International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Research Article - (2020)Volume 8, Issue 3
Background: Eggshell membrane supplementation has been shown to reduce exercise-induced joint pain and stiffness and lower urinary excretion of C-terminal cross-linked telopeptide of type-II collagen (uCTX-II), a marker of cartilage degradation, in healthy, postmenopausal women. This study was conducted to evaluate whether the combination of eggshell membrane and omega-3 polyunsaturated fatty acids (ES-OM3) versus placebo would reduce levels of uCTX-II and alleviate joint pain or stiffness immediately following exercise and 12 hours post exercise in healthy men and women.
Methods: The study was a two-week randomized, double-blind, placebo-controlled trial. Eighty-five healthy men and women (40-70 years) were randomly assigned to receive either 500 mg eggshell membrane with 1,500 mg fish oil concentrate (n=43) or 2,000 mg placebo (n=42) daily. Subjects performed an aerobic step exercise regimen (40 to 100 steps per leg) on alternating days. The primary endpoint was any statistically significant reduction in exercise-induced cartilage turnover as determined by levels of uCTX-II versus placebo, evaluated after 1 and 2 weeks of exercise. Secondary endpoints were changes in exercise-induced joint pain or stiffness versus placebo, evaluated immediately after exercise and 12 hours post exercise.
Results: ES-OM3 produced a significant reduction in levels of uCTX-II versus placebo after 1 (-12.9%, p=0.035) and 2 weeks of exercise (-17.7%, p=0.019). Compared with placebo, ES-OM3 supplementation significantly relieved joint pain (p<0.05) immediately after exercise and 12 hours after exercise (Days 3,5 and 7 and Days 2 and 8 respectively) and significantly improved levels of stiffness (p<0.05) immediately after exercise and 12 hours after exercise (Day 3, and Days 2,4,6 and 8, respectively).
Conclusion: In healthy adults who performed an aerobic step exercise regimen for two weeks, daily administration of a novel nutraceutical (Move3®), consisting of eggshell membrane and fish oil (ES-OM3) decreased levels of CTX-II, a biomarker for cartilage degradation. ES-OM3 supplementation provided rapid relief of joint pain and stiffness immediately after exercise and 12 hours post exercise. Use of a nutraceutical such as ES-OM3 (Move3®) is a promising new approach for alleviating joint pain and stiffness associated with exercise.
Trial registration: NCT 04215198
Eggshell membrane; Omega-3 polyunsaturated fatty acids, Joint pain and stiffness, Cartilage degradation; Delayed onset muscle soreness, Nutraceutical
AE: Adverse Event; BMI: Body Mass Index; Cr: Urinary Creatinine; CTX-II: C-Terminal Cross-Linked Telopeptideof Type-Ii Collagen; DHA: Docosahexaenoic Acid; DOMS: Delayed Onset Muscle Soreness; ECG: Electrocardiogram; ELISA: Enzyme-linked Immunosorbent Assay (ELISA); EPA: Eicosapentaenoic Acid; FDA: Food and Drug Administration; GAGs: Glycosaminoglycans; IRB: Institutional Review Board; IL-1β: Interleukin-1beta; NSAIDs: Non-Steroidal Anti-inflammatory Drugs; OA: Osteoarthritis; OTC: Over-The-Counter; PUFAs: Polyunsaturated Fatty Acids; RA: Rheumatoid Arthritis; SAE: Serious Adverse Event; TNF-α: Tumor Necrosis Factor- Alpha, uCTX-II: Urinary CTX-II Biomarker
Musculoskeletal conditions are common throughout life, although their prevalence increases with age [1]. They affect muscles, bones, joints, and associated tissues such as tendons, and ligaments [1]. A recent survey indicated that half of all Americans live with a musculoskeletal condition, the same number as those with cardiovascular disease [2]. Musculoskeletal conditions are typically painful and frequently limit mobility, dexterity and functional ability. The most disabling musculoskeletal conditions are associated with osteoarthritis (OA), rheumatoid arthritis (RA), back and neck pain and bone fractures [1].
Although numerous studies have demonstrated the beneficial effects of exercise in relieving symptoms of OA and improving balance and joint flexibility, injuries from sports and exercise can induce sprains and strains and other injuries, especially when exercise is done too intensely, with too great a load, or too long a period [3]. The discomfort often manifests as either pain or stiffness in joints. For both the novice and experienced athlete, delayed onset muscle soreness (DOMS) also may occur following unaccustomed physical activity or when individuals return to exercise after a period of reduced activity [4]. Due to the importance of exercise for overall health and disease prevention, there is a need to evaluate new treatment alternatives that can not only protect joints from potential detrimental effects of exercise but also reduce the resulting pain and stiffness.
