabetes [1,2]. Current guidelines for adults suggest that they participate in at least 150 minutes of moderate-intensity physical activity per week [1,3]. Unfortunately, modern society has become increasingly sedentary with only 5% of U.S. adults participating in 30 minutes of physical activity per day [4], and roughly 23% to 36% of adults in much of the westernized world getting the recommended amount of physical activity per week [4,5]. Regular exercise provides the better-known benefits already described, but new research is beginning to reveal benefits to longevity, as well. In a study of 16,741 older women (mean age 72.0 ± 5.7 years), as few as 4,400 steps per day was significantly related to lower mortality rates and mortality rates progressively decreased before leveling at approximately 7,500 steps per day [6]. In another study examining over 30,000 U.S. adults, replacing as little as 30 minutes of sedentary time with 30 minutes of either lightintensity physical activity or moderate to vigorous physical activity showed a significant association with a 17% or 35% reduction in mortality risk, respectively [7]. Even relatively short-term exercise regimens may have long term benefits for participants. One study found that sedentary individuals who participated in an 8-month exercise regimen still had some residual benefits 10-years later, such as a greater preservation of cardiorespiratory fitness and waist circumference [8].
Joint disorders, including Osteoarthritis (OA) make it hard to maintain physical activity, [1] but physical activity is essential for optimal health. Numerous studies have demonstrated the beneficial effects of exercise in relieving OA symptoms [9,10], particularly for weight-bearing joints such as the knees or hips, and it is recommended by the American College of Rheumatology as a non-pharmacologic therapy for OA [11]. There is even some clinical evidence to suggest that exercise may play a role in the prevention of OA [12]. One study on adults at a high risk of developing OA suggests that moderate, regular exercise encourages cartilage to adapt to the mechanical loading demands incurred by increasing Glycosaminoglycan (GAG) content [13]. Even in the absence of OA or other diagnosed joint and connective tissue disorders, adults may experience joint pain or discomfort as a result of physical activity. This is supported by data recently released by the National Center for Health Statistics from the 2017 National Health Interview Survey [14]. While 30.3% of adults aged 45-64 were reported to have a diagnosis of arthritis, 38% of individuals in the same age group reported that they were experiencing chronic joint symptoms which referred to pain, aching, or stiffness within or around a joint (with the exception of the back or neck) that had lasted at least 3 months at the time of questioning [14]. Even the fear of pain could be a barrier to exercise participation. In a study comprised of individuals with a history of acute back pain, a fear of pain was a major factor that hindered increasing physical activity [15]. Less physical activity in turn, may increase the risk for obesity with recent evidence confirming that obese individuals have thinner cartilage and greater cartilage strain [16], which may spur degeneration of cartilage over time. Therefore, a chondroprotective dietary supplement, derived from eggshell membrane, may have use even in healthy populations.
Eggshell membrane lies between the albumin and the calcified shell of the egg. It is characterized by its bi-layered, mesh-like structure formed by fibrous proteins such as collagen [17]. The membrane also contains other constituents that may contribute to its joint health benefits which include Glycosaminoglycans (GAGs) such as chondroitin sulphate, derma tan sulphate and hyaluronic acid [18]. While eggs have a long history of consumption around the world, it wasn’t until the early 21st century when eggshell membrane began to be investigated for its nutraceutical applications. Among the first, ESM Technologies, LLC (Carthage, MO USA) developed a method to remove the membrane from eggshells using steam and physical abrasion [19]. A partial, enzymatic process to aid digestibility followed by dry blending is used to create the finished ingredient (NEM® brand eggshell membrane), which can be incorporated into capsules, tablets, chews, and soft gels. NEM® has been the subject of numerous studies. It was shown to reduce the expression of various pro-inflammatory cytokines, including the key mediators of inflammation Interleukin-1beta (IL-1β) and Tumor Necrosis Factor-Alpha (TNF-α), both in vitro [20], and in vivo [21]. Multiple joint health efficacy studies have also been conducted utilizing NEM®, including an animal efficacy trial in dogs [22], and human clinical trials in subjects with pre-existing joint disorders [23], and osteoarthritis [24-26]. Interestingly, NEM® was found to reduce C-terminal Cross-linked Telopeptide of Type-II collagen (CTX-II), a biomarker of cartilage degradation, in rat models of both OA [27], and Rheumatoid Arthritis (RA) [28], as well as in naturally occurring joint disease in dogs [22]. CTX-II is known to be elevated in diseased populations such as those with OA, but has also been found to be elevated in articularly healthy subsets of the population including overweight adults [29], athletes that participate in high impact endurance sports such as cross country running [30], and postmenopausal women [29]. A innovative clinical trial conducted in 2018 showed that NEM® supplementation conferred joint health benefits to articularly healthy, postmenopausal women demonstrated by reductions in post-exercise joint pain and stiffness and lowered excretion of urinary CTX-II [31].