Recently, a randomized, double-blind, placebo-controlled clinical study demonstrated that eggshell membrane (NEM®, ESM Technologies, LLC, Carthage, MO) supplementation reduced exercise-induced joint pain and stiffness and lowered the urinary excretion of C-terminal cross-linked telopeptide of type-II collagen (CTX-II), a marker of cartilage degradation, in healthy, postmenopausal women [3]. Eggshell membrane lies between the albumin and the calcified shell of the egg. It is characterized by its bi-layered, mesh-like structure formed by fibrous proteins such as collagen Type I [5]. Eggshell membrane contains chondroitin sulfate, dermatan sulfate, hyaluronic acid, and other glycosaminoglycans (GAGs), and has been shown to reduce the expression of pro-inflammatory cytokines such as interleukin-1beta (IL-1β) and tumor necrosis factor-alpha (TNF- α) [6,7]. In previous studies, NEM® supplementation reduced joint pain and stiffness in humans with OA [8-10], and reduced CTX-II levels in rat models of OA [11] and RA [12], and in dogs with naturally-occurring joint disease [13].
The omega-3 (n-3) and omega-6 (n-6) long-chain polyunsaturated fatty acids (PUFAs) have been shown to play an important role in the hormonal regulation of bone formation [14,15]. The omega-3 PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are found mainly in marine foods, particularly in cold water fish like salmon, sardines, and herring. The modern “Western” diet is typically low in omega-3 PUFAs and high in omega-6 PUFAs [16,17]. The Western diet with its high n-6 content (n-6 to n-3 ratio>15:1) has been associated with numerous inflammatory disorders such as heart disease, diabetes, colitis, and RA [16-18].
PUFAs are essential fatty acids and precursors to eicosanoids such as prostaglandins, thromboxanes, leukotrienes, and resolvins [14]. Omega-6 and omega-3 PUFAs produce a different series of eicosanoids. The eicosanoids produced by omega-6 PUFAs tend to be more pro-inflammatory than those generated by omega-3 PUFAs [14]. A product of omega-6 PUFAs, prostaglandin PGE2, is a potent mediator and regulator of cartilage formation and resorption [19]. At high levels, PGE2 increases the degradation of cartilage [20]. At low levels, PGE2 stimulates bone formation and cartilage production by increasing insulin-like growth factor [15]. Omega-3 PUFAs (EPA and DHA) mediate the degradation effects of high levels of PGE2 [14]. Animal studies have shown that an omega-3 PUFA diet reduced disease in OA-prone animals by increasing GAGs content [14,21,22]. In animals fed omega-3 PUFAs, most cartilage and subchondral bone parameters were changed toward those seen in a non-pathological strain of animals [14].
The aim of this randomized, double-blind, placebo-controlled study is to evaluate whether an investigational product called Move3® consisting of the combination of NEM® and fish oil concentrate (EPA and DHA [kd-pür®] from KD Pharma, Germany) (called ES-OM3 herein) versus placebo would reduce cartilage turnover (as determined by CTX-II) and alleviate joint pain or stiffness immediately following exercise and 12 hours post exercise in healthy adults.
Study design and population
This was a single-center, randomized, double-blind, placebocontrolled, parallel-group trial (NCT 04215198) conducted in accordance with the FDA’s principals of Good Clinical Practice (Title 21, Code of Federal Regulations, Parts 50 and 56 and ICH E6) and the Helsinki Declaration of 1975 as revised in 1983. This study was conducted by QPS Bio-Kinetic (Springfield, MO), a contract research organization. The study began in September 2018 and the final follow-up was conducted in November 2018. Clinical procedures were conducted in a single clinical site located in Springfield, Missouri. The study was approved by the QPS Bio-Kinetic Clinical Applications Investigational Review Board (Springfield, MO). Written informed consent was obtained from subject.
One-hundred each-forty-three (143) male and female subjects from Missouri were screened to participate in the study. Inclusion criteria were: aged between 40-75 years without a diagnosis of joint or connective tissue disorder of the lower extremities (i.e., ankles, knees, or hips); no persistent lowerextremity joint pain at rest, but could have experienced mild periodic lower-extremity joint pain rated ≤ 2 on an 11-point ordinal pain scale (0=no pain, 10=worst pain imaginable); sufficiently healthy to perform moderate exercise (e.g., using an aerobics step platform) as judged by a medical examination including vital signs (i.e. resting heart rate, blood pressure) and an electrocardiogram (ECG). Subjects who were currently taking analgesic or related medications were eligible to participate following a 14-day washout period for non-steroidal antiinflammatory drugs (NSAIDs), a 7-day washout period for opioids, and a 90-day washout period for injected steroids or anti-depressants. Subjects currently taking dietary supplements for joints such as glucosamine, chondroitin sulfate, curcumin, Boswellia, etc. were eligible after a 3-month washout period. Exclusion criteria were: any continued use of prescriptions or over-the-counter (OTC) pain relief medications including NSAIDs, analgesics, opioids, and anti-depressants prescribed for painful conditions such as fibromyalgia; taking remissioninducing drugs such as methotrexate or immunosuppressive medications or had received them within the past 3 months. Subjects were also excluded if they were currently receiving or planning to receive blood-thinning drugs during the study period; had a confounding inflammatory disease or condition (gout, pseudo gout, lupus, Paget ’ s disease, chronic pain syndrome, etc.) that would interfere with assessment of lowerextremity joint pain; participated in activities involving intensive use of the lower extremities (i.e. running, jogging, sports, bicycling, dancing, etc.) two or more days per week or participated in activities that involved moderate use of the lower extremities (i.e. walking, golfing, yoga, etc.) three or more days per week. Other exclusionary criteria were a body weight ≥ 275 pounds (125 kg) and a known allergy to eggs/egg products or fish/fish products, or being pregnant or breastfeeding. Subjects previously enrolled in a research study involving an investigational product (drug, device, or biologic) or a new application of an approved product within 30 days of screening were also not eligible to participate.
At screening, using a 9-inch tall aerobics step platform, subjects performed 40 steps per leg, increasing by ten at a time per leg up to 100 steps per leg, until they experienced at least a 1 unit change in discomfort (either pain or stiffness) from their resting rating. This number of steps was then assigned to that subject for the remainder of the study.
After satisfying all inclusion/exclusion criteria at screening, eligible subjects were then randomized to receive either ES-OM3 or placebo using a permuted block randomization table consisting of four subjects per block. Eighty-five (85) subjects between the ages of 40 and 70 years were eligible to participate (n=85) in the study and were randomly assigned to either the ES-OM3 treatment group (n=43) or placebo (n=42). All but one subject (assigned to the ES-OM3 group) completed the study as per the protocol. The subject who dropped out developed choledocholithiasis just prior to the final exercise visit (Figure 1: CONSORT flow diagram).
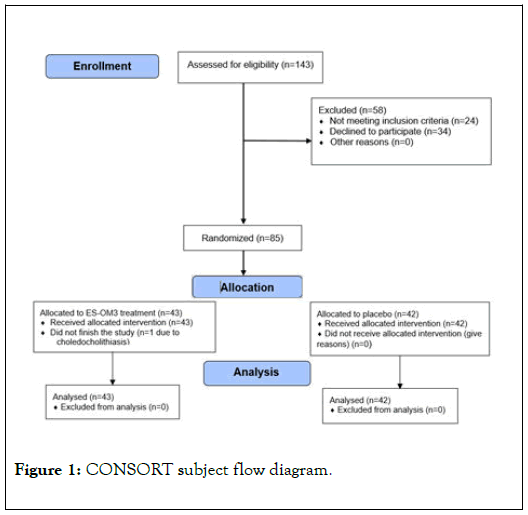
Figure 1. CONSORT subject flow diagram.
Treatment consisted of two daily oral capsules containing a total of either 2,000 mg of Move3® (500 mg of eggshell membrane +1,500 mg fish oil concentrate containing 1,200 mg EPA and DHA) or 2,000 mg placebo. Treatment compliance was checked during clinic visits by an interview conducted by study personnel and by counting the number of unused study capsules. Acetaminophen was allowed for pain relief rescue, if necessary. Subjects recorded the time and amount of acetaminophen taken in subject diaries. All participants, clinical staff, and study management staff remained blinded to treatment assignment throughout the study.
Clinical procedures
The aim of the study was to evaluate whether ES-OM3 would reduce cartilage turnover (via the CTX-II biomarker) or alleviate joint pain or stiffness, either directly following exercise or 12 hours post exercise, versus placebo. An 11-point ordinal scale (0=no pain or stiffness, 10=worst pain or stiffness imaginable) was used to assess the amount of pain/stiffness experienced at baseline and at rest before beginning the exercise regimen and after each exercise event.
Subjects followed their assigned exercise regimen at the clinical site on alternating days for 2 consecutive weeks (i.e. Sunday, Tuesday, Thursday, Saturday, Monday, Wednesday, Friday). Resting (before exercise) pain and stiffness scores were recorded in a questionnaire. The questionnaire consisted of recording a resting score, an assessment of pain/stiffness immediately after exercise (just after and up to 1 hour post exercise) and an approximately 12-hour post exercise assessment. Subjects provided blood and urine samples (from the 2nd void in the morning) at the clinical site about 24 hours after each weekly exercise period, i.e., on Friday of Week 1 and on Saturday of Week 2.
The primary endpoint was any statistically significant change from baseline in the urinary CTX-II biomarker (uCTX-II) (corrected for creatinine) between treatment groups after 1 week and 2 weeks of exercise. Secondary endpoints were any statistically significant changes from baseline in either exerciseinduced joint pain or stiffness (immediately after exercise and 12 hours post exercise) between treatment groups as recorded in the subject questionnaire.
Safety assessments included physical examinations, adverse event (AE) monitoring, assessment of tolerability of investigational products, and changes in vital signs. Subjects underwent a thorough medical examination at screening and upon study completion by a licensed physician. A complete medical history, vital signs (resting heart rate, blood pressure, respirations, and temperature), and ECG were recorded. Subject diaries were reviewed during each clinic visit. Any discomfort beyond what would normally be expected with exercise, tolerability problems, or any other AEs were recorded and reported in accordance with applicable FDA regulations.