While previous trials on NEM® brand eggshell membrane included populations with diseases such as osteoarthritis [24- 26], and healthy women of a specific menopausal status [31], the current study sought to evaluate the joint health effects of NEM® brand eggshell membrane in both healthy men and women to determine if this broader population may directly benefit from supplementation through reduced joint pain and stiffness and cartilage turnover, and indirectly by reducing barriers to greater participation in physical activity.
Study design
This study was conducted utilizing the services of a clinical contract research organization (QPS Bio-Kinetic; Springfield, MO USA) according to a single center, randomized, double-blind, placebo-controlled design in accordance with the U.S. Food and Drug Administration’s principles of Good Clinical Practice (Title 21, Code of Federal Regulations, Parts 50 and 56 and ICH E6) and the Declaration of Helsinki (1996 version) (Clinical Trial Registration Number NCT03679923). The study protocol was approved by a registered Institutional Review Board (IRB) and subjects provided their written informed consent in order to participate. After meeting all inclusion/exclusion criteria at screening, eligible subjects were then randomized to receive either NEM® or placebo in the order in which they were enrolled in the study using a permuted block randomization table consisting of four subjects per block. A Consolidated Standards of Reporting Trails (CONSORT) trial participant flow diagram is shown in Figure 1. Treatment consisted once daily orally of either 500 mg of NEM® or placebo. Treatment compliance was checked at clinic visits by participant interview and by counting the number of unused doses of the study capsules. Acetaminophen was allowed for pain relief rescue, if necessary. Subjects recorded the time and amount of acetaminophen taken in subject diaries. All participants, clinical staff, and study management staff remained blinded to treatment assignment throughout the study.
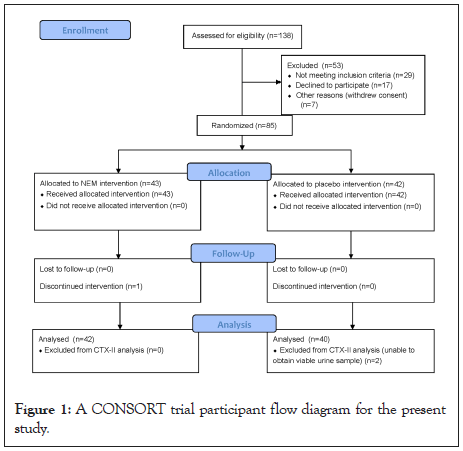
Figure 1: A CONSORT trial participant flow diagram for the present study.
The intent of this study was to evaluate whether NEM® would reduce cartilage turnover (via the CTX-II biomarker) or alleviate joint pain or stiffness, either directly following exercise or 12 hours’ post-exercise, versus placebo. Participants performed 40 to 100 steps per leg utilizing a 9-inch tall aerobics step platform at the clinical site. At screening, subjects performed 40 steps per leg, increasing by ten at a time per leg up to 100 steps per leg, until they experienced at least a 1 unit change in discomfort (either pain or stiffness) from their resting rating. This number of steps was then assigned to that subject for the remainder of the study. Participants then followed their assigned exercise regimen on alternating days for 2 consecutive weeks (i.e., Sunday, Tuesday, Thursday, Saturday, Monday, Wednesday, Friday). Additionally, participants were required to provide blood and urine samples approximately 24 hours after the final weekly exercise visit, which is on Friday of Week 1 and Saturday of Week 2. Changes in pain and stiffness (both immediate and 12-hour) and changes in CTXII levels were compared to those of the placebo group.
Patients
All healthy men and women, 40-75 years of age, who had not been diagnosed with a joint or connective tissue disorder of the lower extremities (i.e., ankles, knees, or hips) were considered for enrollment in the study. In order to be eligible to participate, subjects must not have had persistent lower-extremity joint pain at rest, however subjects could have experienced mild periodic lower-extremity joint pain rated ≤ 2 on a 10-point ordinal scale. Subjects must have been sufficiently healthy to perform moderate exercise as judged by a medical examination including vital signs (i.e., resting heart rate, blood pressure, etc.) and an Electro Cardiogram (ECG). Subjects were required to suspend all current prescription or Over-The-Counter (OTC) pain relief medications i.e., Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), analgesics, opioids, anti-depressants prescribed for painful conditions such as fibromyalgia, or joint supplements] in order to participate in the study. Subjects that were currently taking analgesic or related medications were eligible to participate in the study following a 14 day washout period for NSAIDs, a 7 day washout period for opioids, and a 90 day washout period for injected steroids or anti-depressants. Subjects currently taking joint supplements such as glucosamine, chondroitin sulfate, curcumin, Boswellia, etc., were only eligible after a 3-month washout period. Subjects could not meet any of the classification criteria (other than age >50 years) according to the American College of Rheumatology for either OA [32], or RA [33]. Subjects were also excluded if they were currently receiving remission-inducing drugs such as methotrexate or immunosuppressive medications or had received them within the past 3 months. They were further excluded if they had a confounding inflammatory disease or condition (gout, pseudo gout, lupus, Paget’s disease, chronic pain syndrome, etc.) that would interfere with assessment of lower-extremity joints. Moreover, subjects were excluded if they participated in activities involving intensive use of the lower extremities (i.e., running/ jogging, sports, bicycling, dancing, etc.) two or more days per week or participated in activities that involved moderate use of the lower extremities (i.e., walking, golfing, yoga, etc.) three or more days per week. Other exclusionary criteria were: Body weight 275 pounds (125 kg) or greater, a known allergy to eggs or egg products, or pregnant or breastfeeding women. Subjects previously enrolled in a research study involving an investigational product (drug, device, or biologic) or a new application of an approved product, within 30 days of screening were also excluded from participating in the trial.
Clinical endpoints/treatment response
The primary endpoint for the study was any statistically significant reduction in exercise-induced cartilage turnover (via the CTX-II biomarker) versus placebo evaluated at 1 week and 2 weeks of treatment. Treatment response (reduction) was defined as either a blunted increase in mean urinary CTX-II (corrected for creatinine) in response to exercise or a decrease from baseline in the NEM® group compared to the placebo group. Urine samples were collected from the 2nd void of the morning within 24 hours of completing the final exercise period for each week. Secondary endpoints were any statistically significant reductions in either exercise-induced joint pain or stiffness versus placebo evaluated daily via participant questionnaire. The questionnaire consisted of an immediate assessment (during or up to 1-hour post-exercise) and a 12-hour assessment. Each assessment asked the participant to rate their joint pain and stiffness on a 10-point ordinal scale with zero equating to no pain (or stiffness) and 10 equating to severe pain (or stiffness). A similar 10-point ordinal scale was also used to evaluate participant joint pain and stiffness at rest (average from the prior seven days) and during screening assessment.
Assessment of urinary CTX-II
Urine samples were collected from the 2nd void of the morning. Fifteen milliliters from each urine specimen were centrifuged at 3,000 rpm for 10 minutes at 12°C-25°C. Following centrifuging, two 5 mL aliquots were removed by pipette and were immediately placed in a -20°C freezer. Samples were stored frozen (-20°C) until analysis. Only a single aliquot of the pair was subsequently thawed for initial analysis, to avoid repeated freeze/thaw cycles that might result in aberrant repeat assay values. Urinary concentrations of CTX-II were measured via Enzyme-Linked Immuno Sorbent Assay (ELISA) using a commercial immunoassay (Urine CartiLaps® EIA; Immunodiagnostic Systems, Inc.; Gaithersburg, MD USA) and urinary creatinine was measured via a colorimetric assay (Creatinine (urinary) Colorimetric Assay Kit; Cayman Chemical Company, Inc.; Ann Arbor, MI USA) according to manufacturers’ instructions using a SpectraMax Plus 384 microplate reader (Molecular Devices, LLC; Sunnyvale, CA USA). Urinary CTX-II concentrations were subsequently normalized by dividing uCTXII expressed in micrograms per liter (μg/L) by urinary Creatinine (Cr) expressed in Millimoles Per Liter (mmol/L), the results of which were reported as nanograms of CTX-II per Millimole of Creatinine (ng/mmol Cr). Samples were assayed in duplicate and all assays were repeated (n=4). Duplicate assay values that were within 90% agreement were subsequently averaged. Repeated assay means that were within 90% agreement were further averaged. If repeat assay means differed by more than 10%, an additional assay was performed in duplicate with the remaining frozen aliquot of urine. The additional repeat assay mean was either substituted for the original outlier, or if sufficient agreement wasn’t reached with either original repeat assay mean, then all three were averaged. About 31% of the uCTX-II assays were repeated three times, primarily due to inter-assay variability. Overall intra-assay coefficients of variation were 5.12 and 1.72 for uCTX-II and Cr, respectively.