Investigational products
ES-OM3 consisted of NEM® (provided by ESM Technologies, LLC; Carthage, MO)USA), which is derived from eggshell membrane and manufactured via a patented process [23] and kd-pür® fish oil concentrate (provided by KD Pharma, Germany, Lot#1709055), that contained ≥ 480 mg of EPA and 320 mg DHA per gram in the form of triglycerides to form Move3.® Two#18 oblong softgel capsules, brown in color, contained a total 500 mg of NEM® plus 1,500 mg of kd-pür® (720 mg of EPA and 480 mg DHA). The placebo consisted of two#18 oblong softgel capsules, brown in color, that contained 2,000 mg of corn oil. Treatment and placebo softgel capsules were identical in appearance, odor, and taste and were stored in closed containers at ambient temperature. Subjects were instructed to take two softgels daily with water, approximately the same time each morning prior to eating breakfast.
Assessment of urinary CTX-II (uCTX-II)
Analysis of uCTX-II has been described previously [3] and is briefly summarized here. Concentrations of uCTX-II were measured via enzyme-linked immunosorbent assay (ELISA) using a commercial immunoassay (Urine CartiLaps® EIA; Immunodiagnostic Systems, Inc.; Gaithersburg, MD). Urinary creatinine (Cr) was measured via a colorimetric assay (Creatinine Urinary Colorimetric Assay Kit; Cayman Chemical Company, Inc.; Ann Arbor, MI) using a SpectraMax Plus 384 microplate reader (Molecular Devices, LLC; Sunnyvale, CA). uCTX-II concentrations were subsequently normalized by dividing uCTXII expressed in micrograms per liter (μg/L) by Cr expressed in millimoles per liter (mmol/L), the results of which were reported as nanograms of uCTX-II per millimole of Cr (ng/mmol Cr). Samples were assayed in duplicate and all assays were repeated (n=4).Duplicate assay values that were within 90% agreement were subsequently averaged. Repeated assay means that were within 90% agreement were further averaged. If repeat assay means differed by more than 10%, an additional assay was performed in duplicate with the remaining frozen aliquot of urine. The additional repeat assay mean was either substituted for the original outlier, or if sufficient agreement wasn’t reached with either original repeat assay mean, then all three were averaged. About 33% of the uCTX-II assays were repeated three times, primarily due to inter-assay variability. Overall, intra-assay coefficients of variation were 5.32 and 1.90 for uCTX-II and Cr, respectively.
Sample size calculation and statistical analysis
The hypothesis for this study was that the ES-OM3 treatment group would be superior to that of the placebo group in limiting the increase in uCTX-II levels resulting from moderate exercise. A 17% absolute change in the mean treatment response (i.e., uCTX-II would increase by an average of at least 17% more in the placebo group than in the ES-OM3 treatment group) was expected based upon results from the previously published study using NEM® alone [3]. A sample size of 84 subjects was needed to achieve a statistical power of 80% to detect a clinically meaningful difference between treatment groups, assuming a rate of response of -8% for the ES-OM3 treatment group and a rate of response of +9% for the placebo group, with no dropouts.
Descriptive statistics were calculated for mean age, height, weight, body mass index (BMI), and number of steps per leg. Kruskal-Wallis test for multiple independent samples was used to compare treatment group baseline data and to validate randomization. Following evaluation for normality (D’Agostino- Pearson), to determine the overall trend, post-baseline betweengroup statistical analyses were completed using either an independent group t-test (for uCTX-II) or repeated measures univariate analysis of variance (rm-ANOVA) for levels of pain and stiffness. Items found to have statistical significance with rm-ANOVA were then compared using a Kruskal-Wallis test for multiple independent samples. Post-baseline within-group statistical analyses were completed using either a paired sample ttest (level of stiffness immediately after exercise) or a pairedsample Wilcoxon test (levels of pain and stiffness immediately after exercise and levels of pain and stiffness 12 hours post exercise). Absolute Treatment Effects (TEabs) were calculated for each outcome value as the net difference between ES-OM3 versus placebo for the mean change (as a percent) from baseline (or resting) to end of Week 1 and Week 2. Negative values for percent differences within groups and between groups and TEabs values represent superior results for the treatment group. Selected statistically significant TEabs values are presented in the results section. All statistical tests used a significance level of p<0.05. Analysis of the primary and all secondary endpoints was conducted on the per protocol population. MedCalc® Software (version 18.11.3) was used for the statistical analyses [24].
Demographic data for the treatment groups, including mean pain scores at baseline/resting, are provided in Table 1. The compliance rate, determined from capsule count, was 99.8% (601/602) in the ES-OM3 group and 99.8% (587/588) in the placebo group. Two subjects in the placebo group were unable to provide urine samples. One subject in the placebo group underwent a colonoscopy near the end of Week 1 and was given fentanyl and benzodiazepine (Versed®); therefore, the subject’s data collected just prior to the colonoscopy were carried forward for the remainder of the study. There were no statistically significant differences between treatment groups in any demographic or baseline parameter (Table 1).
| ES-OM3 | Placebo | |
|---|---|---|
| (n=43) | (n=42) | |
| Age (years) | 55.3 ± 7.6 | 54.5 ± 8.3 |
| Sex | ||
| Female(%) | 31(72) | 31(74) |
| Male(%) | 12(28) | 11(26) |
| Height(cm) | 167 ± 13 | 168 ± 7 |
| Weight(kg) | 84.7 ± 21.1 | 84.0 ± 18.6 |
| BMI(kg/m2) | 29.9 ± 6.6 | 29.7 ± 6.5 |
| uCTX-II, ng/mmol Cr | 315 ± 185 | 273 ± 212 |
| Resting Pain Score | 0.6 ± 0.8 | 0.9 ± 0.8 |
| Resting Stiffness Score | 1.0 ± 1.1 | 1.3 ± 1.2 |
| Number of Steps per Leg | 47.9 ± 12.8 | 45.0 ± 10.9 |
| Baseline Pain Score | 2.2 ± 1.3 | 2.4 ± 1.3 |
| Baseline Stiffness Score | 2.1 ± 1.5 | 2.5 ± 1.7 |
Except where indicated otherwise, values are reported as mean ± standard deviation. BMI body mass index, uCTX-II urinary c-terminal crosslinkedtelopeptide of type-II collagen
Table 1: Participant baseline demographic data.
Table 2 shows the mean scores and within-group differences for each of the outcome measures (uCTX-II, pain, and stiffness) at baseline/resting and after 1 and 2 weeks of exercise. Withingroup statistically significant changes (improvements) from baseline in the ES-OM3 treatment group were observed for uCTX-II at 2 weeks (-12.4%, p=0.009). No significant withingroup differences were observed for uCTX-II in the placebo group. For the ES-OM3 group, within-group statistically significant changes (improvements) were also observed for pain experienced immediately after exercise at 1 week (-31.8%, p=0.012) and at 2 weeks (-40.9%, p<0.001), and for stiffness experienced immediately after exercise at 2 weeks (-28.6%, p=0.016) (Table 2). For the placebo group, within-group statistically significant changes (improvements) from baseline were observed for pain experienced immediately after exercise at 2 weeks (-25.0%, p=0.029) and for stiffness experienced immediately after exercise at 2 weeks (-32.0%, p=0.014).
| Weeks | Treatment | ||
| Post-treatment | ES-OM3 (%) | Placebo (%) | |
| uCTX-II | Resting (n=43, 40) | 315 ± 185 | 273 ± 212 |
| 1 (n=43,40) | 296±193 (-6.0) | 292±228(+7.0) | |
| 2 (n=43,40) | 276±175(12.4)* | 288±269(+5.5) | |
| Immediate Pain | Baseline(n=43,42) | 2.2 ± 1.3 | 2.4 ± 1.3 |
| 1(n=43,42) | 1.5±1.5(-31.8)* | 2.1±1.5(-12.5) | |
| 2(n=43,42) | 1.3±1.4(40.9)** | 1.8±1.5(25.0)* | |
| 12-hour Pain | Resting(n=43,42) | 0.6 ± 0.8 | 0.9±0.8 |
| 1(n=43,42) | 0.8±1.2(+33.3) | 1.5±1.7(+66.7) | |
| 2(n=43,42) | 0.6±0.9(0.0) | 1.0±1.5(+11.1) | |
| Immediate stiffness | Baseline(n=43,42) | 2.1 ± 1.5 | 2.5 ± 1.7 |
| 1(n=43,42) | 1.7±1.4(-19.0) | 2.0±1.5(-20.0) | |
| 2(n=43,42) | 1.5±1.4(-28.6)* | 1.7±1.7(-32.0)* | |
| 12-hour Stiffness | Resting(n=43,42) | 1.0 ± 1.1 | 1.3 ± 1.2 |
| 1(n=43,42) | 0.9±1.1(-10.0) | 1.5±1.6(+15.4) | |
| 2(n=43,42) | 0.7±0.9(-30.0) | 1.1±1.4(-15.4) |
Except where indicated otherwise, values are reported as mean ± standard deviation. uCTX-II urinary c-terminal crosslinked telopeptide of type-II collagen.*p<0.05, **p<0.01, within-group from resting or baseline, percent difference in parenthesis
Table 2: Mean uCTX-II, pain and stiffness scores immediately after exercise and 12-hours post exercise for the ES-OM3 treatment and placebo groups at baseline (or resting) and after 1 and 2 weeks of exercise.
Large between-group (placebo versus ES-OM3) statistically significant changes (improvements) were observed for pain immediately after exercise (-28.6%, p=0.049) at 1 week, pain 12 hours after exercise (-46.7%, p=0.035) at 1 week, and stiffness 12 hours after exercise at 1 week (-40%, p= 0.042).
As measured by TEabs, for the primary outcome measure (uCTX-II), supplementation with ES-OM3 produced a significant absolute treatment effect versus placebo after 1 (TEabs-12.9%, p=0.035) and 2 weeks of exercise (TEabs -17.7%, p=0.019) (Figure 2).