Investigational product
The investigational product, NEM®, is derived from eggshell membrane and is manufactured via a patented process [34]. During the manufacture of NEM® the eggshell membrane is partially hydrolyzed utilizing a gentle enzymatic process, as opposed to using harsh indiscriminant chemicals, to enhance gastrointestinal absorption while preserving the membrane’s natural biological activity. For this clinical study, 500 mg of NEM® (Lot #8100010) or placebo (500 mg of excipients) were provided in #0 vegetarian capsules by ESM Technologies, LLC (Carthage, MO USA). Treatment and placebo capsules were identical in appearance, odor, and taste and were stored in closed containers at ambient temperature. Participants were instructed to take one capsule daily with water, approximately the same time each morning prior to eating breakfast.
Safety/adverse events
Secondary objectives of the study were to evaluate tolerability and safety or any adverse reactions associated with ingestion of NEM®. Subjects underwent a thorough medical examination at screening and upon study completion by a licensed physician including a complete medical history, physical examination, vital signs (resting heart rate, blood pressure, respirations, and temperature), and ECG. Additionally, participants’ selfassessment diaries were reviewed at each clinic visit and any discomfort beyond what would be expected with exercise or any other adverse events were recorded and reported in accordance with applicable FDA regulations. Adverse events and serious adverse events were assessed by the clinical investigator and were followed until resolution, as necessary. Serious adverse events were required to be reported to the clinical monitor immediately.
Statistical analysis
The hypothesis for this study was that the treatment group would be superior to that of the placebo group in limiting the increase in uCTX-II levels resulting from moderate exercise. A 17% absolute change in the mean treatment response (uCTX-II would increase by an average of at least 17% more in the placebo group than in the treatment group) was expected based upon results from the previously published healthy, post-menopausal women trial [31]. We estimated that a sample size of 84 subjects would need to be enrolled to provide the study with a statistical power of 80% to detect a clinically meaningful difference between the treatment group and the placebo group, assuming a rate of response of -8% for the treatment group and a rate of response of +9% for the placebo group, with no dropouts. Since the actual enrollment for the study was 85 subjects with one dropout, this should be sufficient to provide adequate safety and comparative effectiveness information. Descriptive statistics were calculated including mean age, height, weight, Body Mass Index (BMI), and number of steps per leg, and comparisons of this demographic data were made with a Kruskal-Wallis test for multiple independent samples at baseline to validate randomization. Following evaluation for normality (D’Agostino-Pearson), post-baseline between-group statistical analyses were done utilizing either an independent group t-test (CTX-II) or repeated measures univariate Analysis of Variance (rm-ANOVA) (pain and stiffness). Items found to have statistical significance with rm-ANOVA were then compared using a Kruskal-Wallis test for multiple independent samples. Post-baseline within-group statistical analyses were done utilizing either a paired sample t-test (immediate pain and stiffness) or a paired sample Wilcoxon test (recovery pain and stiffness). In all cases, statistical significance was accepted at p<0.05. Analysis of the primary endpoint, as well as all secondary endpoints was conducted on the per protocol population. MedCalc® Software (version 18.11.3) was used for all statistical analyses [35].
Recruitment began in September 2018 at a single clinical site in Missouri and the final follow-up was conducted in November 2018. One-hundred-thirty-eight (138) male and female subjects were screened and a total of 85 subjects between the ages of 40 and 72 years were enrolled in the trial and underwent randomization. Forty-two subjects (49.4%) were randomized to the placebo group and 43 subjects (50.6%) were randomized to the NEM® treatment group. All subjects that completed the study did so per the protocol and there was one dropout (1.2%) due to flu-like symptoms prior to their first exercise visit. Compliance with the study treatment regimen was good in both treatment groups, as judged by capsule count at clinic visits.
Participant demographic data (Table 1) was initially evaluated to verify randomization. There were no abnormalities in any of these evaluations (not shown). One placebo subject underwent a colonoscopy near the end of Week 1 and was given fentanyl and Versed®, therefore their last observation prior to the procedure was carried forward for the remainder of the study time points. Viable urine samples were unable to be obtained from two placebo subjects.
| NEM (n=42) | Placebo (n=42) | |
|---|---|---|
| Age, yrs | 53.5 ± 8.5 | 54.5 ± 8.3 |
| Sex | ||
| Female (%) | 26 (62) | 30 (73) |
| Male (%) | 16 (38) | 11 (27) |
| Height, cm | 167 ± 15 | 168 ± 7 |
| Weight, kg | 80.3 ± 20.1 | 84.0 ± 18.6 |
| BMI, kg/m2 | 28.3 ± 6.2 | 29.7 ± 6.5 |
| uCTX-II, ng/mmol Cr | 222 ± 131 | 273 ± 212 |
| Resting pain score | 0.8 ± 0.9 | 0.9 ± 0.8 |
| Resting stiffness score | 1.1 ± 1.1 | 1.3 ± 1.2 |
| Number of steps per Leg | 49.3 ± 17.0 | 45.0 ± 10.9 |
| Baseline pain score | 2.0 ± 1.2 | 2.4 ± 1.3 |
| Baseline stiffness score | 2.1 ± 1.2 | 2.5 ± 1.7 |
Note: Except where indicated otherwise, values are reported as mean ± standard deviation. There were no statistical differences between treatment groups in any of the listed parameters. Baseline pain and stiffness scores were obtained from performing the number of steps per leg determined at screening.
Abbreviations: BMI: Body Mass Index, calculated as weight in kilograms divided by the square of height in meters; uCTX-II: Urinary c-terminal Cross-linked Telopeptide Of Type-II collagen, reported as Nanograms per millimole of Creatinine (ng/mmol Cr).
Table 1: Participant baseline demographic data.
A clinical comparison of valid subjects was carried out to obtain mean scores for each of the outcome measures (CTX-II, pain, and stiffness) after one week and two weeks of exercise (Table 2). Absolute Treatment Effects (TEabs) for all outcome measures were calculated as the net difference of treatment versus placebo for the mean change in treatment effect from baseline (or resting) for each group expressed as a percent. Negative values represent superior results in the treatment group. Statistical analysis of the primary outcome measure (CTX-II) revealed that supplementation with NEM® produced a significant treatment response versus placebo after both one week (TEabs -19.2%, p=0.008) and two weeks of exercise (TEabs -18.8%, p=0.031) (Figure 2). Within-group changes were also statistically significant for uCTX-II for the NEM® treatment group for both weeks (Day 7, -12.0%, p=0.034) (Day 14, -13.0%, p=0.014), whereas neither week was significant for the placebo group.
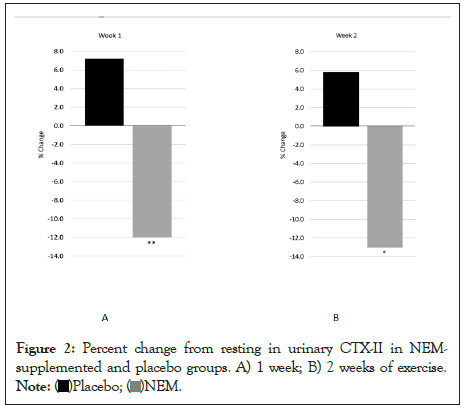
Figure 2: Percent change from resting in urinary CTX-II in NEMsupplemented
and placebo groups. A) 1 week; B) 2 weeks of exercise.
| Weeks | Treatment | ||
|---|---|---|---|
| Post-treatment | NEM | Placebo | |
| uCTX -II | Resting (n=42,40) | 222 ± 131 | 273 ± 212 |
| 1 (n=42,40) | 195 ± 98* | 292 ± 228 | |
| 2 (n=42,40) | 193 ± 103* | 288 ± 269 | |
| Immediate pain | Baseline (n=42,42) | 2.0 ± 1.2 | 2.4 ± 1.3 |
| 1 (n=42, 42) | 1.2 ± 0.9** | 2.1 ± 1.5 | |
| 2 (n=42,41) | 1.2 ± 1.2** | 1.8 ± 1.5* | |
| 12-hour pain | Resting (n=42,42) | 0.8 ± 0.9 | 0.9 ± 0.8 |
| 1 (n=42,42) | 0.9 ± 1.0 | 1.5 ± 1.7# | |
| 2 (n=42,42) | 0.7 ± 1.1 | 1.0 ± 1.5 | |
| Immediate stiffness | Baseline (n=42,42) | 2.1 ± 1.2 | 2.5 ± 1.7 |
| 1 (n=42,42) | 1.5 ± 1.0** | 2.0 ± 1.5# | |
| 2 (n=42,42) | 1.3 ± 1.1** | 1.7 ± 1.7* | |
| 12-hour stiffness | Resting (n=42,42) | 1.1 ± 1.1 | 1.3 ± 1.2 |
| 1 (n=42,42) | 1.0 ± 1.2 | 1.5 ± 1.6 | |
| 2 (n=42,42) | 0.8 ± 1.1 | 1.1 ± 1.4 | |
Note: Values are reported as mean ± standard deviation. Abbreviations: uCTX-II: urinary C-Terminal Cross-linked Telopeptide of Type-II collagen, reported as Nanograms per millimole of Creatinine (ng/mmol Cr). **P<0.01; *P<0.05; # P<0.10 within-group from baseline or resting.