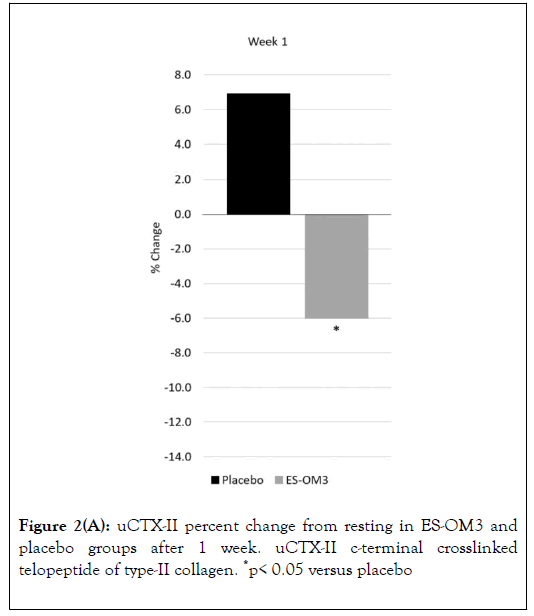
Figure 2A. uCTX-II percent change from resting in ES-OM3 and placebo groups after 1 week. uCTX-II c-terminal crosslinked telopeptide of type-II collagen. *p< 0.05 versus placebo
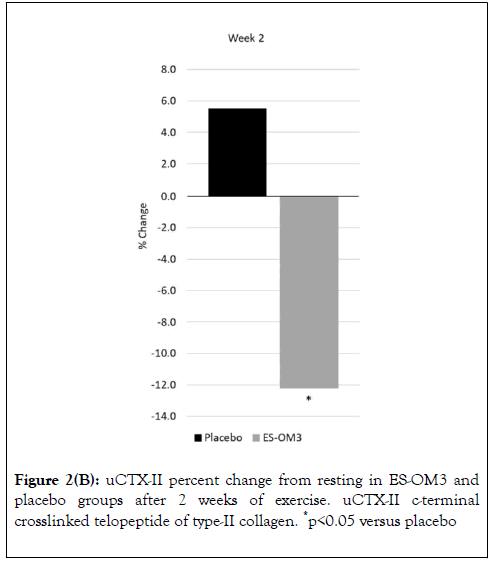
Figure 2B. uCTX-II percent change from resting in ES-OM3 and placebo groups after 2 weeks of exercise. uCTX-II c-terminal crosslinked telopeptide of type-II collagen. *p<0.05 versus placebo
Trend data indicated that significant differences between treatment groups for level of pain immediately after exercise occurred on Days 3,5 and 7 (Figure 3A). However, the overall trend for pain immediately after exercise in the ES-OM3 group versus placebo fell just shy of statistical significance (p=0.065) (Figure 3A). The overall trend for pain 12 hours post exercise (recovery) in the ES-OM3 group was significantly different from placebo (p = 0.019); significant differences in levels of pain 12 hours post exercise occurred on Days 2 and 8 (Figure 3B).
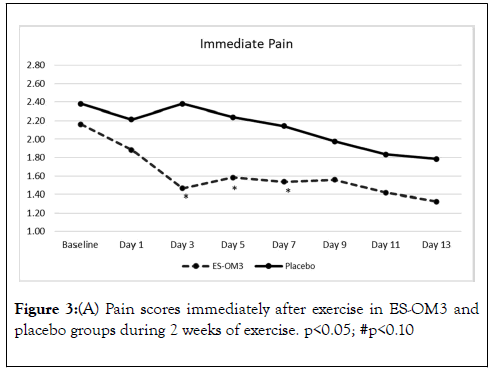
Figure 3A. Pain scores immediately after exercise in ES-OM3 and placebo groups during 2 weeks of exercise. p<0.05; #p<0.10
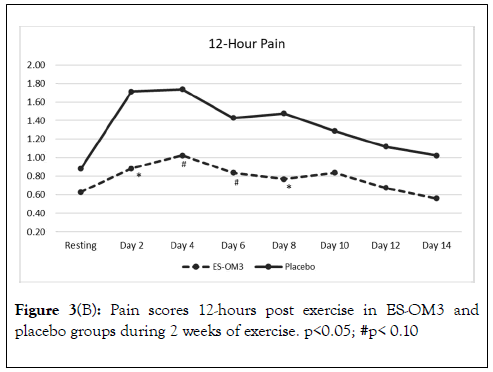
Figure 3B. Pain scores 12-hours post exercise in ES-OM3 and placebo groups during 2 weeks of exercise. p<0.05; #p< 0.10
The overall trend for stiffness immediately after exercise in the ES-OM3 group was not significantly different from placebo (p=0.442) (Figure 4A), but a significant difference in the level of stiffness immediately after exercise occurred on Day 3 (p<0.05). The overall trend for stiffness 12 hours post exercise in the ESOM3 group was significantly different from placebo (p=0.031); significant differences in levels of stiffness 12 hours post exercise occurred on Days 2,4,6 and 8 (Figure 4B). Both treatment groups experienced less stiffness 12 hours post exercise (recovery) as the study progressed. However, the ES-OM3 treatment group showed a greater benefit (Day 14, TEabs -11.8%).