Table 2: Mean scores for CTX-II and (immediate and 12-hour) Pain and Stiffness in NEM-supplemented and placebo groups at baseline (or resting) and after 1 week and 2 weeks of exercise.
The overall trend for immediate pain was significantly different from placebo (p=0.004) and this difference occurred at Day 1 and continued through Day 11 with Day 13 showing a positive trend (p<0.10) for being different from placebo (Figure 3A). Within group differences for the NEM® treatment group were also significant for immediate pain for both weeks (Day 7 -41.2%, p<0.001) (Day 14 -38.8%, p<0.001), whereas only week 2 was significant for the placebo group (Day 14 -25.0%, p<0.026). The overall trend for recovery (12-hour) pain was not significantly different from placebo (p=0.057), despite the fact that recovery pain had returned to resting levels for the NEM treatment group (Day 14 TEabs -27.6%), while placebo group recovery pain levels remained elevated (Figure 3B).
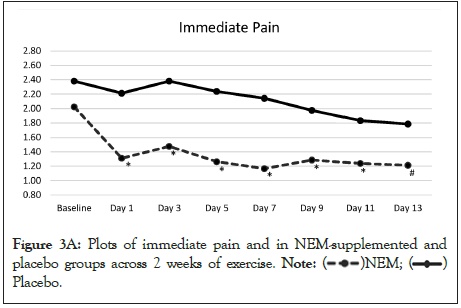
Figure 3A: Plots of immediate pain and in NEM-supplemented and placebo groups across 2 weeks of exercise.  Placebo.
Placebo.
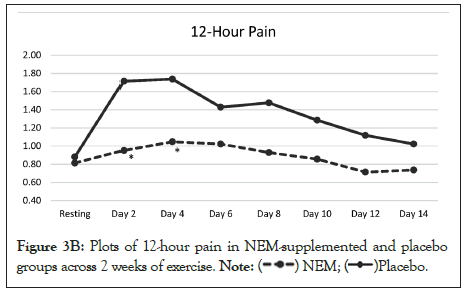
Figure 3B: Plots of 12-hour pain in NEM-supplemented and placebo groups across 2 weeks of exercise. Placebo.
Placebo.
The overall trend for immediate stiffness was significantly different from placebo (p=0.028) and this difference occurred at Days 5, 7 and 11 with Day 3 and Day 9 showing a positive trend (p<0.10) for being different from placebo (Figure 4A). Within group differences for the NEM® treatment group were also significant for immediate stiffness for both weeks (Day 7 to 30.4%, p=0.004) (Day 14, -36.0%, p=0.001), while there was a trend for improvement for week 1 and week 2 was significant for the placebo group (Day 7, -21.9%, p=0.052) (Day 14, -31.4%, p=0.014). The overall trend for recovery (12-hour) stiffness was not significantly different from placebo (p=0.112), despite the fact that recovery stiffness fell well below resting levels for the NEM treatment group and substantially more so than the placebo group (Figure 4B). Both groups experienced less recovery stiffness from performing the exercise regimen as the study progressed, however the NEM® treatment group felt a greater benefit (Day 14, TEabs -18.2%).
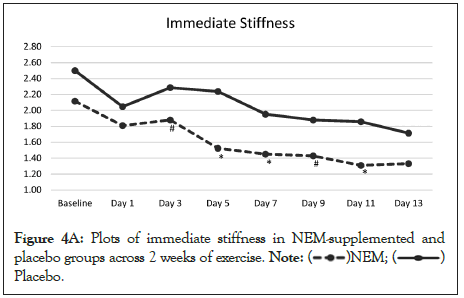
Figure 4A: Plots of immediate stiffness in NEM-supplemented and
placebo groups across 2 weeks of exercise.  Placebo.
Placebo.
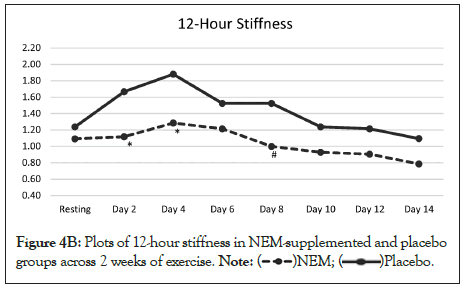
Figure 4B: Plots of 12-hour stiffness in NEM-supplemented and placebo groups across 2 weeks of exercise. Placebo.
Placebo.
There were twelve (12) non-exercise-related Adverse Events (AEs) reported by the treatment group, the most common of which were 5 instances of cold/flu/sinus congestion and 4 instances of headache. None of the treatment group AEs was judged by the clinical investigator to be associated with treatment. There were eighteen (18) non-exercise-related AEs reported by the placebo group, the most common of which were 7 instances of headache and 2 instances of cold/flu/sinus congestion. None of the placebo group AEs was judged by the clinical investigator to be associated with treatment. There were no serious adverse events reported during the study in either treatment group. There were 7 instances of acetaminophen use in the placebo group (avg. 10.2 mg/subject/day) and 4 instances in the NEM treatment group (avg. 5.1 mg/subject/day) with the vast majority of these instances being related to either headache or cold/flu/sinus congestion. The treatment was reported to be well tolerated by study participants.
Despite the well-studied benefits of exercise, participation rates in physical activity in much of the westernized world remain low [5,4]. Removing or limiting as many barriers to exercise as possible may be a crucial factor in raising participation rates. This trial was designed to evaluate the effects of NEM® brand eggshell membrane in relieving exercise-induced pain, stiffness, and cartilage turnover, some of the major barriers to participation, in articularly healthy men and women. NEM® demonstrated meaningful beneficial effects for all three of these clinical endpoints.
NEM® provided significant relief from the immediate pain resulting from exercise compared to placebo, with the greatest effects seen during the first week of the exercise regimen (ranging from 25.9% to 31.2% better than placebo during this time). Notably, the effect on pain was immediately realized on the first day of the exercise regimen. Although just shy of significance, NEM® provided substantial benefits for recovery pain (12 hours post-exercise) as well during the first week (ranging from 39.3% to 80.3% better than placebo). In a focus group study examining barriers to physical activity, those who were less active mentioned feelings of discomfort, including pain, more often than those who were active [36]. A systematic review examined studies for factors relating to not participating in strength training by older adults and pain was cited as an individual-level barrier [37]. Based upon the effects of NEM® in relieving exercised-induced pain elucidated in this trial, it is clear that NEM® could help to remove one of the major barriers to engaging in physical activity. Future research could examine how NEM® may promote physical activity in various populations by improving exercise tolerability using an electronic monitoring device such as a pedometer.
NEM® also provided significant relief from immediate stiffness resulting from exercise, however not surprisingly this effect was muted compared to pain relief (averaging slightly below 10% better than placebo during the first week), as stiffness occurs more frequently 24-48 hours after exercise as joint inflammation ensues. Although not significant overall, this delayed trend is borne out through substantial reductions in recovery stiffness (12 hours post-exercise) which averaged almost 29% better than placebo during the first week of exercise. Joint stiffness is a symptom that is often considered uncomfortable, which like pain would be a barrier to participating in physical activity.
While NEM® supplementation in this study did not achieve statistical significance overall for either recovery pain or recovery stiffness where the previous similarly designed clinical trial evaluating NEM® had done so [31], there were notable differences in the both the study population and design that likely explain these shortcomings. The previous trial evaluated supplementation in only post-menopausal women, whereas the current trial examined both healthy men and women. Postmenopausal women are thought to have cartilage that is more sensitive to strain than their pre-menopausal counterparts. This is supported by the fact that uCTX-II has been shown to be about 2-fold higher in post-menopausal women than in age-matched pre-menopausal women [29]. The present study included women irrespective of menopausal status. Additionally, the present study included men who, on average, are taller and would have a lesser knee flexion angle when performing the step regimen, which would produce less knee strain. A taller step was used in the present study (9 inches) compared to the previous study (6 inches), nevertheless men in the placebo group experienced on average a 2.3% increase in uCTX-II from the first week of exercise, whereas women in the placebo group experienced on average an 8.8% increase in uCTX-II over the same time period. This nearly 4-fold difference strongly supports the fact that men experienced less knee strain from performing the step exercise, despite the step height increase. The largest effect, however, is likely a result of the proportion of subjects that were non-responders. That is, there were 9 subjects (5F, 4M) in the NEM® treatment group and 5 female subjects in the placebo group that experienced no 12-hour pain from performing the exercise regimen. Similarly, there were 5 subjects (3F, 2M) in the NEM® treatment group and 4 female subjects in the placebo group that experienced no 12- hour stiffness from performing the exercise regimen. In essence, this reduced the study population by 11%-17% as no treatment effect was even possible in these participants. In future studies, it may be necessary to use step platforms of differing height for subjects depending on subject height or their total length to help mitigate these issues. It may also be beneficial to examine exercise regimens that create greater strain in the joints, through higher intensity, duration, or frequency of lower body activity.