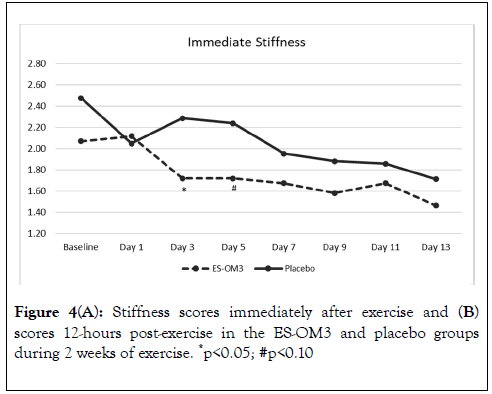
Figure 4A. Stiffness scores immediately after exercise and (B) scores 12-hours post-exercise in the ES-OM3 and placebo groups during 2 weeks of exercise. *p<0.05; #p<0.10
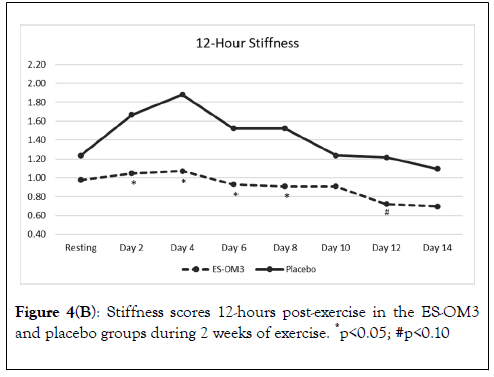
Figure 4B. Stiffness scores 12-hours post-exercise in the ES-OM3 and placebo groups during 2 weeks of exercise. *p<0.05; #p<0.10
The investigational products were well tolerated by all study participants. There were fifteen (15) non-exercise-related AEs reported in the ES-OM3 treatment group, the most common of which were 6 instances of headache and 2 instances each of cold/flu/sinus congestion, and nausea. None of the ES-OM3 treatment group AEs were judged by the principal investigator to be associated with the investigational product. There were eighteen (18) non-exercise-related AEs reported in the placebo group, the most common of which were 7 instances of headache and 2 instances of cold/flu/sinus congestion. None of the placebo group AEs were judged to be associated with the investigational product. There was one serious adverse event (SAE) reported during the study in the ES-OM3 treatment group. A subject developed acute choledocholithiasis that required surgical intervention. This was deemed unrelated to the treatment by the principal investigator due to the patient’s medical history. There were 7 instances of acetaminophen use in the placebo group (avg.10.4 mg/subject/day) and no instances of acetaminophen use in the ES-OM3 treatment group.
This randomized, double-blind, placebo-controlled study conducted in healthy adults aimed to evaluate whether ES-OM3 (Move3®), a nutraceutical product containing a combination of eggshell membrane (NEM®) and concentrated omega-3 PUFAs (EPA and DHA), would reduce cartilage turnover (via the CTXII biomarker) or alleviate joint pain or stiffness, either directly following exercise or 12 hours after exercise, versus placebo. In a previous clinical trial conducted in postmenopausal women [3], NEM® alone produced a within-group significant change (improvement) for uCTX-II at 1 week and a significant TEabs effect versus placebo after both weeks of exercise. In the present study, for uCTX-II in the ES-OM3 group, a within-group significant improvement was observed at 2 weeks and a significant TEabs effect versus placebo was also seen after both weeks of exercise.
Notably, uCTX-II level in the ES-OM3 group was reduced by 12.4% but increased by 5.5% in the placebo group after 2 weeks of treatment and exercise. Like eggshell membrane, omega-3 fatty acid PUFAs mediate the expression of pro-inflammatory eicosanoids and cytokines, increase cartilage GAG content, and Ruff KJ, et al. Int J Phys Med Rehabil, Vol.8 Iss.3 No:1000551 7 modify cartilage turnover. As shown in OA-prone animals (guinea pigs), omega-3 PUFAs produce cartilage and subchondral bone parameter changes similar to those seen in a non-pathological strain of animals [14]. The present study clearly demonstrates that ES-OM3 has the potential to reduce the level of CTX-II and prevent or delay cartilage degradation processes after exercise.
In line with the results reported in the previous trial [3], the overall trend for the ES-OM3 treatment group for pain immediately after exercise was not significantly different from placebo. In the present study, significant differences from placebo in the level of pain immediately after exercise occurred early and for an extended period of time (on Days 3,5 and 7).
The overall trend for pain 12 hours post exercise (recovery) was significantly different from placebo; significant differences occurred on Days 2 and 8. These results are corroborative of the results reported in the previous trial [3]. In the present study, the recovery effect was greatest during the first week of exercise. ESOM3 provided early relief from pain 12 hours after exercise, with the effect seen on Day 2.
In the present study, for subjects in the ES-OM3 group, the overall trend for stiffness immediately after exercise was not significantly different from placebo, but a significant difference versus placebo occurred on Day 3. Similar to the previous study [3], for subjects in the ES-OM3 group, the overall trend for stiffness 12 hours post exercise was significantly different from placebo; significant differences versus placebo occurred at Days 2,4,6 and 8. For those subjects given ES-OM3, there was a rapid improvement in both immediate stiffness compared with placebo (Day 3) and recovery from stiffness (Day 2).