Perhaps the most promising and novel effect of NEM® supplementation confirmed in this trial is its chondroprotective effect via reduction of uCTX-II. NEM® supplementation significantly reduced CTX-II in comparison to placebo at both weeks 1 and 2 with a -19.2% and -18.8% absolute treatment effect, respectively. It is worth noting that uCTX-II levels for NEM-treated subjects not only increased less than in placebo subjects but were actually below resting levels for the NEM subjects. The present study confirms in healthy men and women the cartilage-sparing effects from NEM® supplementation observed in the prior postmenopausal women study [31], and first observed in rats [27,28], and dogs [22]. The implications of this prevention of cartilage degradation may be even more important in some subsets of the population including the obese. While historically this might have once been a rather small proportion of the population, in 2006 the obese represented 26.4% of the population while an early release of data from 2018 indicates that obesity rates have continued to rise with 31.7% of U.S. adults ages 20 and older being considered obese [38]. Because of the increased cartilage strain that obese individual’s experience [16], the clinically demonstrated chondroprotective effects of NEM® may serve as a good concomitant intervention for individuals undertaking proper nutritional and exercise interventions to reduce excess weight.
While the mechanism by which NEM® supplementation reduces CTX-II is currently unknown, one hypothesis is that it is a result of oral tolerance [31]. Oral tolerance refers to a reduced response of the peripheral immune system to ingested material after repeated consumption. NEM® contains various types of collagen proteins (types I, V and X collagen). Collagen also serves as a component of cartilage in humans, and antibodies to type II collagen have been found in up to half of individuals with osteoarthritis [39]. The ingestion of NEM® may in turn help to reduce the body’s autoimmune response to collagen within the joints. Substantive support for this hypothesis is derived from in vitro data demonstrating that eggshell membrane hydrolyzate activates NF-κB in both human primary cells and a human leukemic cell line [40], and that NEM® was subsequently shown in vivo to markedly reduce inflammation and cartilage destruction in a collagen-induced arthritis rat model [28], which is known to be mediated by NF-κB [41]. The regulation and activity of NF- κB plays a fundamental role in the proper functioning of the immune system and its dysregulation very likely plays a role in the development of inflammatory diseases.
There was a moderate number of AEs in the trial, all of which were deemed unrelated to treatment, and no serious AEs occurred. Although there were a greater number of AEs in the placebo group (60%) versus the NEM® group (40%), there were no obvious trends in these occurrences. Overall use of acetaminophen was also greater in the placebo group, but again there were no obvious trends and the vast majority of these instances were related to either headache or cold/flu/sinus congestion. No side effects were noted in this trial, nor in any of the six prior trials published to date [23-26,31]. Food-derived natural products such as NEM® would be expected to have a robust safety profile, and this has been confirmed throughout its clinical research, excluding the obvious egg allergy concern.
In conclusion, NEM® brand eggshell membrane, 500 mg once daily, provided rapid relief for exercise-induced joint pain (Day 1) and stiffness (Day 5) immediately following exercise. Moreover, a substantial chondroprotective effect was demonstrated from supplementation with NEM® through a lasting decrease in the cartilage degradation biomarker CTX-II. Treatment with NEM® was reported to be well-tolerated. The beneficial effects of NEM® versus placebo in exercise-induced joint pain, stiffness and cartilage turnover described here for the first time in a broad healthy population (men and women, age 40-72), should help people of this age group to get active and stay active, ultimately maintaining healthy joints longer. Reducing pain and stiffness from exercise and the concern of damaging cartilage is tantamount to reducing these major barriers to participation in increased physical activity and exercise.
The sponsor would like to thank all of the study participants. The study sponsor was ESM Technologies, LLC. KJR & MB are employed by the sponsor. KM and SAD are employed by the CRO and have no other competing interests.
The study sponsor was ESM Technologies, LLC.
KJR and MB are employed by the sponsor. KM and SAD are employed by the CRO and have no other competing interests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar][PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Ruff KJ, Morton KM, Duncan SA, Back M (2024) Eggshell Membrane (NEM®) Reduces Exercise-Induced Joint Pain, Stiffness and Cartilage Turnover in Healthy Men and Women: A Randomized Controlled Trial. Int J Phys Med Rehabil. 12:722.
Received: 18-Jan-2024, Manuscript No. JPMR-24-29220; Editor assigned: 22-Jan-2024, Pre QC No. JPMR-24-29220 (PQ); Reviewed: 07-Feb-2024, QC No. JPMR-24-29220; Revised: 15-Feb-2024, Manuscript No. JPMR-24-29220 (R); Published: 23-Feb-2024 , DOI: 10.35248/2329-9096.24.12.722
Copyright: © 2024 Ruff KJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.