DOMS frequently occurs following physical activity [4]. The intensity of discomfort (e.g., stiffness) usually develops in a delayed fashion, increasing within the first 24 hours after exercise and peaks between 24 and 72 hours following exercise [4]. It is clinically meaningful that ES-OM3 quickly improves stiffness after exercise. Stiffness is commonly used as an indirect measure of joint inflammation [4]. There is strong evidence that eggshell membrane and particularly omega-3 PUFAs mediate the highly pro-inflammatory effects of certain cytokines, eicosanoids, and reactive oxygen species associated with inflammation [14,25]. Thus, a nutraceutical product containing a combination of these products may play an important role in relieving localized joint stiffness and/or inflammation resulting from exercise. Further studies are warranted to determine the long-term influence of ES-OM3 on cartilage degradation in healthy subjects who exercise frequently.
The use of a different population and study design may explain why the administration of ES-OM3 did not achieve statistical significance versus placebo for the overall trend for stiffness immediately after exercise while NEM® alone [3] did. In this study, both men and women were considered. Men who, on average, are taller than women have a smaller knee flexion angle when performing the step exercise. The smaller knee flexion angle likely results in less knee strain. Post-menopausal women may have cartilage that is more sensitive to strain than their premenopausal counterparts. This is supported by the fact that the uCTX-II has been reported to be about 2-fold higher in postmenopausal women compared with age-matched premenopausal women [26]. The present study included women irrespective of menopausal status. Another possible reason for the difference in results is that there were several nonresponders: 5 subjects (3F, 2M) in the placebo group who experienced no stiffness immediately after performing the exercise regimen. This reduced the sample size of the placebo group by 12%. As a result, a statistically significant treatment effect could not be achieved. In future studies, it may be necessary to use step platforms of differing heights for subjects depending on their height and/or their tibial length. It may also be beneficial to examine exercise regimens that create greater strain in the joints, through higher intensity, duration, or frequency of lower body activity.
All the AEs (n=33) and SAEs (n=1) reported in the trial were deemed unrelated to treatment. There were slightly more AEs in the placebo group (n=18) than in the ES-OM3 group (n=15); this difference was not statistically significant. There was no acetaminophen used in the treatment group, whereas the placebo group had 7 instances of use. Although acetaminophen use was low in the placebo group and both groups had similar instances of headache (placebo=7, ES-OM3=6), it is interesting that the ES-OM3 treatment group did not report a similar rate of acetaminophen use. There is some preliminary evidence that increasing dietary omega-3 fatty acids while reducing omega-6 fatty acids can reduce both the duration and intensity of headaches in chronic sufferers [27]. Although the present study was short in duration, the repeated exercise regimen may have enhanced the metabolism of the omega-3 fatty acids leading to an apparent reduction in nociception. No side effects were noted in this trial. This may be related to the fact that kd-pür® is a concentrated formulation of EPA and DHA resulting in a much smaller dose of total oil consumed. NEM® also has not been shown in any of the previously published trials to have known side effects [8-10].
A two-week daily administration of a novel nutraceutical (Move3®), consisting of eggshell membrane and fish oil (EPA and DHA) (ES-OM3) decreased levels of CTX-II, a biomarker for cartilage degradation. ES-OM3 supplementation provided rapid relief from joint pain and stiffness immediately after exercise and 12 hours post exercise. Administration of ES-OM3 was well-tolerated in all subjects. The use of a nutraceutical such as ES-OM3 (Move3®) is a promising new approach for alleviating joint pain and stiffness associated with exercise.
The scientific advice and role in designing this trial from the late Dr. David Barnes is gratefully acknowledged.
KJR, MB, and ASR co-wrote the manuscript. KM and SAD selected the subjects, conducted site monitoring and performed the statistical analysis. AI coordinated the provision of investigational products and helped revise the manuscript. All Ruff KJ, et al. Int J Phys Med Rehabil, Vol.8 Iss.3 No:1000551 8 authors reviewed the results and approved the final version of the manuscript.
The study was sponsored by KD Pharma Group and was conducted under license from ESM Technologies, LLC (U.S. Patent 9,983,214). The sponsor had no role in the collection, analyses, or interpretation of data.
AI is employed by the sponsor. KJR and MB are employed by the licensor. KM and SAD are employed by the QPS Bio- Kinetic. ASR received funds from the sponsor to help draft the manuscript. The authors do not have any other competing interests.
Citation: Ruff KJ, Morton K, Duncan SA, Back M, Ismail A, Ryan AS (2020) Eggshell Membrane+Fish Oil Combination (Move3®) Reduces Exercise-Induced Joint Pain, Stiffness and Cartilage Turnover in Healthy Adults: Results from a Randomized, Double-Blind, Placebo-Controlled Study. Int J Phys Med Rehabil 8:551. DOI: 10.35248/2329-9096.20.08.551
Received: 22-May-2020 Accepted: 05-Jun-2020 Published: 12-Jun-2020 , DOI: 10.35248/2329-9096.20.08.551
Copyright: © 2020 Ruff KJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : The study was sponsored by KD Pharma Group and was conducted under license from ESM Technologies, LLC (U.S. Patent 9,983,214). The sponsor had no role in the collection, analyses, or interpretation of data.